The identification of signaling pathways critical to myeloma growth and progression has yielded an array of novel agents with clinical activity. Multiple myeloma (MM) growth is IL-6 dependent, and IL-6 is secreted in an autocrine/paracrine fashion with signaling via the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway. We hypothesized that combining a Ras pathway inhibitor (lonafarnib, SCH66336) with a proteasome inhibitor (bortezomib, Velcade, PS-341) would enhance myeloma-cell killing. MM cell lines and primary human cells were used to test either single agent bortezomib, lonafarnib, or the combination on MM signaling and apoptosis. Combination therapy induced synergistic tumor-cell death in MM cell lines and primary MM plasma cells. Cell death was rapid and associated with increased caspase 3, 8, and 9 cleavage and concomitant down-regulation of p-AKT. Down-regulation of p-AKT was seen only in combination therapy and not seen with either single agent. Cells transfected with constitutively active p-AKT, wild-type AKT, or Bcl-2 continued to demonstrate synergistic cell death in response to the combination. The order of addition was critically important, supporting bortezomib followed by lonafarnib as the optimal schedule. The combination of a proteasome inhibitor and farnesyl transferase inhibitor demonstrates synergistic myeloma-cell death and warrants further preclinical and clinical studies.
Introduction
Multiple myeloma (MM) is a clonal plasma-cell disorder characterized by multiorgan dysfunction as a result of bone and bone marrow infiltration by malignant cells, and the systemic sequelae of circulating paraprotein.1 The past 5 years have seen a myriad of new treatment options available for myeloma patients, including the rapid integration of novel, non-chemotherapy-based interventions such as thalidomide,2,3 lenalidomide (CC-5013, Revlimid),4 and the recently approved proteasome inhibitor bortezomib (PS-341, Velcade).5 While these new agents have demonstrated significant activity in the setting of relapsed and refractory myeloma, most patients do not achieve an objective response. To improve response rates, it is necessary to understand resistance pathways for these novel agents or to target multiple signaling pathways in a manner that limits tumor-cell escape and results in enhanced cell death. The proteasome inhibitor bortezomib is an agent that has multiple different potential mechanisms of action including, but not limited to, inhibition of nuclear translocation of nuclear factor kB (NFkB), activation of the c-Jun N-terminal kinase (JNK) pathway, regulation of cyclin-dependent kinases, and regulation of other apoptotic proteins.6-8
The farnesyl transferase inhibitors (FTIs) have historically been agents directed at the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway,9,10 however, emerging data suggests that among patients with responses to the FTIs, these occur independent of Ras mutations.11 The clinical and preclinical experience with the FTIs in human cancers has been mixed. In preclinical models of solid and liquid tumors, there has been impressive activity both using in vivo and in vitro models,12 but the clinical activity of FTIs in human myeloma has been suboptimal. In the large phase 2 trial from Alsina et al, in which tipifarnib was used at a fixed dose to treat relapsed myeloma, there were no objective responses, although more than 50% of patients demonstrated stable disease. However, Alsina and colleagues did demonstrate achievement of the molecular end point of the study, inhibiting farnesylation as measured by reduction in farnesyltransferase activity and HDJ-2 protein farnesylation. Nonetheless, reduction in this putative target for the FTIs was not correlated with responses or disease stabilization.13
Our initial hypothesis was that combining proteasome inhibition with FTI would target two separate signaling pathways in myeloma cells, thereby disrupting multiple signaling pathways, abrogating drug resistance and ultimately enhancing tumor-cell death. The choice to combine farnesyl transferase inhibition with proteasome inhibition stems from the observation that both pathways appear to be nonoverlapping and that an agent such as lonafarnib could inhibit interleukin-6 (IL-6)-mediated signaling,14 a known survival pathway for myeloma cells.15,16
In this report, we present data that demonstrate significant and synergistic myeloma-cell death when bortezomib and lonafarnib are added in combination at low, clinically achievable doses and that the combination results in brisk caspase 3, 8, and 9 cleavage, with rapid down-regulation of phospho-AKT (p-AKT). Additionally, we have demonstrated that order of addition is critically important, and suboptimal sequencing results in less down-regulation of p-AKT. Finally, neither exogenous IL-6, insulin-like growth factor-1 (IGF-1), nor the overexpression of AKT or Bcl-2 was able to abrogate the observed synergy seen with combination therapy. These preclinical data, combined with the clinical experience using bortezomib and lonafarnib to treat myeloma, serve to form the basis for further clinical trials combining these agents.
Materials and methods
Tissue culture and MM-derived cell lines
Dexamethasone-sensitive, MM.1S, and dexamethasone-resistant, MM.1R, RPMI8226, and U266 cell lines were maintained in RPMI 1640 medium with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (25 mM) and without l-glutamine supplemented with 5% or 10% fetal bovine serum (FBS), 100 U/mL penicillin/streptomycin (P/S), 1 × nonessential amino acids, and sodium pyruvate (1 mM) (CellGro, Herndon, VA). All MM cell lines were supplemented with 0.05 M 2-mercaptoethanol (Sigma, St Louis, MO). All MM cell lines were kindly provided by Kenneth Anderson and Dharminder Chauhan from the Dana Farber Cancer Institute (Boston, MA).
Bortezomib and lonafarnib
Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA) and prepared in 100 mM stock in dimethyl sulfoxide (DMSO). Lonafarnib was provided by Schering-Plough Research Institute (Piscataway, NJ) and was prepared in 20 mM stock in DMSO and diluted to the appropriate concentrations with RPMI complete medium.
MTT cytotoxicity assay
MM cells (50 000) were seeded in the RPMI 1640 complete media (96-well plates, Costar, Corning, NY). To establish a dose response to bortezomib and lonafarnib, cells were incubated for a minimum of 24 hours up to 72 hours in 200 μL volume. Following continuous exposure, 20 μL 3-(4, 5-dimethylthiozol-2-yl-2, 5-diphenyltetrazolium bromide (MTT kit from American Type Culture Collection, Manassas, VA) dye was added 4 hours prior to the end of incubation. Bortezomib or lonafarnib was added and incubated at several incubation time points, usually between 24 hours and 72 hours. The insoluble formazan complex was solubilized with 100 μLof the detergent, followed by incubation for a minimum of 4 hours, and the absorbance was measured at 570 nm.
Determination of apoptosis
Apoptosis was determined using annexin V staining (BD Biosciences, San Jose, CA) performed as recommended by the manufacturer. Fluorescent events were collected using Diva software on a fluorescence-activated cell-sorter scanner (FACS) Aria (BD Biosciences), and files were analyzed using FlowJo (Treestar, Ashland, OR) software to discriminate live cells (annexin V [-]) from apoptotic cells (annexin V [+]). In some experiments, cells also were stained with propidium iodide (PI) (50 μg/mL, Sigma). From 50 000 to 200 000 ungated events were collected per sample. Human cells were gated in the CD138+ fraction to evaluate induction of apoptosis in the myeloma-cell fraction of unfractionated bone marrow.
Isobologram analysis
The interaction between bortezomib and lonafarnib was analyzed using the CalcuSyn software program (Biosoft, Ferguson, MO). Data from cell-viability assays (MTT) were expressed as fraction of cells with growth affected (FA) in drug-treated versus untreated cells. This program is based upon the Chou and Talalay method17 according to the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug 1 and 2 that have the same x effect when used alone. When the combination index (CI) is equal to 1, this equation represents a conservative isobologram and indicates additive effects. CI values of less than 1.0 indicate synergy, while CI values greater than 1 indicate antagonism.
Western blotting
MM cells treated with bortezomib and lonafarnib were frozen and suspended in 1 × lysis buffer (Cell Signaling Technology, Beverly, MA) and supplemented with protease inhibitors (Active Motif, Carlsbad, CA) as recommended by the manufacturers. Cell extracts were prepared by sonication (15 seconds) and spun down for 10 minutes at 18 000g. Protein estimation was performed routinely using the Pierce Coomassie protein assay kit (Pierce Biotechnology, Rockford, IL). Thirty micrograms of protein was loaded in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Precast 12.5% gels; Bio-Rad Laboratories, Hercules, CA) and transferred to membranes by electroblotting in a Criterion Wet Gel Transfer Apparatus (Bio-Rad) at 100 V for 1 hour. The membranes were blocked by 1 × Tris-buffered saline (TBS) (Cell Signaling Technology) containing 5% milk. Antibodies against caspase 8, caspase 9, caspase 3, poly(ADP-ribose) polymerase (PARP), Bcl-XL/Bcl-2-association death promoter (BAD), Phos-BAD, Bcl-2, Phos-Bcl-2, Bcl-XL, AKT, Phos-AKT (473), Phos-P38 MAPK, and AKT siRNA were purchased from Cell Signaling Technology.
Isolation of CD138+ from MM patients, treatment, and detection of apoptosis
Bone marrow from the MM patients was diluted with 1 × phosphate-buffered saline (PBS) with 1 mM EDTA (ethylenediaminetetraacetic acid) and layered gently on a lymphocyte separation medium (Cellgro). The samples were centrifuged for 30 minutes at 19 000g. The interphase cells were carefully collected without red blood cells and washed twice with 1 × PBS containing 1 mM EDTA. Approximately 200 000 cells were plated in each well of a 96-well plate. The cells were incubated with desired concentrations of bortezomib and lonafarnib. The cells were washed after 40 hours with 1 × PBS twice and stained with annexin V and propidium iodide as per the recommendations of the manufacturers. The samples were analyzed using FACS Aria, and the files were analyzed using FlowJo software. Approval was obtained from the Emory University Institutional Review Board for the use of patient samples. Informed consent was obtained according to the Declaration of Helsinki.
AKT siRNA studies
Immediately prior to transfection, MM.1R cells (150 000 cells) were plated in each well of a 12-well plate containing 6 mL complete medium (10% FBS in RPMI 1640). For each condition, in a separate tube, 250 μL serum-free medium was taken with 15 or 75 μL of TransIT-siQuest transfection reagent (Mirus, Madison, WI), respectively, for 15 or 50 nm AKT siRNA (Cell Signaling Technology). The incubation conditions followed manufacturer recommendations before adding the cocktail mixture to the medium. After 24 hours, the cells were harvested and stained with annexin V to analyze the level of apoptosis, and the extracts were prepared for Western blot analysis.
AKT kinase assay for GSK α and β
AKT kinase assay was performed using Western blots according to the manufacturer's specifications (Cell Signaling). Briefly, MM cells were lysed in 1 × lysis buffer. Total cell lysate (400 μg proteins) was incubated with anti-AKT antibody and immunoprecipitated using sepharose beads. Beads were resuspended in kinase buffer with purified glycogen synthase kinase 3 (GSK3) substrate and adenosine triphosphate (ATP). The reaction was stopped by boiling in SDS-PAGE sample-loading buffer, run through SDS-PAGE, and electroblotted to a membrane. The membrane was blotted with phos-GSK α and β antibody.
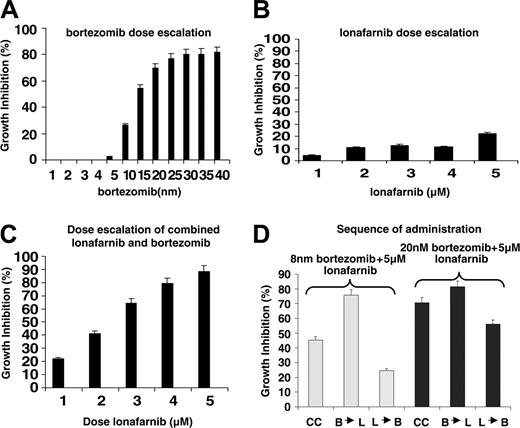
Dose escalation demonstrates significant growth inhibition resulting from the combination of bortezomib and lonafarnib that is sequence dependent in myeloma cell lines. (A) MM.1S cells were plated in 96-well plates in triplicate and were treated with bortezomib at nanomolar concentrations for 48 hours. MTT assay was performed after 48 hours, and proliferation is represented as percent growth inhibition. (B) MM.1S cells were plated as in panel A and treated with lonafarnib in micromolar concentrations, and cell proliferation was assessed using an MTT assay. (C) MM.1S cells were plated as in panel A and treated concurrently with bortezomib (8 nm) and lonafarnib (increasing 1-5 μM), and cell proliferation was assessed using an MTT assay. (D) RPMI8226 cells were treated with bortezomib and lonafarnib either concurrently (C) or in sequence first with bortezomib followed by lonafarnib (B → L) or lonafarnib followed by bortezomib (L → B) after 9 hours. MTT assay was performed after 72 hours. C indicates concurrent; B, bortezomib; and L, lonafarnib.
Dose escalation demonstrates significant growth inhibition resulting from the combination of bortezomib and lonafarnib that is sequence dependent in myeloma cell lines. (A) MM.1S cells were plated in 96-well plates in triplicate and were treated with bortezomib at nanomolar concentrations for 48 hours. MTT assay was performed after 48 hours, and proliferation is represented as percent growth inhibition. (B) MM.1S cells were plated as in panel A and treated with lonafarnib in micromolar concentrations, and cell proliferation was assessed using an MTT assay. (C) MM.1S cells were plated as in panel A and treated concurrently with bortezomib (8 nm) and lonafarnib (increasing 1-5 μM), and cell proliferation was assessed using an MTT assay. (D) RPMI8226 cells were treated with bortezomib and lonafarnib either concurrently (C) or in sequence first with bortezomib followed by lonafarnib (B → L) or lonafarnib followed by bortezomib (L → B) after 9 hours. MTT assay was performed after 72 hours. C indicates concurrent; B, bortezomib; and L, lonafarnib.
[3H]thymidine incorporation assay
To determine the rate of DNA synthesis, cells were plated onto 96-well plates at 50 000 cells per well in RPMI 1640 medium containing 5% FBS and incubated for 48 hours and the desired concentrations of IL-6, IGF-1, or no cytokines (R&D Biosystems, Minneapolis, MN) in a final volume of 200 μL. Eight hours prior to harvesting, cells were exposed to RPMI 1640 containing 1 μCi (0.037 MBq) [3H]thymidine (Perkin Elmer, Wellesley, MA). Each assay was performed in triplicate. After 48 hours, cells were washed twice with 1 × PBS, twice with cold 5% trichloroacetic acid for 10 minutes at 4°C, and solubilized in 200 μL of 0.1 N NaOH/1% SDS at room temperature. The solubilized DNA was harvested for liquid scintillation counting. The results are expressed based on the counts of radioactivity incorporated into the DNA and the change from the controls.
Transient transfections
Both AKTactivated and AKTwild type constructs (Upstate Biotechnology, Charlottesville, VA) were transiently transfected in an MM.1S cell line using the Cell Line Nucleofector Kit V (Amaxa Biosystems, Cologne, Germany), according to the published methods. 2.5 μg of the AKT constructs and 2 μg of pmax GFP construct were suspended in a total volume of 100 μL of the nucleofector solution containing 2 × 105 to 2 × 106 cells. Cells were pulsed using a Gene Pulser (BioRad) and transferred to a 6-well plate following the manufacturer's instructions. The cells were incubated overnight in 500 μL complete medium containing 10% FBS. Cells were washed in cold PBS twice before they were treated with bortezomib and lonafarnib. After 24 or 48 hours of treatment, an aliquot of cells was stained with annexin V-APC to determine apoptosis, and the remaining cells were lysed to analyze the AKT expression by Western blotting. In order to confirm successful cotransfection, cells after transfection were sorted using a high-speed cell sorter (FACS Aria) to confirm that the GFP-positive fraction was successfully cotransfected with the appropriate vector.
Results
Increased growth inhibition occurs when bortezomib is combined with lonafarnib; sequence of administration is critical in enhancing this effect
As reported before, bortezomib is cytotoxic against standard myeloma cell lines at doses varying between 20 and 50 nm. Doses below 20 nm induce modest levels of growth inhibition within the first 24 hours (Figure 1A). Lonafarnib induces minimal growth inhibition using doses ranging from 1 μM to 5 μM (Figure 1B), levels that have been shown in phase 1 trials to be clinically achievable.18 Next, myeloma-cell growth was evaluated using the combination of 8 nm bortezomib and increasing doses of lonafarnib, with the combination resulting in more than 80% growth inhibition in myeloma cell lines (Figure 1C). Similar degrees of growth inhibition with either single-agent bortezomib, lonafarnib, or the combination were seen with other myeloma cells as well (MM.1R, RPMI8226, U266, data not shown). We then evaluated order of addition for both agents on the synergistic effect of the combination (Figure 1D). When cells were first exposed to lonafarnib, (L → B: lonafarnib followed by bortezomib 9 hours later), there was less growth inhibition than seen when cells were exposed to both agents simultaneously (C-Concurrent). This effect was seen when using both high-dose bortezomib (20 nm) or low-dose bortezomib (8 nm). When bortezomib was administered first, followed 9 hours later by lonafarnib (B → L: bortezomib followed by lonafarnib 9 hours later), growth inhibition was increased compared with concurrent administration. This effect was seen both with high-dose and low-dose bortezomib, though it was more pronounced for the low-dose combination. Similar order of addition effects were noted in other myeloma cell lines as well (MM.1R, RPMI 8226, and U266, data not presented for other cell lines).
The effect of bortezomib and lonafarnib combination is synergistic in MM cell lines
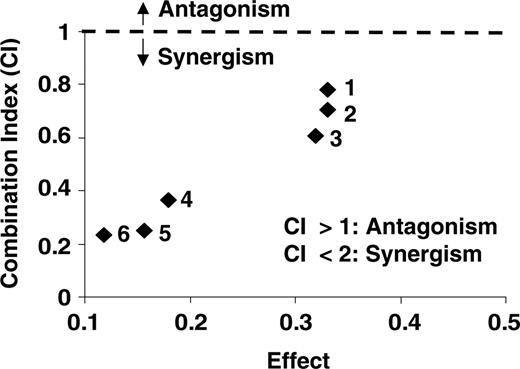
The interaction between the bortezomib and lonafarnib was analyzed using the CalcuSyn software program to determine whether this combination has additive, synergistic, or antagonistic cytotoxicity. Using the Chou and Talalay method17 to calculate a combination index (CI), we generated an isobologram of varying concentrations of bortezomib with lonafarnib in MM.1S cells (Figure 2; Table 1). When doses ranging from 6, 8, and 20 nm bortezomib were combined with 5-μM concentrations of lonafarnib, the CI value ranged from 0.777, 0.366, and 0.236, respectively, for each combination of bortezomib and lonafarnib, further supporting synergy when bortezomib is combined with lonafarnib, with increasing synergy seen with increasing doses of both agents.
Summary of combination indexes (CIs) generated from the isobologram at increasing concentrations of bortezomib and lonafarnib for MM.1R cell line
Combination . | Bortezomib (nM) . | FAbort . | Lonafarnib (μM) . | FAlona . | FAComb . | CI . |
|---|---|---|---|---|---|---|
| 1 | 6 nm | 0.522 | 5 | 0.570 | 0.334 | 0.777 |
| 2 | 8 nm | 0.488 | 5 | 0.570 | 0.317 | 0.366 |
| 3 | 8 nm | 0.488 | 4.5 | 0.726 | 0.335 | 0.717 |
| 4 | 20 nm | 0.423 | 3.5 | 0.664 | 0.315 | 0.607 |
| 5 | 20 nm | 0.423 | 4 | 0.727 | 0.157 | 0.255 |
| 6 | 20 nm | 0.423 | 5 | 0.570 | 0.117 | 0.236 |
Combination . | Bortezomib (nM) . | FAbort . | Lonafarnib (μM) . | FAlona . | FAComb . | CI . |
|---|---|---|---|---|---|---|
| 1 | 6 nm | 0.522 | 5 | 0.570 | 0.334 | 0.777 |
| 2 | 8 nm | 0.488 | 5 | 0.570 | 0.317 | 0.366 |
| 3 | 8 nm | 0.488 | 4.5 | 0.726 | 0.335 | 0.717 |
| 4 | 20 nm | 0.423 | 3.5 | 0.664 | 0.315 | 0.607 |
| 5 | 20 nm | 0.423 | 4 | 0.727 | 0.157 | 0.255 |
| 6 | 20 nm | 0.423 | 5 | 0.570 | 0.117 | 0.236 |
CI less than 1.0 indicates synergy, while CI greater than 1.0 indicates antagonism.
FAbort indicates fraction growth inhibition by bortezomib alone; FAlona, fraction growth inhibition by lonafarnib alone; and FAComb, fraction growth inhibition by bortezomib + lonafarnib.
Synergy calculations confirm that the benefit of the combination is more than additive. MM.1S cells were plated in triplicate and concurrently treated with bortezomib (up to 10 nm) and lonafarnib (up to 6 μM) for 48 hours. MTT assays were performed to assess growth inhibition. Dose-effect curve and isobologram obtained for the MM.1S cell line are shown.
Synergy calculations confirm that the benefit of the combination is more than additive. MM.1S cells were plated in triplicate and concurrently treated with bortezomib (up to 10 nm) and lonafarnib (up to 6 μM) for 48 hours. MTT assays were performed to assess growth inhibition. Dose-effect curve and isobologram obtained for the MM.1S cell line are shown.
Increased apoptosis when bortezomib is combined with lonafarnib
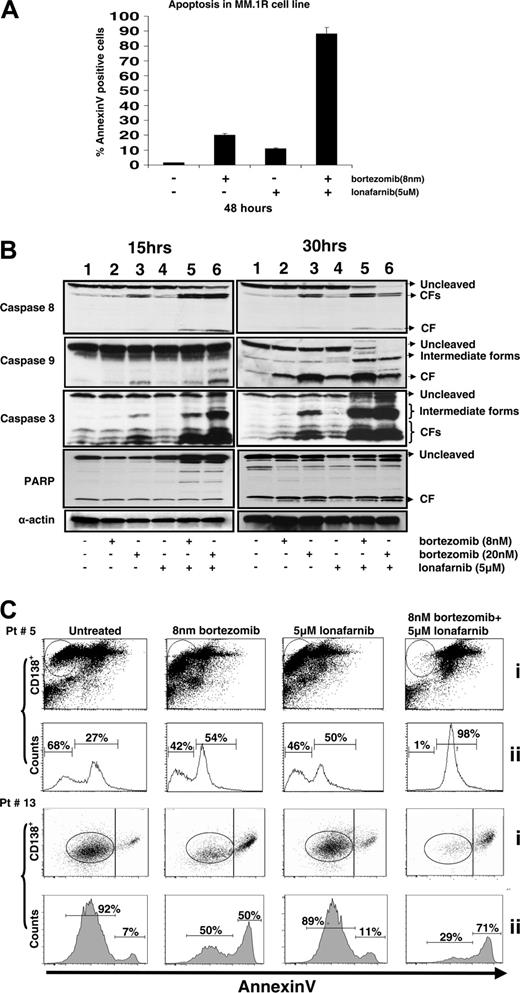
The relative impact of this combination on apoptosis was tested by assessing PARP cleavage, caspase cleavage, and annexin V staining among treated cells. Annexin V positivity was seen in 80% of the MM.1R cells exposed to the drug combination, indicating early apoptosis. On the other hand, single agent bortezomib (8 nM) or lonafarnib (5 μM) resulted in only 20% and 10% annexin staining, respectively (Figure 3A). The combination of bortezomib and lonafarnib cleaved more caspase 3 and 9 than either drug alone (Figure 3B). Compared with caspase activities of 8 and 3, there was more caspase 9 cleavage demonstrated with lonafarnib alone (lane 4), suggesting that the combination effect may be rapid and broad activation of both the intrinsic and extrinsic caspase pathways.
Primary human MM cells were induced to undergo rapid apoptosis when bortezomib was combined with lonafarnib
We next evaluated the combination effect in primary MM cells from patients. Whole bone marrow from patients with relapsed myeloma was treated as outlined in the cell-line experiments, and the CD138+ cells were analyzed for response to the combination of bortezomib and lonafarnib using flow cytometry. Two representative patients are shown in Figure 3C. The differences in the number of cells undergoing apoptosis are represented in a histogram plot in which patients #5 and #13 demonstrated a measurable difference in anti-MM activity. In both of these human tumor samples, apoptosis is increased in the combination compared with either single agent, but in addition to a higher apoptotic fraction, the fraction of annexin V-negative CD138+ cells is markedly reduced in the combination treatment, suggesting that there is a “right shift” of tumor cells toward the annexin V axis, with a profound reduction of live CD138+ cells (within the circles in each frame). Similar degrees of primary myeloma-cell death have been observed with other samples and further support the hypothesis that the combination is synergistic not only in myeloma cell lines but also in primary tumor cells.
AKT Phos473 is down-regulated more efficiently when bortezomib is combined with lonafarnib
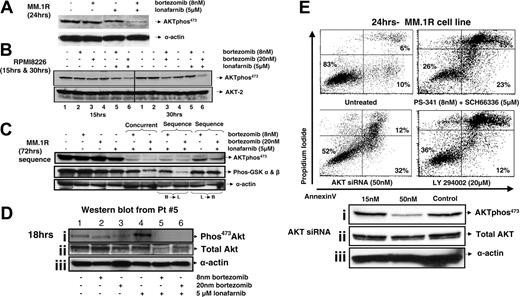
We characterized the changes in the phosphorylation of AKT when MM cells were treated with the combination of bortezomib and lonafarnib. Down-regulation on p-AKT expression was seen across 4 different cell lines (MM.1R and RPMI8226 are shown) and occurred as early as 15 hours after exposure (Figure 4A,B). Furthermore, the down-regulation of p-AKT is not accompanied by a down-regulation in total AKT initially, but is associated with a down-regulation of the p-GSK α and β, confirming the inhibitory effects of the combination on the PI3K/AKT axis (Figure 4A). We have previously demonstrated that order of addition is of critical importance to the combination effect (Figure 1D) and tested the effect of order of addition on p-AKT down-regulation (Figure 4C). In the B → L sequence there was more cell death and inhibition of p-AKT473 than seen with the L → B sequence. In aggregate, these data suggest that the phosphorylation of AKT is inhibited as a consequence of B → L and that L alone or L → B is not associated with significant apoptosis or down-regulation of p-AKT.
Demonstration of synergistic apoptosis induction as measured by annexin V staining in cell lines and primary human myeloma cells that is associated with rapid caspase cleavage. (A) MM.1R cells were plated and concurrently treated with bortezomib (8 nm) and lonafarnib (5 μM). After 48 hours, cells were pooled and stained with annexin V. The percent annexin V-positive cells is represented as an index of apoptosis for either single-agent or combination therapy. (B) RPMI8226 cells were plated and concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 15 and 30 hours, Western blotting was performed for expression of caspase 8, caspase 9, caspase 3, and PARP. Antibodies were used to evaluate the amount of cleaved and uncleaved forms of these proteins. Lane 1, untreated control; lane 2, 8 nM bortezomib; lane 3, 20 nM bortezomib; lane 4, 5 μM lonafarnib; lane 5, 8 nM bortezomib + 5 μM lonafarnib; lane 6, 20 nm bortezomib + 5 μM lonafarnib. (C) Bone marrow samples collected from MM patients were treated with bortezomib and lonafarnib for 40 hours. Cells were pooled and stained with annexin V and PI as described in “Materials and methods.”
Demonstration of synergistic apoptosis induction as measured by annexin V staining in cell lines and primary human myeloma cells that is associated with rapid caspase cleavage. (A) MM.1R cells were plated and concurrently treated with bortezomib (8 nm) and lonafarnib (5 μM). After 48 hours, cells were pooled and stained with annexin V. The percent annexin V-positive cells is represented as an index of apoptosis for either single-agent or combination therapy. (B) RPMI8226 cells were plated and concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 15 and 30 hours, Western blotting was performed for expression of caspase 8, caspase 9, caspase 3, and PARP. Antibodies were used to evaluate the amount of cleaved and uncleaved forms of these proteins. Lane 1, untreated control; lane 2, 8 nM bortezomib; lane 3, 20 nM bortezomib; lane 4, 5 μM lonafarnib; lane 5, 8 nM bortezomib + 5 μM lonafarnib; lane 6, 20 nm bortezomib + 5 μM lonafarnib. (C) Bone marrow samples collected from MM patients were treated with bortezomib and lonafarnib for 40 hours. Cells were pooled and stained with annexin V and PI as described in “Materials and methods.”
Synergistic apoptosis is associated with down-regulation of p-AKT in cell lines and primary human myeloma cells dependent upon order of addition and can be mimicked with the use of other AKT-specific inhibitors. (A) MM.1R cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 24 hours, the cells were collected, and Western blots were performed with phos-AKT473 antibody. (B) RPMI8226 cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 15 and 30 hours, the cells were collected, and Western blots were performed with Phos-AKT473 antibody in order to determine the time course for p-AKT reduction. (C) Kinase assay for AKT was performed using GSK α and β as a substrate. (D) Primary human MM cells were treated for 18 hours with either single agent or the combination of bortezomib and lonafarnib, and p-AKT or total AKT expression was analyzed using Western blot analysis. (E) MM.1R cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM) or LY294002 (20 μM) for 24 hours. Cells were harvested and stained with annexin V and PI as described in “Materials and methods.” Similarly, MM.1R cells were treated with AKT (1 and 2) siRNA, and after 24 hours, cells were collected, washed, and treated with bortezomib and lonafarnib for another 24 hours.
Synergistic apoptosis is associated with down-regulation of p-AKT in cell lines and primary human myeloma cells dependent upon order of addition and can be mimicked with the use of other AKT-specific inhibitors. (A) MM.1R cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 24 hours, the cells were collected, and Western blots were performed with phos-AKT473 antibody. (B) RPMI8226 cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM). After 15 and 30 hours, the cells were collected, and Western blots were performed with Phos-AKT473 antibody in order to determine the time course for p-AKT reduction. (C) Kinase assay for AKT was performed using GSK α and β as a substrate. (D) Primary human MM cells were treated for 18 hours with either single agent or the combination of bortezomib and lonafarnib, and p-AKT or total AKT expression was analyzed using Western blot analysis. (E) MM.1R cells were concurrently treated with bortezomib (8 nM or 20 nM) and lonafarnib (5 μM) or LY294002 (20 μM) for 24 hours. Cells were harvested and stained with annexin V and PI as described in “Materials and methods.” Similarly, MM.1R cells were treated with AKT (1 and 2) siRNA, and after 24 hours, cells were collected, washed, and treated with bortezomib and lonafarnib for another 24 hours.
Western blot analysis of human primary MM cells following treatment with either single agent or combined bortezomib and lonafarnib for 18 hours demonstrates down-regulation of p-AKT, further suggesting that total AKT is reduced as well, paralleling the experience we have demonstrated using MM cell lines (Figure 4A,D).
We next tested to see if AKT inhibition using siRNA or the PI3 kinase by inhibitor LY294002 could induce similar levels of apoptosis seen with the combination of bortezomib and lonafarnib, to further explore the importance of AKT on the cellular events we have noted with combination therapy. The level of apoptosis induced by AKT siRNA alone was compared with LY294002 (20 μM) as well as with the bortezomib and lonafarnib combination for 24 hours (Figure 4E). These data further suggest that the inhibition of AKT phoshorylation is an early event and that other methods to inhibit AKT function show similar degrees of apoptosis induction and caspase activation.
Phos-Bcl-2 is cleaved more efficiently by the combination of bortezomib and lonafarnib
We next determined the phosphorylation and the down-regulation of the antiapoptotic family members Bcl-2 and Bcl-XL. Our data indicate that phos-Bcl-2 and total Bcl-XL are cleaved in RPMI8226 cells in a dose-dependent manner in the presence of bortezomib and lonafarnib much more efficiently than by bortezomib alone. Because of the known impact of bortezomib on JNK activation, we tested the effects in our model and demonstrated a reduction in JNK1 expression and an increase in c-Jun expression, more marked for the combination. Interestingly, p-38 MAPK was increased in the highest bortezomib/lonafarnib combination, suggesting that surviving cells may do so by increasing p-38 MAPK expression. These data, together with inhibition of AKT phosphorylation, suggested that the combination of bortezomib and lonafarnib can directly target p-AKT and p-Bcl-2 rapidly and that the rapid effect on apoptosis may be a direct effect on the mitochondria.
IL-6 and IGF-1 inhibition of MM-cell growth
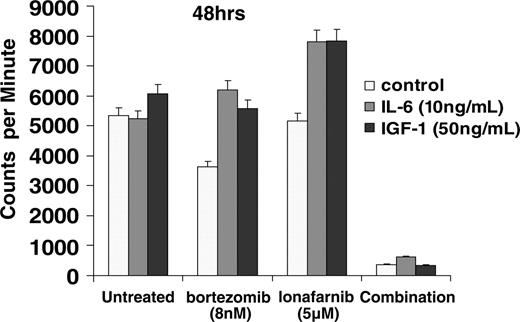
We next attempted to evaluate whether the combination of bortezomib and lonafarnib could overcome the MM-cell growth and proliferation induced by the known stimulatory cytokines, IL-6 and IGF-1. MM.1R cells were treated with either single agent or combined bortezomib and lonafarnib, in the presence of IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) for 48 hours. Bortezomib and lonafarnib alone were unable to overcome the protective effects of these growth factors (Figure 5). However, neither of these growth factors were able to abrogate the growth inhibitory effects of the combination of bortezomib and lonafarnib, further supporting the potential clinical benefit for the combination.
Exogenous cytokines cannot overcome the antiproliferative effect of the combination of bortezomib and lonafarnib. MM.1R cells were incubated for 48 hours with exogenous IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) in a final volume of 200 μL. Cell proliferation was then measured using thymidine incorporation in the presence or absence of IL-6 or IGF-1.
Exogenous cytokines cannot overcome the antiproliferative effect of the combination of bortezomib and lonafarnib. MM.1R cells were incubated for 48 hours with exogenous IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) in a final volume of 200 μL. Cell proliferation was then measured using thymidine incorporation in the presence or absence of IL-6 or IGF-1.
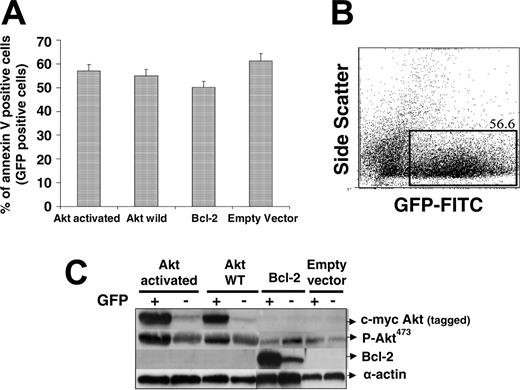
Overexpression of AKT or Bcl-2 was not able to inhibit the effects of the combination in transfected myeloma cell lines. AKT constructs for the activated form, wild-type, Bcl-2, and empty vectors were cotransfected with GFP into MM.1S cells. Cells were then treated with control, bortezomib, lonafarnib, or the combination, and were subsequently stained with APC-annexin V. (A) The fraction of annexin V-positive, GFP-positive cells treated with bortezomib and lonafarnib when transfected with constitutively active AKT, WT AKT, WT Bcl-2, or empty vector. (B) Gating strategy to isolate the fraction of GFP-positive cells that were then analyzed for annexin V staining. (C) Western blot analysis of the AKT and Bcl-2-expressed proteins among either GFP(+) or GFP(-) sorted cells, confirming successful transfection with the different vectors.
Overexpression of AKT or Bcl-2 was not able to inhibit the effects of the combination in transfected myeloma cell lines. AKT constructs for the activated form, wild-type, Bcl-2, and empty vectors were cotransfected with GFP into MM.1S cells. Cells were then treated with control, bortezomib, lonafarnib, or the combination, and were subsequently stained with APC-annexin V. (A) The fraction of annexin V-positive, GFP-positive cells treated with bortezomib and lonafarnib when transfected with constitutively active AKT, WT AKT, WT Bcl-2, or empty vector. (B) Gating strategy to isolate the fraction of GFP-positive cells that were then analyzed for annexin V staining. (C) Western blot analysis of the AKT and Bcl-2-expressed proteins among either GFP(+) or GFP(-) sorted cells, confirming successful transfection with the different vectors.
Transfection with constitutively active AKT does not abrogate the combination effect
To further evaluate the impact of AKT signaling on the combination effect, MM.1S cells were transiently transfected with constitutively active AKT, wild-type (WT) AKT, Bcl-2, or empty vector, and then treated with either single agent bortezomib, lonafarnib, or the combination, as we have described (Figure 6). Cells successfully transfected with one of these vectors were assessed for annexin V staining by gating on the fraction of cells that expressed GFP (Figure 6B). GFP-positive cells were sorted using the FACS Aria and then blotted to confirm successful transfection of the appropriate vector (Figure 6C). These data suggest that while either single agent had modest activity in any of the 4 transfected groups (data not shown), the combination treatment continued to induce significant apoptosis regardless of AKT constitutive activation, WT overexpression, or Bcl-2 overexpression. These data suggest that while AKT overexpression is known to be a powerful resistance mechanism for myeloma cells, the combination of lonafarnib and bortezomib is sufficient to overcome this potential resistance mechanism in MM cells.
Discussion
The rapid emergence of novel targeted agents is a result of a vastly improved understanding of disease biology.19,20 While the success of agents such as imatinib and rituximab is encouraging, it is unlikely that most targeted agents will induce significant responses when given alone, as most human malignancies are a result of multiple aberrant pathways that are not easily reversed with a single agent. Bortezomib represents an exciting new treatment option for patients with myeloma, yet only 38% of patients will achieve either a complete or partial remission using bortezomib with or without dexamethasone.5,21,22 However, preclinical and clinical data evaluating the effect of combining bortezomib with other agents such as doxil,23 thalidomide,24 or melphalan25 have demonstrated significantly improved response rates. This further supports the hypothesis that in addition to the direct effects of bortezomib on inducing apoptosis, bortezomib also enhances cellular responses to other agents.8,26 The initial impetus for testing the combination of bortezomib and lonafarnib stems from the hypothesis that targeting 2 separate and critical pathways should improve responses compared with targeting a single pathway through either agent alone. In addition, it has been well documented that IL-6 is a major myeloma growth factor secreted both by myeloma cells themselves and by bone marrow stromal cells that support their growth.16,27 IL-6-mediated growth and proliferation is mediated by the Ras/Raf/MAPK pathway, a known target for the FTIs.28,29
To date several FTIs have been evaluated in preclinical and clinical trials,30 including BMS-214664, L-778123, R115777 (tipifarnib), and SCH-66336 (lonafarnib). Overall, FTIs have been shown to have mild toxicities, but also modest activity with the exception of acute myeloid leukemia (AML).31,32 SCH-66336 (lonafarnib) was one of the first FTIs to undergo clinical testing, with early hints of activity in a number of different tumor types33,34 as well as the demonstration of improved responses when combined with cytotoxic agents.33 A subsequent confirmatory phase 3 trial failed to demonstrate a benefit for the combination among a group of unselected patients with lung cancer.35 Lonafarnib was tested in conjuction with the tyrosine kinase inhibitor inatinib and was noted to enhance imatinib-induced apoptosis of imatinib-sensitive and imatinib-resistant cell lines and primary human chronic myeloid leukemia (CML) cells, independent of the presence of an activated ras oncogene.36,37
Clinical data using the FTI R115777 or tipifarnib in the setting of AML and myelodysplastic syndrome (MDS) have been presented by a number of different groups and have demonstrated promising results. A phase 1 trial from Karp et al45 evaluated the use of tipifarnib among patiens with relapsed/refractory AML as well as a few patients with CML and ALL. In that trial, the maximum tolerated dose (MTD) was noted to be 1200mg twice daily, though FT inhibition was noted at doses as low as 300 mg twice daily. Responses were noted among 30% of patients. A subsequent phase 2 trial from Europe in relapsed or refractory AML patients demonstrated a 17% response rate,46 while a United States trial among patients deemed not eligible for therapy with newly diagnosed AML demonstrated a 34% response rate.47
In the setting of multiple myeloma, laboratory data from Le Gouill et al38 evaluated the effects of the FTI R115777 and its effects on myeloma-cell death in vitro and demonstrated significant in vitro cell death, however, the doses used to result in apoptosis were above the levels that can be clinically achieved. Data from Alsina et al13 tested the efficacy of single agent R115777 (tipifarnib) in a phase 2 trial for patients with relapsed myeloma. Although the therapy was well tolerated, there were no true objective responses, with 64% of patients achieving stable disease with a median duration of response among the stable disease patients of 4 months. It was interesting to note that farnesylation was inhibited using this dose and that degree of FTI was not correlated with the achievement of stable disease.13 This is not a surprising finding, as it is known that the FTIs are proapoptotic, with a mechanism of action that is continuing to be further defined.39,40 Gene Chip array analysis from Raponi and colleagues tested the effects of the FTI tipifarnib on AML-cell growth using both cell lines and human AML blasts. In their analysis, the suggestion was that FTI-induced apoptosis was more delayed than that seen using standard cytotoxic agents.41 We found similar results in our preclinical data, where the effects of single FTI alone were delayed and resulted primarily in growth inhibition, rather than overapoptosis as was seen with bortezomib. This may be due to the wide impact on other signaling pathways that are mediated via farnesylation, and because the full impact of farnesyl transferase inhibition is not rapidly seen. The net effect from these studies and numerous others is that as a class of agents, FTIs are not able to effect significant cytotoxic tumor-cell death but are ideal agents to consider in combination regimens due to the fact that their effects can enhance the effects of many other targets.42 Whether this is a result of effect on cell-survival pathways or other nonoverlapping mechanisms for inducing cell death is unclear.
Our preclinical studies represent the first published data combining FTIs and bortezomib and clearly demonstrate that synergy occurs when these agents are combined at doses at or below what can be clinically achieved in humans. Pharmacokinetic studies using lonafarnib in lung cancer have demonstrated that trough levels of lonafarnib were in the 1- to 1.5-μM range, and peak levels were between 2 and 2.5 times higher than the trough. Thus, we choose a dose of 5 μM in order to be in or near the range of what could be clinically achieved. It is important to notice from Figure 1C that the synergy we report was observed using doses of 3, 4, or 5 μM of lonafarnib and that the effect was dose dependent. We did not test doses higher than 5 μM because there were no clinical data suggesting that level could be clinically achieved.33 The combination effect is associated with rapid and early caspase activation to a greater extent than is seen with either agent alone, and concomitant to the rapid induction of cell death, is a rapid reduction in p-AKT expression both in cell lines and primary human tumor cells. This reduction is noted as early as 15 hours after exposure and is seen to a much greater extent in combination therapy than with either agent given alone.
The implications of the down-regulation of p-AKT are important, as they are associated with down-regulation of other downstream targets such as GSK α β. Thus, down-regulation of p-AKT results in upstream inhibition of the entire PI3K pathway, which can have major implications for malignant-cell survival. Using the AKT-specific inhibitor Ly294002 or siRNA directed against AKT1, we have demonstrated an amount of induced cell death similar to that seen using the combination of bortezomib and lonafarnib, but this is insufficient to claim causality. However, when the order of additional experiments are taken into account, it appears that sequencing lonafarnib before bortezomib not only induces less apoptosis than concomitant or bortezomib prior to lonafarnib administration (Figure 1D), but also is associated with less down-regulation of p-AKT (Figure 4C). Interestingly, when we transfected MM.1S cells with constitutively active AKT, overexpressed WT AKT, or Bcl-2, neither of those known resistance factors were able to inhibit the combination effect when compared to the empty vector control. Based upon our observations, two possible explanations exist regarding the association between p-AKT and the significant apoptosis we report with the combination. First, it is possible that the combination effect on p-AKT is so potent that even constitutive activation is insufficient to blunt the effect when both agents are administered. Second, it is possible that the down-regulation of p-AKT is an epiphenomenon, and while it is associated with the combination effect, it is not required for the combination effect. While our search to understand this has focused on AKT, it is possible that the combination effect is mediated by ras effector genes such as RASSF1, RASSF2, Noey2, Rig, or RRP22. Conventional thinking is that the FTIs could serve to inactivate these important tumor suppressor genes, but the impact of concomitant proteasome inhibition either before or after FTI administration may effect the balance of functional ras family oncogenes and tumor suppressors, allowing them to play a more significant role.43,44 Whichever the explanation, p-AKT could certainly be used as a biomarker for response and dosing in phase 1 clinical trials combining these agents.
Data evaluating the effect of order of administration demonstrate that while concomitant administration is effective, sequencing bortezomib before lonafarnib is more effective, and the converse is less effective at inducing myeloma-cell apoptosis. This order of addition effect is of great importance and needs to be tested with all targeted agent combinations. Based upon the order of addition experiments, we have designed our clinical trial to further validate this approach by sequencing clinical drug exposure and delaying the initiation of the FTI until after patients have received the first dose of bortezomib. While this cannot be done practically for each bortezomib dose, this can be done at the beginning of each cycle to maximize the potential for responses. Reasons for this difference in this sequence-specific apoptosis may be related to the relative effects of lonafarnib on cell-cycle or cyclin-dependent kinases (CDKs) that may be antagonistic to the effects of proteasome inhibition. Inattention to these details suggested by preclinical data may result in clinical trials that do not demonstrate activity. Similar sequence-specific effects have been demonstrated with bortezomib and cytotoxic agents, as well as with other targeted agents in preclinical work.
In conclusion, we describe convincing preclinical data, using myeloma cell lines and primary human myeloma cells to test the effect of the combination of bortezomib and lonafarnib, that demonstrate significant induction of apoptosis accompanied by rapid down-regulation of p-AKT. The clinical experience with these drugs as single agents and the synergistic killing of myeloma cells demonstrated in the present study support the development of a phase 1 clinical trial testing the combination in patients with relapsed or refractory myeloma.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-06-2584.
Supported in part by a Multiple Myeloma Research Foundation fellowship (E.D.) and by a Career Development Award from the Lymphoma Research Foundation (S.L.).
Declaration of financial interest: S.L. is a consultant for and on the Speakers' Bureau of, Millennium Pharmaceuticals, Inc.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal