Abstract
Tumor antigen–specific CD4+ and CD8+ T lymphocytes, especially interferon-γ (IFN-γ)–producing type-1 helper T (Th1) and type-1 cytotoxic T (Tc1) cells, play a crucial role in tumor eradication. Adoptive transfer using tumor-specific Th1 and Tc1 cells is a promising therapeutic strategy for tumor immunotherapy. However, its clinical application has been hampered because of difficulties in generating tumor-specific Th1 cells from patients with tumors. To overcome this problem, we have developed an efficient method to prepare tumor-specific Th1 and Tc1 cells. T-cell receptor (TCR) α and β genes obtained from an HLA-A24–restricted, Wilms tumor 1 (WT1) peptide–specific Tc clone were lentivirally transduced to polyclonally activated Th1 and Tc1 cells. As expected, TCR gene-modified Tc1 cells showed cytotoxicity and IFN-γ production in response to peptide-loaded lymphoblastoid cell lines, WT1 gene–transduced cells, and freshly isolated leukemia cells expressing both WT1 and HLA-A24. Surprisingly, we further demonstrated that Th1 cells transduced with HLA-class I–restricted TCR genes also showed both cytotoxicity and cytokine production in an HLA-A24–restricted manner. In contrast to gene-modified Tc1 cells, Th1 cells produced high amounts of interleukin-2 (IL-2) in addition to IFN-γ, which is beneficial for induction of antitumor cellular immunity. Thus, TCR gene–modified HLA-class I–restricted Th1 and Tc1 cells are a powerful strategy for the application to adoptive immunotherapy of human cancer.
Introduction
It has been shown that tumor-specific type-1 immunity, which is controlled mainly by tumor antigen–specific type-1 helper T (Th1) and type-1 cytotoxic T (Tc1) cells, play a critical role in tumor eradication.1-3 Tumor-specific Tc1 cells can directly destroy tumor cells when they recognize tumor antigenic peptide bound by major histocompatibility complex (MHC) class I molecules. On the other hand, Th1 cells produce interleukin-2 (IL-2) and interferon-γ (IFN-γ) by recognition of antigenic peptide presented on MHC class II molecules. Although few tumor cells express MHC class II molecules essential for Th cell activation, it is widely accepted that Th cells, especially Th1 cells, play an important role in eradication of tumor cells via cellular immunity.4-6 This may be because tumor-specific Th1 cells provide local help to enhance antitumor cellular immunity by interacting with tumor antigen-bound MHC class II molecules on antigen-presenting cells (APCs) such as dendritic cells (DCs). In addition, direct interaction of Th cells with MHC class II molecules expressed on tumor cells may result in stronger induction of tumor-specific Tc cells.7-9
Many tumor-associated antigens derived from various types of tumors have been characterized.10,11 The identification of tumor-associated peptides, which are bound by MHC class I molecules and recognized by CD8+ Tc cells, make it possible to induce tumor-specific Tc cells from peripheral blood mononuclear cells (PBMCs) of healthy donors as well as patients with tumors. In contrast to Tc cells, tumor antigen–specific Th cells can be generated only for a limited number of patients with cancer because HLA class II–binding peptides were generally determined for only a few human leukocyte antigen (HLA) types such as DRB1*0401. Moreover, there are significant difficulties in the generation and propagation of tumor-specific Th cells from patients with tumors. Therefore, it is critically important to develop an efficient method to prepare tumor-specific Th1 cells in vitro.
Recently, it has been shown that antigen specificity can be transferred to nonspecific T cells by transducing both T-cell receptor (TCR) α and TCR β genes obtained from antigen-specific T cells.12-19 In most experiments, MHC class I–restricted TCR genes, which were obtained from MHC class I–restricted antigen-specific Tc cells, were transduced into nonspecific Tc cells.12-17 Some investigators also reported successful transduction of MHC class II–restricted TCR genes to nonspecific Th cells to transfer antigen specificity.18,19 Recently, we have established an efficient method to generate antigen-specific mouse Th1 cells from polyclonally activated Th1 cells by transducing MHC class II–restricted TCR genes. We also demonstrated that the gene-modified, antigen-specific Th1 cells exhibited potent antitumor activity both in vitro and in vivo.19 Here, we tried to extend our findings to establish a method for preparing human tumor–specific Th1 cells. However, it is difficult to induce tumor-specific Th cells, which are an essential source for preparing MHC class II–restricted TCR genes, because few MHC class II–binding tumor peptides have been identified. To overcome this problem, we developed an alternative approach for preparing tumor-specific Th1 cells. Our working hypothesis is that transfer of tumor antigen specificity into nonspecifically activated Th cells from patients by gene transduction with MHC class I–restricted TCR genes is an effective method for preparing tumor antigen–specific Th1 cells from many patients. Our working hypothesis is based on the following evidence: (1) it is easy to induce MHC class I–restricted tumor-specific Tc cells, because hundreds of MHC class I–binding tumor peptides from a variety of tumor cells have been identified; (2) MHC class I–restricted TCR genes recognizing tumor-antigen peptide bound by various HLA genotypes are available from these Tc clones; and (3) it has already been shown that MHC class I–restricted tumor antigen specificity can be transferred to CD4+ T cells as well as to CD8+ T cells.20
In the present study, we developed a novel strategy to induce both tumor-specific Th1 and Tc1 cells by lentiviral transduction of HLA-A24–restricted TCR α and β chain genes isolated from a WT1-specific Tc clone. TCR gene-transduced Th1 and Tc1 cells, expressing tumor-specific TCR complex on the cell surface, exhibited both cytotoxicity and cytokine production in response to WT1 tumor peptide–pulsed HLA-A24+ lymphoblastoid cell lines (LCLs) and freshly isolated HLA-A24+ WT1+ leukemia cells. Thus, it is feasible to prepare human tumor–specific Th1 cells from polyclonally activated Th cells if we obtain MHC class I–restricted TCR genes. We believe that our established method will contribute to the development of a novel tailor-made immunotherapy of human tumors using gene-modified tumor-specific Th1 and Tc1 cells.
Materials and methods
Construction of lentiviral vector containing WT1-specific TCR genes
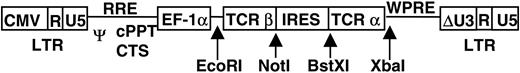
The generation and characterization of the HLA-A24–restricted WT1 peptide (CMTWNQMNL)–specific Tc clone, TAK-1, were described previously.21,22 Total RNA of TAK-1 was extracted by using Isogene reagent (Nippon Gene, Tokyo, Japan) and converted to cDNA by reverse transcription using oligo-dT primer and superscript II reagent (Invitrogen, Carlsbad, CA). 5′-rapid amplification of cDNA ends–polymerase chain reaction (RACE-PCR) was performed by SMART RACE cDNAAmplification Kit (Clontech Laboratories, Palo Alto, CA) using gene-specific antisense primers for the constant region of TCR α and β chain genes. PCR products were inserted into pCR II-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced. Coding regions of TCR α and β chain genes were amplified with primers containing restriction enzyme sites and inserted between BstXI and XbaI sites and EcoRI and NotI sites, respectively, of a lentiviral self-inactivating vector (CSII-EF-MCS-IRES-hrGFP).23,24 A schematic of the lentivirus plasmid vector carrying TCR α and β chain genes (CSII-β-IRES-α) is shown in Figure 1. Other constructs used to produce the lentivirus vector have been described elsewhere.25
Production of recombinant lentivirus particles
Production of lentivirus vectors was carried out as described previously.23-25 Briefly, 293T cells were transfected with CSII-β-IRES-α together with packaging construct (pMDLg/p RRE), REV expression construct (pRSVrev), and envelope construct (pMD.G) by the calcium phosphate method. After 16 hours, culture medium was replaced with fresh medium containing 10 μM forskolin (Sigma, St Louis, MO), and the cells were incubated for 48 hours. Lentivirus containing supernatant was cleared by 0.45-μm filter and concentrated to about 1/100 by ultracentrifugation. The concentrated lentivirus stock was stored at –80°C until use.
Schematic of a self-inactivating vector carrying WT1-specific TCR α and β chain genes. The coding region of WT1-specific TCR genes was obtained from cDNA of the WT1-specific Tc clone, TAK-1, by a 5′-RACE–PCR method. Genes encoding WT1-specific TCR α and β chains were inserted into self-inactivating lentivirus vector (CS II-MCS-IRES-GFP) as described in “Materials and methods,” and CS II-β-IRES-α was constructed. Ψ indicates packaging signal; RRE, rev responsive element; cPPT, central polypurine tract; CTS, central termination sequence; EF-1α, human elongation factor 1α subunit gene promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; LTR, long terminal repeat; and IRES, internal ribosome entry site.
Schematic of a self-inactivating vector carrying WT1-specific TCR α and β chain genes. The coding region of WT1-specific TCR genes was obtained from cDNA of the WT1-specific Tc clone, TAK-1, by a 5′-RACE–PCR method. Genes encoding WT1-specific TCR α and β chains were inserted into self-inactivating lentivirus vector (CS II-MCS-IRES-GFP) as described in “Materials and methods,” and CS II-β-IRES-α was constructed. Ψ indicates packaging signal; RRE, rev responsive element; cPPT, central polypurine tract; CTS, central termination sequence; EF-1α, human elongation factor 1α subunit gene promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; LTR, long terminal repeat; and IRES, internal ribosome entry site.
Generation of TCR gene–transduced Th1 and Tc1 cells
PBMCs were isolated from healthy donors by density gradient using Ficoll-Paque reagent (Amersham Biosciences AB, Uppsala, Sweden) and were activated with 20 μg/mL phytohemagglutinin-P (PHA; Honen, Tokyo, Japan). After 36 hours, cells were harvested, washed, and infected with lentivirus vector on a 96-well plate precoated with both RetroNectin (25 μg/mL; Takara, Ohtu, Japan) and anti-CD3 monoclonal antibody (mAb; 5 μg/mL; Pharmingen, San Diego, CA). Cells were expanded for about 10 days, and a fraction of the cells was stained with phycoerythrin (PE)–conjugated anti-CD4 or anti-CD8 mAb (Nichirei, Tokyo, Japan) together with fluorescein isothiocyanate (FITC)–conjugated Vβ5.1 mAb (Immunotech, Marseille, France) to determine transduction efficiency. Fluorescence intensity of the cells was measured by a FACSCalibur instrument and analyzed by CellQuest software (BD Biosciences, San Jose, CA). The remaining cells were stained with PE-conjugated anti-CD4 mAb and FITC-conjugated anti-CD8 mAb and sorted into CD4+ and CD8+ T cells by a FACSVantage instrument (BD Biosciences). In some experiments, sorted CD4+ and CD8+ cells were restimulated by LCLs pulsed with WT1 peptide and expanded. To polarize cells into type-1 T cells, interleukin-12 (IL-12; 50 U/mL; kindly donated from Genetics Institute, Cambridge, MA), IFN-γ (10 ng/mL; PeproTech, Rocky Hill, NJ), IL-2 (100 U/mL; kindly donated from Shionogi Pharmaceutical Institute, Osaka, Japan), and anti–IL-4 mAb (5 μg/mL; Pharmingen) were added for 10 days.
Evaluation of antitumor activity
LCLs with various combinations of HLA types were generated and maintained in our laboratory. HLA-A24+ LCLs, which express WT1, were generated by retroviral transduction of WT1 gene. Reverse transcription (RT)–PCR analysis showed that WT1 gene–transduced LCLs expressed strong level of WT1 mRNA, while their parental LCLs showed no WT1 mRNA expression (data not shown). Primary leukemia cells were obtained from patients with leukemia by a density gradient method. Antitumor activity of TCR gene-transduced Th1 and Tc1 cells was investigated against HLA-A24+ LCLs pulsed with 10 μg/mL WT1 peptide (CMTWNQMNL), which binds to HLA-A24 and is recognized by the TAK-1 Tc clone. HLA-A24 binding peptide derived from human cytomegalovirus (CMV) pp65 protein (QYDPVAALF)26 was used as control irrelevant peptide. Cytotoxicity against peptide-loaded or unloaded LCLs or leukemia cells was measured by 4-hour 51Cr-release assay. To evaluate antigen-specific cytokine production, 1 × 105 TCR gene-transduced or control T cells were cocultured with 5 × 104 stimulator cells for 20 hours. Cytokine levels in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA). In some experiments, 10 μg/mL mAb against HLA-A2 (BB7-2) or -A24 (A11.1M) were added to determine MHC restriction.
Approval was obtained from the institutional review board of the Institute for Genetic Medicine, Hokkaido University, Hokkaido University School of Medicine, and Ehime University School of Medicine for these studies. Informed consent was provided according to the Declaration of Helsinki.
Results
Lentiviral transduction of TCR genes into T cells
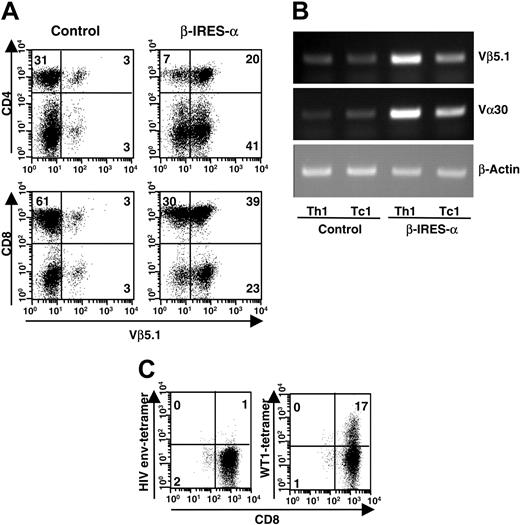
PBMCs from healthy donors were nonspecifically activated with PHA and plate-bound anti-CD3 mAb under type-1 T-cell–inducing conditions (IL-12, IFN-γ, IL-2, and anti–IL-4 mAb) and infected with a lentivirus carrying HLA-A24–restricted WT1-specific TCR α and β chain genes. The subtypes of the variable region of the transduced TCR α and β chain genes were determined as Vα30 and Vβ5.1, respectively, from their DNA sequences. Cells were expanded for 10 days under type-1 conditions, and expression of the transduced TCR β chain was determined by staining with FITC-conjugated anti-Vβ5.1 mAb to evaluate transduction efficiency. As shown in Figure 2A, the percentage of Vβ5.1 expressing CD4+ and CD8+ T cells was remarkably increased after TCR gene transfer. Transduction efficiency was estimated at about 70% in CD4+ T cells and 60% in CD8+ T cells. Expression of Vβ5.1 and Vα30 mRNA was also investigated by RT-PCR using CD4+ and CD8+ T cells isolated from control or TCR gene–transduced T cells (Figure 2B). Consistent with the intensity of cell-surface expression of TCR Vβ5.1, the mRNA expression levels of TCR Vβ5.1 were increased in TCR gene–transduced Th1 and Tc1 cells compared with control cells. We also confirmed that TCR gene–transduced Th1 and Tc1 cells show increased levels of Vα30 mRNA expression compared with control cells. Expression of WT1 peptide–specific TCR complex was investigated by staining with HLA-A24 tetramer loaded with WT1 peptide, which reacted to the original HLA-A24–restricted WT1-specific CTL clone, TAK-1 (data not shown). As shown in Figure 2C, TCR gene–transduced Tc1 cells were stained with WT1 tetramer, demonstrating that the transduced TCR α and β chains recognized HLA-A24–restricted WT1 peptide as well as native TCR complex. On the other hand, such positive staining was not observed in TCR gene–transduced Tc1 cells treated with HLA-A24 tetramer loaded with unrelated HLA-A24–binding peptide derived from HIV envelope (Figure 2C). Control Tc1 cells were not stained with WT1 or HIV tetramer (data not shown). These data indicated that class I–restricted TCR α and β chain genes of the WT1-specific Tc clone were successfully expressed by nonspecific Th1 and Tc1 cells by lentiviral transduction of TCR genes.
Expression of transduced WT1-specific TCR. PBMCs were activated by PHA and plate-bound anti-CD3 mAb in the presence of type-1–inducing conditions and infected with a lentivirus carrying WT1-specific TCR α and β chain genes or mock virus. Ten days after infection, expression of the transduced TCR (Vα30 and Vβ5.1) was examined. (A) Cells were stained with FITC-conjugated anti-TCR Vβ5.1 mAb and either PE-conjugated anti-CD4 or anti-CD8 mAb. Fluorescence intensity of the cells was measured by a FACSCalibur instrument and analyzed by CellQuest software. (B) After isolation of CD4+ and CD8+ T cells by a FACSVantage instrument, mRNA was extracted and converted to cDNA by reverse transcription. TCR Vα30 and Vβ5.1 cDNA were amplified by PCR, separated on 1% agarose gel, and visualized with ethidium bromide. (C) TCR gene–transduced Tc1 cells were stained with PE-conjugated HLA-A24 tetramer loaded with WT1 peptide or HIV envelope peptide. Then, cells were stained with FITC-conjugated anti-CD8 mAb. Staining profile of the cells was measured by a FACSCalibur instrument and analyzed by CellQuest software. In panels A and C, percentage of cells in each quadrant is indicated in cytometer plots.
Expression of transduced WT1-specific TCR. PBMCs were activated by PHA and plate-bound anti-CD3 mAb in the presence of type-1–inducing conditions and infected with a lentivirus carrying WT1-specific TCR α and β chain genes or mock virus. Ten days after infection, expression of the transduced TCR (Vα30 and Vβ5.1) was examined. (A) Cells were stained with FITC-conjugated anti-TCR Vβ5.1 mAb and either PE-conjugated anti-CD4 or anti-CD8 mAb. Fluorescence intensity of the cells was measured by a FACSCalibur instrument and analyzed by CellQuest software. (B) After isolation of CD4+ and CD8+ T cells by a FACSVantage instrument, mRNA was extracted and converted to cDNA by reverse transcription. TCR Vα30 and Vβ5.1 cDNA were amplified by PCR, separated on 1% agarose gel, and visualized with ethidium bromide. (C) TCR gene–transduced Tc1 cells were stained with PE-conjugated HLA-A24 tetramer loaded with WT1 peptide or HIV envelope peptide. Then, cells were stained with FITC-conjugated anti-CD8 mAb. Staining profile of the cells was measured by a FACSCalibur instrument and analyzed by CellQuest software. In panels A and C, percentage of cells in each quadrant is indicated in cytometer plots.
WT1 peptide–specific cytotoxicity mediated by TCR gene–transduced Th1 and Tc1 cells
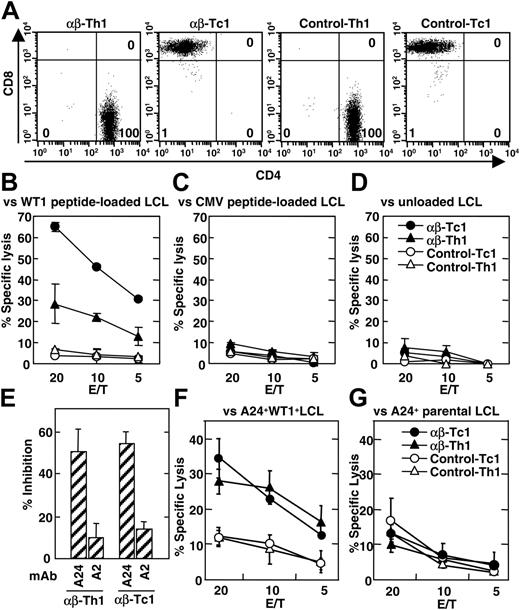
To investigate WT1-specific reactivity of TCR gene–transduced CD4+ Th1 and CD8+ Tc1 cells, CD4+ Th1 and CD8+ Tc1 cells were enriched from heterogeneous T-cell populations, which had been transduced with WT1-specific TCR genes and expanded under type-1 T-cell–inducing conditions. Fluorescence-activated cell sorter (FACS)–isolated Th1 and Tc1 cells were examined for their CD4 and CD8 expression as shown in Figure 3A before functional assay. The purity of CD4+ Th1 and CD8+ Tc1 cells was greater than 98% in all experiments. Then, the ability of the cells to lyse WT1-expressing target cells was determined by 51Cr-release assay. TCR gene–transduced Tc1 cells showed strong cytotoxicity against WT1 peptide–pulsed HLA-A24+ LCLs but not irrelevant HLA-A24 binding CMV peptides pulsed or peptide–unloaded LCLs (Figure 3B). Control Tc1 cells exhibited no significant cytotoxicity against WT1 peptide–pulsed LCLs. These results indicated that TCR gene–modified Tc1 cells exhibit cytotoxicity against peptide–loaded LCLs in a WT1 peptide–specific manner. TCR gene–transduced Tc1 cells from HLA-A24+ donors could respond to both autologous and allogeneic WT1 peptide–pulsed HLA-A24+ LCLs with similar efficacy. Moreover, HLA-A24+ and HLA-A24– TCR gene–transduced Tc1 cells showed similar levels of cytotoxicity against HLA-A24+ LCLs pulsed with WT1 peptide (data not shown), indicating that cytotoxicity was mediated by the transduced TCR but not by the endogenously expressed TCR.
In addition to Tc1 cells, Th1 cells also showed detectable cytotoxicity against HLA-A24+ WT1 peptide–loaded LCLs when they were transduced with WT1-specific TCR genes (Figure 3B). Cytotoxic activity of TCR gene–transduced Th1 cells was also WT1 peptide specific and restricted to HLA-A24 because they were unable to respond to CMV peptides pulsed or unpulsed LCLs (Figure 3C-D) or HLA-A24– LCLs loaded with WT1 peptide (data not shown). HLA-A24–restricted cytotoxicity of TCR gene–modified Th1 and Tc1 cells was further confirmed by a blocking assay using HLA-A24–specific mAb (Figure 3E). Cytotoxic activity of TCR gene–transduced Th1 and Tc1 cells against WT1 peptide–pulsed HLA-A24+ LCLs was measured in the presence or absence of anti–HLA-A24 mAb or control anti–HLA-A2 mAb. Cytotoxic activity of TCR gene–transduced Tc1 cells was inhibited by the addition of anti–HLA-A24 mAb but not with anti–HLA-A2 mAb (Figure 3E). Similarly, the cytotoxic activity of TCR gene–transduced Th1 cells was also blocked by anti–HLA-A24 mAb but not by anti–HLA-A2 mAb (Figure 3E).
Cytotoxic activity of TCR gene–transduced Th1 and Tc1 cells against naturally processed WT1 peptide was demonstrated using HLA-A24+ LCLs retrovirally transduced with WT1 gene as target cells. Both TCR gene–transduced Th1 and Tc1 cells showed cytotoxic activity against WT1 gene–transduced LCLs (Figure 3F) but not parental WT1-negative LCLs (Figure 3G). Control Th1 and Tc1 cells showed significant cytotoxicity against neither WT1 gene–transduced LCLs nor their parental LCLs (Figure 3F-G). Thus, these results clearly demonstrate that the reactivity of the WT1-specific CD8+ Tc clone was successfully transferred into CD4+ Th1 and CD8+ Tc1 cells by transferring TCR genes.
HLA-A24–restricted antitumor activity of TCR gene–transduced Th1 and Tc1 cells against WT1 peptide–loaded LCLs. (A) WT1-specific TCR α and β chain genes were lentivirally transduced to nonspecific Th1 and Tc1 cells obtained from an HLA-A24+ healthy donor as described in “Materials and methods.” Ten days after infection, TCR gene–transduced and control T cells were stained with FITC-labeled anti-CD4 mAb and PE-labeled anti-CD8 mAb and sorted to CD4+ and CD8+ T cells by a FACSVantage instrument. After isolation, staining profile of Th1 and Tc1 cells was analyzed by FACSCalibur instrument and Cell Quest software. Percentage of cells in each quadrant is indicated in flow cytometer plots. (B-D) Cytotoxic activity of TCR gene–transduced and control Th1 and Tc1 cells against WT1 peptide–pulsed (B), CMV peptide–pulsed (C), or unloaded (D) LCLs (TAK-LCL, which are derived from an HLA-A24+ patient with leukemia) was evaluated by 4-hour 51Cr-release assay. Nonspecific cytotoxicity of Th1 and Tc1 cells against HLA-A24– LCLs was less than 10%. (E) Blocking of cytotoxicity against WT1 peptide–pulsed HLA-A24+ LCLs by anti–HLA-A2 and anti–HLA-A24 mAbs was evaluated at an effector-to-target (E/T) ratio of 20 for Tc1 cells and E/T of 40 for Th1 cells. The percentage of inhibition was calculated by the following formula; % Inhibition = (% cytotoxicity with mAb) / (% cytotoxicity without mAb) × 100. Percentage of cytotoxicity of TCR gene–transduced Tc1 and Th1 against WT1 peptide–loaded LCL was 77% and 57%, respectively. Background cytotoxicity of Tc1 and Th1 against unloaded LCLs was 9% and 13%, respectively. (F-G) Cytotoxic activity of TCR gene–transduced and control Th1 and Tc1 cells against HLA-A24+ LCLs derived from healthy donors and retrovirally transduced with WT1 gene (F) and their parental A24+ LCLs (G) was evaluated by 4-hour 51Cr-release assay. Similar results were obtained using TCR gene–transduced Th1 and Tc1 cells derived from 2 other HLA-A24+ and 2 HLA-A24– healthy volunteers. Error bars indicate standard error (SE) in triplicate samples.
HLA-A24–restricted antitumor activity of TCR gene–transduced Th1 and Tc1 cells against WT1 peptide–loaded LCLs. (A) WT1-specific TCR α and β chain genes were lentivirally transduced to nonspecific Th1 and Tc1 cells obtained from an HLA-A24+ healthy donor as described in “Materials and methods.” Ten days after infection, TCR gene–transduced and control T cells were stained with FITC-labeled anti-CD4 mAb and PE-labeled anti-CD8 mAb and sorted to CD4+ and CD8+ T cells by a FACSVantage instrument. After isolation, staining profile of Th1 and Tc1 cells was analyzed by FACSCalibur instrument and Cell Quest software. Percentage of cells in each quadrant is indicated in flow cytometer plots. (B-D) Cytotoxic activity of TCR gene–transduced and control Th1 and Tc1 cells against WT1 peptide–pulsed (B), CMV peptide–pulsed (C), or unloaded (D) LCLs (TAK-LCL, which are derived from an HLA-A24+ patient with leukemia) was evaluated by 4-hour 51Cr-release assay. Nonspecific cytotoxicity of Th1 and Tc1 cells against HLA-A24– LCLs was less than 10%. (E) Blocking of cytotoxicity against WT1 peptide–pulsed HLA-A24+ LCLs by anti–HLA-A2 and anti–HLA-A24 mAbs was evaluated at an effector-to-target (E/T) ratio of 20 for Tc1 cells and E/T of 40 for Th1 cells. The percentage of inhibition was calculated by the following formula; % Inhibition = (% cytotoxicity with mAb) / (% cytotoxicity without mAb) × 100. Percentage of cytotoxicity of TCR gene–transduced Tc1 and Th1 against WT1 peptide–loaded LCL was 77% and 57%, respectively. Background cytotoxicity of Tc1 and Th1 against unloaded LCLs was 9% and 13%, respectively. (F-G) Cytotoxic activity of TCR gene–transduced and control Th1 and Tc1 cells against HLA-A24+ LCLs derived from healthy donors and retrovirally transduced with WT1 gene (F) and their parental A24+ LCLs (G) was evaluated by 4-hour 51Cr-release assay. Similar results were obtained using TCR gene–transduced Th1 and Tc1 cells derived from 2 other HLA-A24+ and 2 HLA-A24– healthy volunteers. Error bars indicate standard error (SE) in triplicate samples.
Cytokine production by TCR gene–engineered Th1 and Tc1 cells in response to WT1 peptide–pulsed HLA-A24+ LCLs
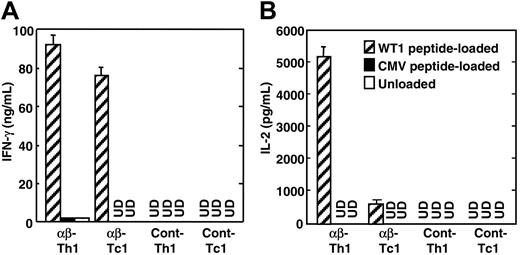
Next, we investigated the antigen-specific cytokine production of TCR gene–transduced Th1 and Tc1 cells. When WT1-specific TCR gene–transduced Tc1 cells were cocultured with HLA-A24+ LCLs loaded with WT1 peptide, high levels of IFN-γ were detected in the culture supernatants (Figure 4A). The supernatants of TCR gene–transduced Tc1 cells cocultured with control CMV peptide–loaded or unloaded LCLs contained undetectable IFN-γ, showing that IFN-γ production from TCR gene–transduced Tc1 cells was WT1 peptide specific. Likewise, TCR gene–transduced Th1 cells produced high levels of IFN-γ in response to LCLs loaded with HLA-A24–binding WT1 peptide. Such IFN-γ production by TCR gene–modified Th1 cells was not induced by stimulation with unloaded LCLs (Figure 4A). Interestingly, in contrast to TCR gene–transduced Tc1 cells, TCR gene–transduced Th1 cells also produced high levels of IL-2 when cocultured with WT1 peptide–loaded LCLs (Figure 4B). In contrast, TCR gene–transduced Th1 and Tc1 cells produced little IL-2 in response to control CMV peptide–pulsed or peptide–unloaded HLA-A24+ LCLs (Figure 4B). IL-4 was not detected in the culture supernatants of TCR gene–transduced Th1 and Tc1 cells, which indicated that TCR gene–modified Th1 and Tc1 cells were successfully polarized into type-1 T cells in terms of cytokine production pattern (data not shown). TCR gene–transduced Th1 and Tc1 cells with similar reactivity were induced from both HLA-A24+ and HLA-A24– donors (data not shown). TCR gene–transduced Th1 and Tc1 cells prepared from HLA-A24+ donors produced similar level of cytokines against autologous and allogeneic WT1 peptide–loaded HLA-A24+ LCLs. Moreover, they produced cytokines in response to stimulation with WT1 peptide–pulsed HLA-A24+ LCLs but not with peptide–pulsed HLA-A24– LCLs (data not shown). Therefore, the capability of these cells to produce cytokines in response to WT1 peptide was mediated by the transduced genes, encoding the HLA-A24–restricted, WT1-specific TCRs.
Cytotoxicity of, and cytokine production by, TCR gene–transduced Th1 and Tc1 cells in response to leukemia cells
Finally, we examined whether TCR gene–transduced Th1 and Tc1 cells exhibit antitumor activity against freshly isolated leukemia cells that present naturally processed WT1 peptide on MHC molecules. Freshly isolated leukemia cells were examined for their WT1 expression by the real-time PCR method, and all leukemia cells used in this experiment were found to show a strong WT1 mRNA expression (see the legend for Figure 5B-G), as reported previously.21 The FACS-sorted TCR gene–transduced Th1 and Tc1 cells were significantly expanded by WT1 peptide–loaded LCLs without changing their purity (Figure 5A). On the other hand, control Th1 and Tc1 cells could not be expanded by stimulation with WT1 peptide–loaded LCLs (data not shown). Th1 and Tc1 cells, transduced with WT1-specific TCR genes and stimulated with WT1 peptide–loaded allogeneic LCLs, showed no significant cytotoxicity against HLA-A24+ LCLs used as stimulator cells (data not shown). Thus, alloreactive T cells were not generated by stimulation with WT1 peptide–pulsed allogeneic LCLs. Tc1 cells, transduced with WT1-specific TCR genes and stimulated with antigenic peptide, exhibited strong cytotoxicity against HLA-A24+ but not HLA-A24– leukemia cells (Figure 5B). Consistent with the cytotoxic activity of TCR gene–transduced Tc1 cells, they also produced significant levels of IFN-γ in response to HLA-A24+ freshly isolated leukemia cells (Figure 5D). Such strong IFN-γ production was not induced by coculture with HLA-A24– leukemia cells.
WT1 peptide–specific cytokine production by TCR gene–transduced Th1 and Tc1 cells. TCR gene–transduced Th1 and Tc1 cells (1 × 105), obtained from nonspecific Th1 and Tc1 cells of the same healthy donor of T cells used in the experiments of Figure 3, were cocultured with WT1 peptide–pulsed, CMV peptide–pulsed, or unloaded HLA-A24+ LCLs (TAK-LCL) (5 × 104). After 20 hours, supernatants were harvested from culture, and their IFN-γ (A) and IL-2 levels (B) were determined by ELISA. UD represents undetected. Similar results were obtained using TCR gene–transduced Th1 and Tc1 cells derived from 2 other HLA-A24+ and 2 HLA-A24– healthy volunteers. Error bars indicate standard error (SE) in triplicate samples.
WT1 peptide–specific cytokine production by TCR gene–transduced Th1 and Tc1 cells. TCR gene–transduced Th1 and Tc1 cells (1 × 105), obtained from nonspecific Th1 and Tc1 cells of the same healthy donor of T cells used in the experiments of Figure 3, were cocultured with WT1 peptide–pulsed, CMV peptide–pulsed, or unloaded HLA-A24+ LCLs (TAK-LCL) (5 × 104). After 20 hours, supernatants were harvested from culture, and their IFN-γ (A) and IL-2 levels (B) were determined by ELISA. UD represents undetected. Similar results were obtained using TCR gene–transduced Th1 and Tc1 cells derived from 2 other HLA-A24+ and 2 HLA-A24– healthy volunteers. Error bars indicate standard error (SE) in triplicate samples.
HLA-A24–restricted cytotoxicity of, and IFN-γ production by, TCR gene–transduced Tc1 and Th1 cells against freshly isolated leukemia cells. TCR gene–transduced CD8+ Tc1 and CD4+ Th1 cells were isolated by a FACSVantage instrument restimulated with WT1 peptide (CMTWNQMNL)–pulsed HLA-A24+ LCLs and expanded in the presence of IL-2. (A) After expansion, TCR gene–transduced Th1 and Tc1 cells were stained with FITC-labeled anti-CD4 mAb and PE-labeled anti-CD8 mAb, and the staining profile of Th1 and Tc1 cells was analyzed by a FACSCalibur instrument and Cell Quest software. Percentage of cells in each quadrant is indicated in flow cytometer plots. (B-C) Cytotoxic activity of TCR gene–transduced Tc1 (B) and Th1 cells (C) against freshly isolated leukemia cells was evaluated by 4-hour 51Cr release assay. (D-E) TCR gene–transduced Tc1 (D) and Th1 cells (E) were cocultured with leukemia cells. After 20 hours, culture supernatant was harvested, and IFN-γ levels in the supernatant were measured by ELISA. WT1 expression level of leukemia cells used in the experiments of Figure 5B-E was determined by quantitative real-time PCR to be HLA-A24+ AML M1 (3.5 × 10–1), AML M21 (2.7 × 10–1), AML M22 (8.6 × 10–2), AML M4 (8.8 × 10–1), ALL L2 (5.3 × 10–1), HLA-A24– AML M2 (1.5 × 100), AML M4 (2.5 × 10–1), and ALL L2 (5.8 × 10–1). Expression levels were expressed as relative values against K562 cells which strongly express WT1. Error bars indicate standard error (SE) in triplicate samples. (F-G) Cytotoxic activity of TCR gene–transduced Tc1 (F) and Th1 cells (G) against HLA-A24+ WT1+ freshly isolated leukemia cells and HLA-A24+ WT1– malignant lymphoma (ML) cells was evaluated by 4-hour 51Cr release assay. WT1 expression level of leukemia and lymphoma cells used in the experiments of Figure 5F-G was determined to be AML1 (1.5 × 100), AML2 (7.2 × 10–1), ML (DLBL) (7.3 × 10–5), ML (Burkitt)1 (3.5 × 10–4), and ML (Burkitt)2 (7.8 × 10–5). AML indicates acute myeloid leukemia; ALL, acute lymphoid leukemia; DLBL, diffuse large B cell lymphoma; and Burkitt, Burkitt lymphoma.
HLA-A24–restricted cytotoxicity of, and IFN-γ production by, TCR gene–transduced Tc1 and Th1 cells against freshly isolated leukemia cells. TCR gene–transduced CD8+ Tc1 and CD4+ Th1 cells were isolated by a FACSVantage instrument restimulated with WT1 peptide (CMTWNQMNL)–pulsed HLA-A24+ LCLs and expanded in the presence of IL-2. (A) After expansion, TCR gene–transduced Th1 and Tc1 cells were stained with FITC-labeled anti-CD4 mAb and PE-labeled anti-CD8 mAb, and the staining profile of Th1 and Tc1 cells was analyzed by a FACSCalibur instrument and Cell Quest software. Percentage of cells in each quadrant is indicated in flow cytometer plots. (B-C) Cytotoxic activity of TCR gene–transduced Tc1 (B) and Th1 cells (C) against freshly isolated leukemia cells was evaluated by 4-hour 51Cr release assay. (D-E) TCR gene–transduced Tc1 (D) and Th1 cells (E) were cocultured with leukemia cells. After 20 hours, culture supernatant was harvested, and IFN-γ levels in the supernatant were measured by ELISA. WT1 expression level of leukemia cells used in the experiments of Figure 5B-E was determined by quantitative real-time PCR to be HLA-A24+ AML M1 (3.5 × 10–1), AML M21 (2.7 × 10–1), AML M22 (8.6 × 10–2), AML M4 (8.8 × 10–1), ALL L2 (5.3 × 10–1), HLA-A24– AML M2 (1.5 × 100), AML M4 (2.5 × 10–1), and ALL L2 (5.8 × 10–1). Expression levels were expressed as relative values against K562 cells which strongly express WT1. Error bars indicate standard error (SE) in triplicate samples. (F-G) Cytotoxic activity of TCR gene–transduced Tc1 (F) and Th1 cells (G) against HLA-A24+ WT1+ freshly isolated leukemia cells and HLA-A24+ WT1– malignant lymphoma (ML) cells was evaluated by 4-hour 51Cr release assay. WT1 expression level of leukemia and lymphoma cells used in the experiments of Figure 5F-G was determined to be AML1 (1.5 × 100), AML2 (7.2 × 10–1), ML (DLBL) (7.3 × 10–5), ML (Burkitt)1 (3.5 × 10–4), and ML (Burkitt)2 (7.8 × 10–5). AML indicates acute myeloid leukemia; ALL, acute lymphoid leukemia; DLBL, diffuse large B cell lymphoma; and Burkitt, Burkitt lymphoma.
As compared with TCR gene–transduced Tc1 cells, TCR gene–transduced Th1 cells showed weak but significant cytotoxicity against freshly isolated leukemia cells in an HLA-A24–restricted manner (Figure 5C). The cytotoxicity of Th1 cells was apparently lower than that of Tc1 cells. Lower cytotoxicity of Th1 cells is considered to be due to an inherent property of CD4+ Th cells. As shown in Figure 5E, TCR gene–transduced Th1 cells produced similar levels of IFN-γ as Tc1 cells in response to freshly isolated HLA-A24+ but not HLA-A24– leukemia cells. Thus, HLA class I–restricted Th1 cells engineered with TCR genes efficiently recognize antigen peptide on leukemia cells. WT1-specific cytotoxicity and IFN-γ production was considered to be mediated by transduced WT1-specific, HLA-A24–restricted TCRs because we succeeded in preparing HLA-A24–restricted WT1-specific T cells from naive T cells from HLA-A24–negative donors. In addition, control nontransduced T cells could not be expanded by stimulation with WT1 peptide–loaded LCLs.
To demonstrate whether TCR gene–transduced Th1 and Tc1 cells surely exert WT1-specific antileukemia activity, we next examined their antitumor activity against WT1– malignant lymphoma cells as control target cells. As shown above (Figure 5B-C), both TCR gene–modified Th1 and Tc1 cells showed a significant cytotoxicity against WT1 expressing freshly isolated AML cells (Figure 5F-G). In contrast, they showed no detectable cytotoxicity against HLA-A24+ malignant lymphoma cells (Figure 5F-G). Consistently, IFN-γ was not detected in the supernatant of TCR gene–modified Th1 and Tc1 cells cocultured with HLA-A24+ lymphoma cells (data not shown). Thus, antitumor activity by TCR gene–transduced Th1 and Tc1 cells against leukemia cells is demonstrated to be WT1 specific. In summary, we demonstrated that HLA-A24–restricted tumor-specific responsiveness of a Tc clone can be successfully transferred into both nonspecifically activated Th1 and Tc1 cells by TCR gene transduction.
Discussion
In the present paper, we have tested an alternative strategy for preparation of tumor-specific Th1 and Tc1 cells by transferring MHC class I–restricted tumor antigen–specific TCR genes. We have previously demonstrated a critical role of both tumor-specific Th1 cells and tumor-specific Tc1 cells for complete rejection of an established tumor mass in animal models.2,3,19,27 It is now generally accepted that tumor-specific CD4+ Th cells, in addition to CD8+ Tc cells, play an important role in tumor rejection in both animal models and humans.2-6,27-30 However, vaccination with both HLA class I and class II binding tumor-specific peptides to patients with cancer was ineffective for inducing simultaneous activation of antitumor Th and Tc cells.31 This is possibly due to strong immunosuppression and the existence of CD4+CD25+ regulatory T cells in tumor-bearing hosts.32-35 Therefore, it is important to develop a more efficient vaccination protocol to activate both tumor-specific Th and Tc cells for effective induction of antitumor immunity in tumor patients.
Adoptive transfer of tumor antigen–specific Th and Tc cells expanded ex vivo represents a promising strategy to treat patients with cancer.36,37 However, generation of tumor-specific Th cells from patients with tumors is very difficult because few tumor-specific peptides that bind with HLA class II have been identified so far.38,39 To develop a novel method to generate both tumor antigen–specific Th1 and Tc1 cells, we investigated the antitumor activity of gene–modified Th1 and Tc1 cells transduced with HLA class I–restricted TCR genes. By using a highly efficient lentiviral gene transfer system, TCR gene transduction to nonspecifically activated Th1 and Tc1 cells resulted in marked surface expression of the transduced TCR (Figures 1, 2). These TCR gene–transduced Th1 and Tc1 cells responded to WT1 peptide antigen restricted by the same HLA molecule of the original Tc clone (Figures 3, 4). Moreover, TCR gene–modified tumor-specific Th1 and Tc1 cells demonstrated antitumor activity in response to WT1 gene–transduced LCLs or HLA-A24+ leukemia cells, which were expressing naturally processed WT1 peptide on MHC molecules (Figure 3F-G and Figure 5B-G). The recognition of leukemia cells by TCR gene–transduced Th1 and Tc1 cells was found to be WT1 specific because WT1-negative HLA-A24+ lymphoma cells failed to stimulate TCR gene–modified T cells (Figure 5F-G). It is interesting to investigate whether the efficacy of recognition by TCR gene–transduced Th1 and Tc1 cells correlates with WT1 expression level of leukemia cells, because the level of WT1 mRNA expression of lung cancer cell lines was found to correlate with their susceptibility against WT1-specific CTL-mediated cytotoxicity.22 However, we could not conclude it in this study because freshly isolated leukemia cells always express strong WT1 without exception, and the number of available leukemia cells is limited.
The most important finding in the present experiments is that MHC class I–restricted TCR genes obtained from tumor-specific Tc clone were successfully transferred to Th1 cells and that these engineered Th1 cells can respond to antigenic peptide in an MHC class I–restricted manner. MHC class I–restricted tumor-specific Th1 cells but not Tc1 cells produced high levels of IL-2 in response to the WT1 peptide (Figure 4). Therefore, TCR gene–transduced Th1 cells are beneficial for enhancing antitumor cellular immunity mediated by cotransferred Tc1 cells and/or host-derived antitumor effector cells. The generation of MHC class I–restricted Th cells by TCR gene transfer has been reported by Clay et al12 and Morgan et al,20 but these investigators demonstrated only that gene–modified Th cells produced IFN-γ in response to stimulation with antigen peptide–pulsed T2 cells but not with naturally presented antigen bound by MHC molecules.12,20 However, we have clearly demonstrated that (1) CD4+ Th1 cells that were transduced with HLA-A24–restricted TCR genes produced IL-2 in addition to IFN-γ (Figure 4) and (2) TCR gene–modified Th1 cells showed substantial cytotoxicity and IFN-γ production in response to leukemia cells expressing naturally processed tumor antigen peptide on MHC molecules (Figure 5). Our gene–modified class I–restricted Th1 cells possibly exerted strong antitumor activities because we prepared Th1 cells under type-1 T-cell–inducing conditions. We showed that Th1 cells that were transduced with HLA class I–restricted TCR genes showed a significant antitumor activity against freshly isolated leukemia cells (Figure 5) that express HLA class II. However, they showed low levels of antitumor activity against HLA-A24+ WT1+ leukemia cell lines that express no HLA class II molecules, while TCR gene–modified Tc1 cells exhibited similar antitumor activity against both leukemia cell lines and freshly isolated leukemia cells. TCR gene–transduced Th1 cells exhibit both cytotoxicity (cytotoxicity at an E/T ratio of 10 is 9% on average; Figure 5B) and cytokine production (235 pg/mL IFN-γ production on average; Figure 5D) by coculture with freshly isolated HLA class II–expressing leukemia cells but not with HLA class II–negative leukemia cell lines (cytotoxicity at an E/T ratio of 10 is 3% and 30 pg/mL IFN-γ production; data not shown). This indicates that, in addition to the recognition of HLA class I–binding antigenic peptide on leukemia cells by class I–restricted TCR complex, ligation of CD4 with HLA class II appeared to be required for full activation in response to naturally processed WT1 antigen peptide. This is consistent with previous observations that the CD4/MHC class II or CD8/MHC class I interactions are required for the reactivity of naturally occurring class I–restricted Th cells or class II–restricted Tc cells, respectively.40-43 However, some naturally occurring class I–restricted Th cells were also reported to exert their function in the absence of CD4/class II ligation.44 In addition, it was reported that some Tc cells recognized antigen in a CD8-independent way.43,45,46 This discrepancy may be explained by different strength of the interaction between the class I–restricted TCR of different class I–restricted Th cells and antigen peptide presented on MHC molecule. Thus, it might be possible to prepare TCR gene–modified Th1 cells that can respond to MHC class II– target cells if nonspecific Th cells are transferred with class I–restricted TCR genes that have a high binding avidity with MHC-bound antigen. In general, most freshly isolated leukemia cells express high levels of MHC class II molecules.47 Therefore, TCR gene–transduced Th1 cells will be useful for most patients with leukemia irrespective of the strength of the interaction between TCR and HLA-bound antigenic peptide. Even in the case of nonhematopoietic tumor cells, most of which express no HLA class II molecules, class I–restricted TCR gene–transduced Th1 cells should exert antitumor activity by recognizing HLA class I–binding tumor antigen peptide cross-primed by professional APCs such as DCs. WT1, which is widely expressed in leukemia cells, is a potential target antigen for immunotherapy of patients with leukemia.48,49 In addition to leukemia cells, WT1 has been found to be expressed on various types of other tumors, including lung, colon, or breast cancer.50-52 Thus, our present method to transduce class I–restricted WT1-specific TCR genes may be applied to patients with various types of tumors.
Recently, we have proposed adoptive immunotherapy using tumor antigen–specific Th1 cells as a useful strategy for tumor immunotherapy (Th1 cell therapy).2,3,19,27 Indeed, combining Th cells with Tc cells is a powerful method to treat patients with cancer.37 As shown in the present experiments, class I–restricted tumor-specific TCR genes can successfully transfer tumor specificity to Th1 and Tc1 cells. Because these genetically engineered tumor-specific Th1 cells are obtained from polyclonally activated Th1 cells, it is possible to prepare a large number of tumor-specific Th1 cells from a relatively small number of PBMCs in a short culture period. In contrast to class II–binding tumor-specific peptides, hundreds of class I–binding peptides have been identified. These peptides should enable us to generate a database of tumor-specific TCR genes from a variety of tumor-specific Tc clones expressing various HLA genotypes. Therefore, we believe that our established TCR gene–modified tumor-specific Th1 cells will be applicable to many patients with cancer and may become a powerful tool for developing a novel tailor-made Th1 cell therapy.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2004-09-3663.
Supported in part by a grant-in-aid for Science Research on Priority Areas and Millennium Project from the Ministry of Education, Culture, Sports, Science, and Technology (T.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Luc Van Kaer (Vanderbilt University School of Medicine, Nashville, TN) for reviewing this paper. We thank Dr Michiko Kobayashi (Genetics Institute, Cambridge, MA) and Takuko Sawada (Shionogi Pharmaceutical Institute Co, Osaka, Japan) for their kind donation of IL-12 and IL-2, respectively.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal