Comment on Yasui et al, page 706
SDX-101, the etodolac R-enantiomer, is an anti-inflammatory drug with a cytotoxic effect on multiple myeloma cells but not on normal peripheral blood mononuclear cells.
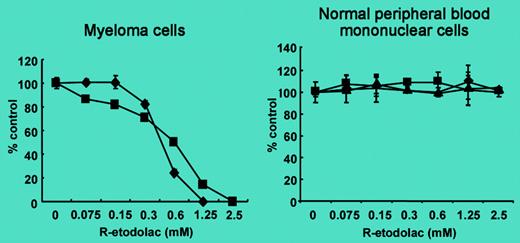
The antiproliferative activity of nonsteroidal anti-inflammatory drugs and their potential use as antitumor agents are under scrutiny.1 A starting point is the demonstration that these drugs have some effects on cancer cells.FIG1
SDX-101 induces growth inhibition in MM cell lines. See the complete figure in the article beginning on page 706.
SDX-101 induces growth inhibition in MM cell lines. See the complete figure in the article beginning on page 706.
In this issue of Blood, Yasui and colleagues provide the first evidence of the cytotoxic effect of SDX-101, the R-enantiomer of etodolac, in different multiple myeloma (MM) cell lines. The authors illustrate SDX-101's antitumor activity against both drug-sensitive and -resistant MM cells and against primary patient MM cells within the bone marrow environment. It is important to bear in mind that these effects were obtained with concentrations without cytotoxicity on normal peripheral blood mononuclear cells and at clinically achievable plasma levels.
Some MM cells were resistant to doxorubicin, dexamethasone, and bortezomib and were hence highly hypermutated. The cytotoxic effect is mediated via caspase-8/-9/-3 activation and apoptosis. In addition, SDX-101 induced down-regulation of cyclin D1 expression in MM cells, resulting in an apoptotic population, as shown by cell-cycle analysis.
As they have previously shown that novel chemotherapeutic agents augment the cytotoxicity of dexamethasone,2 Yasui and colleagues now demonstrate that the combination of SDX-101 plus dexamethasone is highly synergistic. It has been well established that myeloid cell leukemia-1 (Mcl-1) plays an important role in proliferation and inhibition of apoptosis, as well as in the induction of drug resistance3 : the results obtained by Yasui and colleagues thus strongly suggest that up-regulation of the proapoptotic variant Mcl-1S and inhibition of the antiapoptotic variant Mcl-1L are the principal events in the apoptotic activity of SDX-101.
It has previously been reported that the bone marrow microenvironment plays an important role in the pathogenesis of MM.4,5 Yasui and colleagues demonstrate that SDX-101 ablates the growth stimulatory effect of this microenvironment on MM cells. It is interesting to note that SDX-101 lacks cyclooxygenase(COX) inhibitory activity and hence the related side effects and that it is approved for treatment of degenerative joint diseases and rheumatoid arthritis. Results as a whole provide the preclinical framework for clinical trials of SDX-101 alone or in combination with dexamethasone to improve patient outcome in MM. The study is an important contribution to MM research. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal