2,5-dimethyl-celecoxib (DMC) is a close structural analog of the selective cyclooxygenase-2 (COX-2) inhibitor celecoxib that lacks COX-2 inhibitory function. We and others have demonstrated that DMC, despite its inability to block COX-2, is able to potently mimic the antitumor effects of celecoxib in vitro and in vivo. In this current study, we investigated whether DMC would also be able to inhibit the growth of highly drug-resistant tumor cell variants. We focused on human multiple myeloma (MM) cells, as patients with MM frequently develop drug-resistant disease and ultimately succumb to death. Here we show that DMC (and celecoxib) inhibits the proliferation of various multiple myeloma cell lines, including several (multi) drug-resistant variants. Growth inhibition in drug-sensitive and drug-resistant cells is mediated via multiple effects, which include diminished signal transducer and activator of transcription 3 (STAT-3) and mitogen-activated protein (MAP) kinase kinase (MEK) activity, reduced expression of survivin and various cyclins, and is followed by apoptotic cell death. Thus, our study demonstrates that inhibition of proliferation and induction of apoptosis by DMC (and celecoxib) can be accomplished even in highly drug-resistant multiple myeloma cells, and that this effect is achieved via the blockage of multiple targets that are critical for multiple myeloma cell growth and survival.
Introduction
The treatment of patients with multiple myeloma (MM) is far from successful, as patients frequently develop drug-resistant disease and ultimately succumb to death.1 Various intracellular growth and survival pathways have been found activated in MM cells, such as those governed by insulin-like growth factor (IGF-1) receptor, protein kinase B (PKB/Akt), mitogen-activated protein (MAP) kinase, interleukin 6 (IL-6) receptor, and Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) (see Lonial et al2 and Mitsiades et al3 for review). In addition, cyclooxygenase-2 (COX-2) is frequently overexpressed in MM and is an independent predictor of poor outcome.4 Due to the concurrent activation of several mitogenic and survival pathways in multiple myeloma, it is generally assumed that efficient therapy of this disease will require drugs, or drug combinations, that are able to simultaneously target several of these growth-stimulatory components.2,3,5 In this regard, a few novel multitargeted approaches in MM have recently yielded promising results.6-9
Several of the above-mentioned molecular pathways that are activated in MM have been shown—in tumor cells other than MM—to be targeted by the selective COX-2 inhibitor celecoxib and also by its non-COX-2 inhibitory derivative, 2,5-dimethylcelecoxib (DMC).10,11 For instance, it has been shown that treatment of various tumor cell types with celecoxib resulted in decreased activity of the mitogenic MAP kinase pathway, inhibition of the Akt/PKB survival pathway, blockage of crucial cellcycle-regulatory cyclin-dependent kinases (CDKs), and interruption of nuclear factor kappa B (NF-κB) signaling.10-19 In addition, celecoxib was shown to inhibit endoplasmic reticulum Ca2+-adenosine triphosphatases (ATPases) and certain types of carbonic anhydrases.20-22 Intriguingly, in those cases where some of these targets were also examined with DMC, we and others found that this non-COX-2 inhibitory analog was able to potently mimic the inhibitory effects of celecoxib, clearly indicating that inhibition of COX-2 did not play a major role in these processes.10,11,23 Furthermore, it was reported that celecoxib and DMC were able to reduce neovascularization in the chicken chorioallantoic membrane assay23 and suppress tumor formation in mice implanted with human prostate carcinoma or Burkitt lymphoma, thus demonstrating that both drugs exerted similar antitumor activity in vivo.10,11
The experimental use of DMC alongside celecoxib encompasses at least 2 important aspects. One is the critical question whether or not COX-2 is involved in the observed processes.24,25 Because DMC does not inhibit COX-2,10 the affected pathways are unlikely to be governed via the regulation of COX-2 activity; thus, the side-by-side comparison of celecoxib and DMC supports the exposure (or exclusion) of COX-2 involvement. The other important aspect relates to the recently revealed cardiovascular side effects of coxib use in the clinic (ie, the increased risk of heart attack and stroke during the prolonged exposure to elevated dosages of COX-2-inhibitory drugs [celecoxib/Celebrex, rofecoxib/Vioxx, both from Pfizer, New York, NY; and valdecoxib/Bextra from Merck, Whitehouse Station, NJ]).26-28 Bearing in mind that substantially increased daily dosages of these drugs are considered— and probably necessary—for cancer prevention or cancer therapy, the increased risk of cardiovascular failure is of considerable concern.29,30 In this regard, considering that DMC is not a coxib, one could speculate that this drug might not cause these unwanted side effects, and therefore, that it might be safer when used for anticancer purposes.
In view of the above-mentioned multitargeted effects of celecoxib and DMC, we hypothesized that these drugs could be beneficial for inclusion in MM therapy. As a first step in this direction, we decided to determine whether these drugs would be able to exert their antiproliferative effect in various established MM cell lines, including some that had developed substantial resistance to various anticancer drugs that are commonly used in the clinic. Our present study shows that this is indeed the case (ie, both DMC and celecoxib reduced growth and initiated apoptotic cell death in several MM cell lines, independent of their predisposition for drug resistance). These effects appeared to involve the inhibition of several different components of mitogenic and survival pathways, such as STAT3, MAP kinase kinase (MEK), survivin, NF-κB, and various cyclins, but were independent of COX-2. Thus, our data demonstrate that celecoxib and DMC exert multifaceted effects that lead to growth inhibition and apoptosis, even in highly drug-resistant MM cells.
Materials and methods
Materials
Celecoxib is 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.31 DMC is a close structural analog, where the 5-aryl moiety has been altered by replacing 4-methylphenyl with 2,5-dimethylphenyl, resulting in 4-[5-(2,5-dimethylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.10,11 Both compounds were synthesized in our laboratory according to previously published procedures; see Penning et al31 for celecoxib and Kardosh et al10 for DMC. Each drug was dissolved in DMSO at 100 mM (stock solution).
Cell lines and culture conditions
The RPMI8226 human multiple myeloma cell line was obtained from ATCC (Manassas, VA). Various drug-resistant variants thereof were obtained from the laboratory of Dr William Dalton (Moffit Cancer Center, Tampa, FL).32 The 8226/Dox40 variant is resistant to doxorubicin and other drugs (ie, multidrug resistant); 8226/LR5 cells are resistant to melphalan; and 8226/MR20 cells are resistant to mitoxantrone.32,33 The MM1.S and MM1.R human multiple myeloma cell lines were obtained from Dr Nancy Krett (Northwestern University, Chicago, IL).34 MM1.S is sensitive to and MM1.R is resistant to dexamethasone. All cells were propagated in RPMI (GIBCO BRL, Grand Island, NY) supplemented with 5% (8226 cell lines) or 10% (MM1 cell lines) fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin in a humidified incubator at 37°C and a 5% CO2 atmosphere. Contrary to an earlier report,35 no elevated levels of COX-2 were detected in the above cell lines (not shown).
Recombinant human IGF-1 was obtained from R&D Systems (Minneapolis, MN) and reconstituted in phosphate-buffered saline (PBS) to yield a stock solution of 100 μg/mL. Recombinant human IL-6 was purchased from Chemicon (Temecula, CA) and reconstituted in 5 mM acetic acid to 0.5 mg/mL (according to manufacturer's instructions).
Immunoblots and antibodies
Total cell lysates were prepared by lysis of cells with radioimmunoprecipitation assay (RIPA) buffer36 and protein concentrations were determined using the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL). For Western blot analysis, 30 μg of each sample was processed as described.37 All antibodies against cell-cycle-regulatory proteins, as well as anti-PARP and antisurvivin antibodies, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-MEK, phospho-STAT3, phospho-Akt/PKB, STAT3, Akt/PKB, and caspase-3 were from Cell Signaling Technology (Beverly, MA). The secondary antibodies were coupled to horseradish peroxidase, and were detected by chemiluminescence using the SuperSignal substrate from Pierce. All Western and immunoblots were repeated at least once to confirm the results.
Kinase assays
IκB kinase (IKK) assays were performed as described elsewhere.38 Briefly, IKK was immunoprecipitated from cellular lysates and incubated with substrate in the presence of [γ-32P]ATP. As a specific substrate, we used GST-IκBα (1-54), which is a GST-fusion protein containing 54 amino acids from the N-terminus of IκBα.38 The kinase reaction components were separated by polyacrylamide gel electrophoresis and exposed to X-ray film. In addition, the gel was stained with Coomassie blue in order to visually confirm equal loading of each lane.
TUNEL staining
Apoptosis was measured quantitatively with the use of the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay. All components for this procedure were from the ApopTag In Situ Apoptosis Detection kit (Chemicon), which were used according to the manufacturer's instructions (see technical bulletin on Chemicon's website, www.chemicon.com).
MTT assay
Cells were seeded into 96-well plates in a volume of 50 μL per well (2.0-5.0 × 105 cells/mL). The next day, an additional 50 μL of medium containing various concentrations of drug (or solvent DMSO as a control) was added and the cells were incubated for 48 to 72 hours. This was followed by the addition of 10 μL thiazolyl blue tetrazolium bromide (= methylthiazoletetrazolium, MTT; Sigma-Aldrich, St Louis, MO) for 4 hours (stock solution of MTT is 5 mg/mL in PBS). The reaction was stopped and the cell cultures lysed by the addition of 100 μL solubilization solution (10% sodium dodecyl sulfate [SDS] in 0.01 M hydrochloric acid [HCl]). The 96-well plate was left in the cell culture incubator overnight for complete solubilization of the MTT crystals, and the optical density (OD) of each well was determined in an enzyme-linked immunosorbent assay (ELISA) reader at 490 nm. The background value (equal to the OD of control well containing medium without cells + MTT + solubilization solution) was subtracted from all measured values. All experiments were performed at least in quadruplicate.
Results
In this study, we employed several established human multiple myeloma (MM) cell lines and investigated the effects of celecoxib or dimethyl-celecoxib (DMC) on their growth and survival. In addition, various crucial components of intracellular signaling pathways, cell-cycle regulation, and apoptosis were examined. Two different sets of cell lines were used. The first set consisted of RPMI8226 cells and 3 derived drug-resistant variants: 8226/Dox40 cells (resistant to doxorubicin and various other drugs [ie, multidrug resistant]); 8226/LR5 cells (resistant to melphalan); and 8226/MR20 cells (resistant to mitoxantrone).32,33 The second set was a pair of cell lines in which one was sensitive to growth inhibition by dexamethasone (called MM1.S), whereas the other was resistant to treatment by dexamethasone (called MM1.R).34
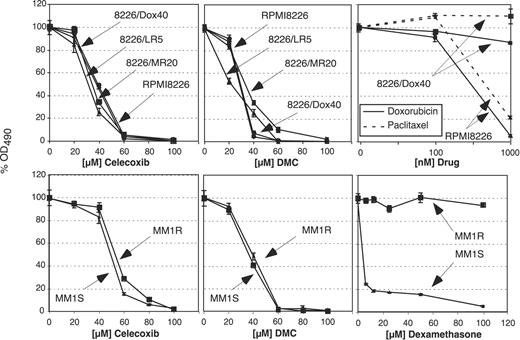
In order to determine the effects of celecoxib and DMC on the proliferation of the different MM cell lines, these cells were exposed to various concentrations of either drug, and cell growth was determined by standard MTT assays. As shown in Figure 1, the growth of all cell lines was similarly inhibited by celecoxib and DMC, and this effect was independent of their status of chemodrug resistance. Overall, DMC exerted slightly stronger growth-inhibitory potency, consistent with earlier observations with the use of prostate carcinoma and lymphoma cells.10,11 As a control, the 2 cell pairs, MM1.S/MM1.R and RPMI8226/8226-Dox40, were treated with dexamethasone or doxorubicin and paclitaxel, respectively. As expected, clear differences in drug resistance were observed, confirming the differential sensitivity of these cells toward conventional chemodrugs (Figure 1).
Reduced survival of MM cell lines in the presence of celecoxib or DMC. Various MM cell lines were treated with different drugs, and cell growth and survival was determined after 48 hours by MTT assay. The top 3 panels show the results with the use of RPMI8226 cells and several drug-resistant variants as indicated. The bottom panels show the results with the cell pair MM1.S and MM1.R. Note that all cell lines are similarly inhibited by celecoxib (left panels) and by DMC (middle panels). As a control to verify drug resistance of these lines, the cells were treated with various drugs, as shown in the right panels. For example, 8226/Dox40 cells demonstrate clear resistance to killing by doxorubicin and paclitaxel, whereas the parental RPMI8226 cells are effectively growth inhibited and killed by these drugs (top right panel). Similarly, the proliferation of MM1.R cells is not affected by dexamethasone up to 100 μM, whereas MM1.S cells are sensitive to concentrations of 5 μM and below (bottom right panel). Shown are the mean values of n equal to 8 plus or minus SE.
Reduced survival of MM cell lines in the presence of celecoxib or DMC. Various MM cell lines were treated with different drugs, and cell growth and survival was determined after 48 hours by MTT assay. The top 3 panels show the results with the use of RPMI8226 cells and several drug-resistant variants as indicated. The bottom panels show the results with the cell pair MM1.S and MM1.R. Note that all cell lines are similarly inhibited by celecoxib (left panels) and by DMC (middle panels). As a control to verify drug resistance of these lines, the cells were treated with various drugs, as shown in the right panels. For example, 8226/Dox40 cells demonstrate clear resistance to killing by doxorubicin and paclitaxel, whereas the parental RPMI8226 cells are effectively growth inhibited and killed by these drugs (top right panel). Similarly, the proliferation of MM1.R cells is not affected by dexamethasone up to 100 μM, whereas MM1.S cells are sensitive to concentrations of 5 μM and below (bottom right panel). Shown are the mean values of n equal to 8 plus or minus SE.
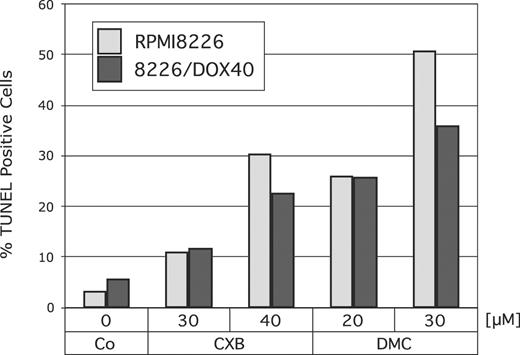
Next, we determined whether treatment with celecoxib or DMC would increase the rate of apoptosis in RPMI8226 cells and also in their multidrug-resistant counterpart, 8226/Dox40 cells. Cells were treated with 30 or 40 μM celecoxib, or with 20 or 30 μM DMC, and apoptosis was determined by TUNEL assay. As shown in Figure 2, both drugs substantially increased the amount of apoptotic cell death in both cell lines. Again, DMC exerted a somewhat stronger effect, in that the potency of 20 μM DMC was comparable to the effects of 40 μM celecoxib.
Induction of apoptosis by celecoxib or DMC. RPMI8226 or drug-resistant 8226/Dox40 cells were cultured in the presence of various concentrations of celecoxib or DMC for 48 hours. Apoptosis was determined using a TUNEL kit as described in “Materials and methods.” Shown is the percentage of TUNEL-positive (ie, apoptotic) cells, as calculated from several hundred cells assessed by visual inspection under the microscope.
Induction of apoptosis by celecoxib or DMC. RPMI8226 or drug-resistant 8226/Dox40 cells were cultured in the presence of various concentrations of celecoxib or DMC for 48 hours. Apoptosis was determined using a TUNEL kit as described in “Materials and methods.” Shown is the percentage of TUNEL-positive (ie, apoptotic) cells, as calculated from several hundred cells assessed by visual inspection under the microscope.
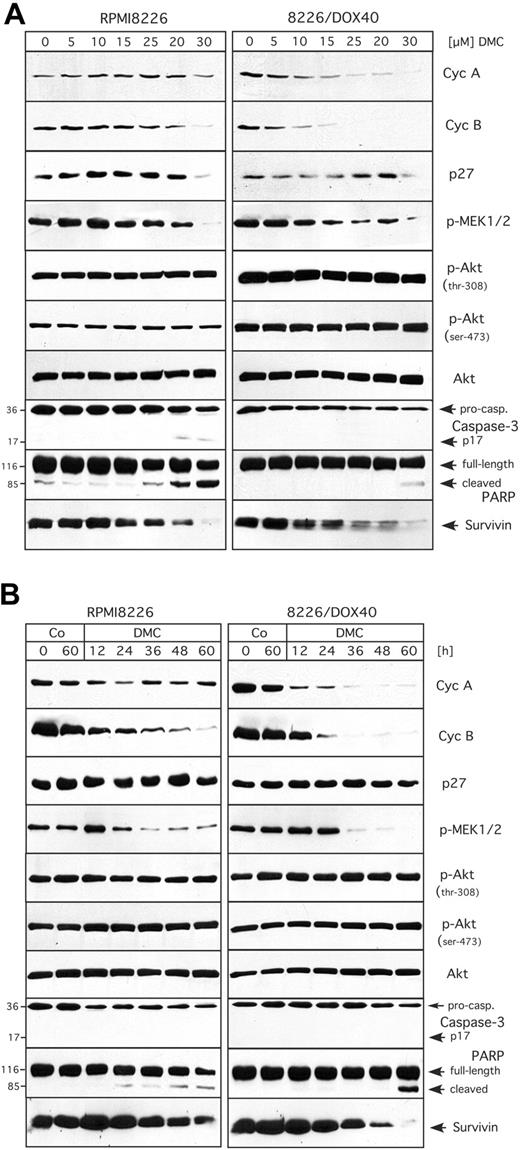
After having established that celecoxib and DMC similarly inhibited cell growth and induced apoptosis in MM cells regardless of the cells' drug-resistance status, we set out to determine the underlying mechanisms for these effects. With the use of glioblastoma and lymphoma cells, we had shown earlier10,14 that celecoxib and DMC caused cell-cycle arrest via the down-regulation of cyclin A and cyclin B, both of which are essential subunits of cyclin-dependent kinases (CDKs) that are absolutely required for cellcycle progression to take place.39,40 We therefore investigated first whether this regulation would also occur in MM cells. For this purpose, RPMI8226 and 8226/Dox40 cells were treated with celecoxib or DMC and the expression levels of various cell-cycle regulatory proteins were analyzed by Western blot analysis. As shown in Figure 3A, there was a strong down-regulation of cyclin A and cyclin B proteins by either drug in the multidrug-resistant 8226/Dox40 cell line, whereas this effect was substantially weaker in RPMI8226 cells. At the same time, no induction of the 2 CDK inhibitory proteins, p21Cip1 and p27Kip1, was observed (Figure 3A). Thus, these results indicated that the inhibition of cell growth induced by either drug did not correlate very well with the observed down-regulation of cyclin expression, suggesting that other mechanisms might participate as well.
Recently, 2 major intracellular signaling pathways had been implicated in mediating the effects of celecoxib and DMC: the cellular survival pathway governed by protein kinase B (PKB/Akt), and the mitogenic mitogen-activated protein (MAP) kinase pathway.10,11,18,41,42 We therefore examined the activity of both of these pathways in drug-treated RPMI8226 and 8226/Dox40 cells by Western blot analysis with the use of antibodies that specifically recognize only the phosphorylated (ie, activated) forms of the respective signaling proteins. As shown in Figure 3B, the activity of the MAP kinase pathway was clearly down-regulated by either drug, as indicated by greatly reduced phosphorylation of MEK1 and MEK2, 2 dual-specificity protein kinases that are central regulatory components of this pathway and responsible for directly phosphorylating (ie, activating) the mitogenic MAP kinases p42 and p44 (ERK1/2).43,44 As was observed for the regulation of cyclins, the inhibitory effects of both drugs were somewhat more potent in the 8226/Dox40 cells compared with the RPMI8226 cells. Surprisingly, however, when the activity (phosphorylation) of PKB/Akt was analyzed, no obvious inhibition was found in either cell type (Figure 3B), indicating that this signaling component might not play a major role in the inhibition of MM cell growth by celecoxib or DMC.
Expression levels and activity of proteins involved in cell cycle, signaling, and apoptosis in response to treatment with celecoxib or DMC. RPMI8226 and 8226/Dox40 cells were cultured in the presence of 30 and 50 μM celecoxib (CXB) or 20 and 40 μM DMC for 48 hours as indicated. In parallel, some cell cultures received 0.5 μM doxorubicin (Dox). Total cellular lysates were prepared and analyzed by Western blotting with specific antibodies or by in vitro kinase assay. (A) Analysis of the cell-cycle regulatory proteins cyclin A (cycA) and cyclin B (cycB), and the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1.
Expression levels and activity of proteins involved in cell cycle, signaling, and apoptosis in response to treatment with celecoxib or DMC. RPMI8226 and 8226/Dox40 cells were cultured in the presence of 30 and 50 μM celecoxib (CXB) or 20 and 40 μM DMC for 48 hours as indicated. In parallel, some cell cultures received 0.5 μM doxorubicin (Dox). Total cellular lysates were prepared and analyzed by Western blotting with specific antibodies or by in vitro kinase assay. (A) Analysis of the cell-cycle regulatory proteins cyclin A (cycA) and cyclin B (cycB), and the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1.
A third intracellular signal transduction pathway, NF-κB, was recently shown to be inhibited by celecoxib in human non-smallcell lung carcinoma and cervical cancer cells.15,17 We therefore determined whether DMC (and celecoxib) would impinge on the activity of this pathway as well. The activity of NF-κB signaling can be revealed by in vitro kinase assays measuring the activity of IκB kinase (IKK), which acts as an activator of NF-κB via the phosphorylation (and thus inactivation) of the NF-κB inhibitor, IκB.38 We therefore determined IKK activity in RPMI8226 and 8226/Dox40 cells treated with DMC or celecoxib. As shown in Figure 3B (bottom panels), DMC prominently inhibited IKK activity in RPMI8226 cells, but not in 8226/Dox40 cells. Similarly, celecoxib displayed a weak inhibitory effect in RPMI8226 cells, but no effect in 8226/Dox40 cells.
Next, in order to obtain insight into the mechanisms of drug-induced cell death, we analyzed representative members of the apoptotic machinery in RPMI8226 and 8226/Dox40 cells. We found that the executioner caspase-3 was activated by both celecoxib and DMC, as indicated by the proteolytic cleavage of procaspase-3 to generate active p17 caspase-3 (Figure 3C). In addition, both drugs generated the typical cleavage products of poly(ADP-ribose) polymerase-1 (PARP-1), the main substrate of caspase-3, thus verifying functional activation of the apoptotic caspase cascade (Figure 3C). In addition, the expression level of another important regulator of apoptosis, survivin, which is an inhibitor of apoptosis that is consistently overexpressed in tumor cells,45,46 was found to be down-regulated by both celecoxib and DMC, and yet again, this effect was somewhat stronger in 8226/Dox40 cells compared with RPMI8226 cells (Figure 3C).
Taken together, the above data indicated that celecoxib and DMC similarly affected critical components of cell-cycle-regulatory and apoptotic pathways, thereby leading to proliferation arrest and apoptosis. In order to gain insight into the regulatory relevance and temporal control of these processes, we next performed a detailed concentration- and time-dependent analysis of these drug-induced events. Because DMC clearly mimicked the effects of celecoxib, we decided to only use DMC, as this drug exerted somewhat stronger overall effects. Thus, RPMI8226 and 8226/Dox40 cells were treated either with increasing concentrations (0-30 μM) of DMC for 48 hours, or with 30 μM DMC for various time points (0-60 hours), and the previously introduced components of cell-cycle regulation, signal transduction, and apoptosis were comparatively analyzed.
The concentration-dependent effects of DMC are presented in Figure 4A. Overall, the most sensitive response was exhibited by cyclin A and cyclin B in 8226/Dox40 cells, where DMC as low as 5 μM reduced the expression levels of these crucial cell-cycle-regulatory proteins. In contrast, in RPMI8226 cells much higher concentrations of DMC were required to impinge on these cyclins. Similarly, the activities of MEK1 and MEK2 were effectively down-regulated by low doses of DMC in 8226/Dox40 cells, whereas RPMI8226 cells required somewhat higher concentrations for this effect to take place. At the same time, there was no apparent inhibition of PKB/Akt activity by DMC in either cell type (Figure 4A), consistent with our observations presented in Figure 3B. With regard to the concentration dependence of apoptotic events induced by DMC, it appeared that RPMI8226 cells were slightly more sensitive than 8226/Dox40 cells, as the activation of caspase-3 and the cleavage of PARP became apparent at somewhat lower concentrations of DMC in RPMI8226 cells (Figure 4A).
These observations indicated that with regard to the inhibition of signal transduction and cell-cycle components, 8226/Dox40 cells were somewhat more sensitive to DMC than RPMI8226 cells, whereas the opposite was true in the case of the induction of executioners of apoptosis. These characteristics were confirmed when the time course of these events was studied (shown in Figure 4B). The down-regulation of cyclins A and B became apparent at around 12 hours of DMC treatment in 8226/Dox40 cells, whereas in RPMI8226 cells cyclin A was barely affected, and cyclin B was down-regulated less effectively. Similarly, the inhibition of the MAP kinase pathway, as exemplified by decreased phosphorylation of MEK1 and MEK2, was more pronounced in 8226/Dox40 cells than in RPMI8226 cells. Conversely, cleavage of PARP became apparent after 24 hours of DMC treatment in RPMI8226 cells, but only after 60 hours in 8226/Dox40 cells (Figure 4B). Thus, in summary, while DMC exerted noticeable differential effects on cell cycle and apoptosis, with inhibition of cell-cycle components favored in 8226/Dox40 cells and induction of apoptosis favored in RPMI8226 cells, the net result in both instances was effective growth inhibition and cell death, as illustrated in Figures 1 and 2. Cell-cycle inhibition of either cell type was further confirmed by determining the distribution of drug-treated cells throughout the cell cycle via fluorescence-activated cell sorting (FACS). Consistent with several previous reports,11,14,23,47 treatment of either cell type with celecoxib or DMC led to increased accumulation of cells in the G1 phase of the cell cycle, which was followed by apoptotic cell death (not shown).
Time and concentration dependence of DMC effects. RPMI8226 and 8226/Dox40 cells were treated with increasing concentrations of DMC for 48 hours (A), or with 30 μM DMC for various time points up to 60 hours (B). Cell lysates were prepared and analyzed by Western blot as detailed in the legend to Figure 3.
Time and concentration dependence of DMC effects. RPMI8226 and 8226/Dox40 cells were treated with increasing concentrations of DMC for 48 hours (A), or with 30 μM DMC for various time points up to 60 hours (B). Cell lysates were prepared and analyzed by Western blot as detailed in the legend to Figure 3.
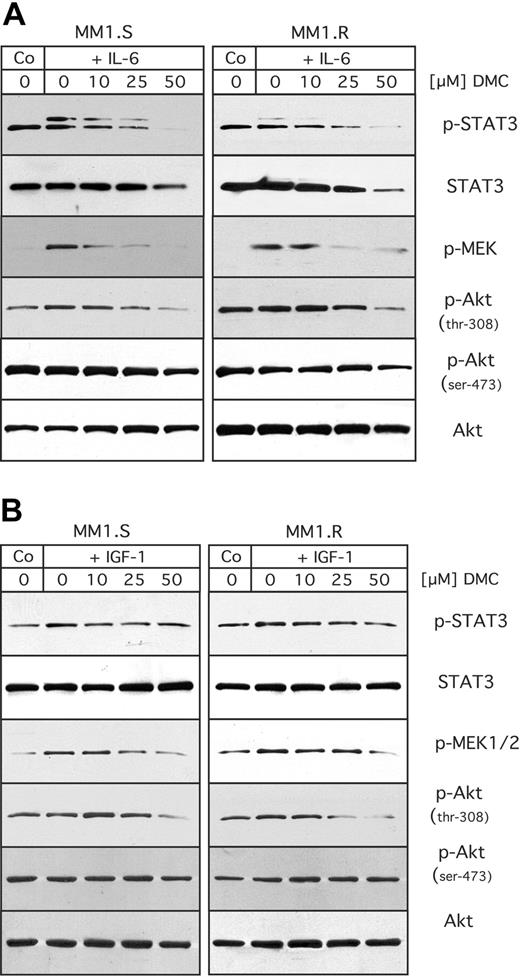
All these presented experiments were performed under in vitro growth conditions supported by fetal bovine serum, which contains an undefined mixture of various growth factors and nutrients. However, as it is known that interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1) in particular are critical growth and survival factors for MM cells,2,3 we decided to investigate whether DMC would be able to overcome the protective effects of these exogenous growth and survival factors. For this purpose, we used the cell pair MM1.S (sensitive to dexamethasone) and MM1.R (resistant to dexamethasone), because the molecular response of these cells to IL-6 and IGF-1 had been well characterized before.7,9,48
MM1.S and MM1.R cells were pretreated with DMC for 1 hour, and then stimulated with either IL-6 or IGF-1, as shown in Figure 5. Stimulation of either cell type with IL-6 generated increased levels of active (ie, phosphorylated) STAT3 and MEK, indicating that treatment with IL-6 activated these pathways. However, DMC was able to block this stimulation in a concentration-dependent manner (Figure 5A). At the same time, no major stimulatory effect of IL-6 on the activity (phosphorylation levels) of Akt could be observed. When these same cells were stimulated with IGF-1, no major increase in STAT3 or Akt phosphorylation was observed, whereas there was a weak stimulation of MEK activity (phosphorylation), which was suppressed by increasing concentrations of DMC (Figure 5B). Thus, these results indicated that DMC was able to abrogate the stimulatory effects of specific growth and survival factors on the intracellular signal transduction machinery.
Inhibitory effects of DMC on stimulation by IL-6 or IGF-1. MM1.S and MM1.R cells were cultured under low serum conditions (RPMI with 1% FBS) overnight. The medium was then replaced with RPMI (without FCS) containing increasing concentrations of DMC as indicated. After 1 hour, the cells were stimulated with either 25 ng/mL of IL-6 (A), or 200 ng/mL of IGF-1 (B). Fifteen minutes later, cells were lysed and analyzed by Western blot analysis as detailed in the legend to Figure 4.
Inhibitory effects of DMC on stimulation by IL-6 or IGF-1. MM1.S and MM1.R cells were cultured under low serum conditions (RPMI with 1% FBS) overnight. The medium was then replaced with RPMI (without FCS) containing increasing concentrations of DMC as indicated. After 1 hour, the cells were stimulated with either 25 ng/mL of IL-6 (A), or 200 ng/mL of IGF-1 (B). Fifteen minutes later, cells were lysed and analyzed by Western blot analysis as detailed in the legend to Figure 4.
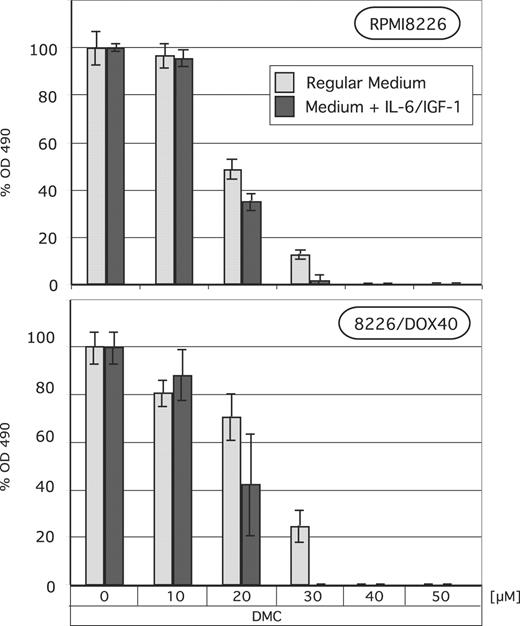
To determine whether the observed abrogation of intracellular signaling would translate into reduced cell growth even in the presence of exogenously added growth and survival factors, we determined MM cell growth and survival in response to DMC treatment after the addition of exogenous IL-6 and IGF-1 to the culture medium. As shown in Figure 6, the presence of these 2 factors did not protect MM cells against DMC-induced cell death; quite in contrast, it appeared that in the presence of added IL-6 and IGF-1 there was slightly more cell death. This finding clearly demonstrates that DMC is able to inhibit MM cell growth even under conditions where important growth and survival factors are present in the cellular milieu. Thus, the inhibitory effects of DMC appear to be dominant and take effect even in the presence of different positive stimuli.
Discussion
The selective COX-2 inhibitor celecoxib appears to hold promise for the treatment and prevention of colorectal cancer and possibly for other cancers as well. Because COX-2 is an oncogene49 and overexpressed in a large number of tumors, it is generally thought that the COX-2-inhibitory function of celecoxib is critical for its antitumor property.50-53 However, several recent studies,11,42,54-58 including two conducted in our laboratory,10,14 have indicated that celecoxib might be unique among the class of coxibs because this particular compound appears to be able to also suppress tumor formation in the absence of COX-2 involvement. For example, all coxibs completely inhibit COX-2 at very low micromolar concentrations in cell culture, yet only celecoxib causes efficient growth arrest and induction of apoptosis—an effect that is furthermore independent of the amount, or even the presence, of intracellular COX-2 (ie, it takes place even in cells that lack COX-2 protein).14,18,54,57,59-62 Additional strong support for COX-2-independent antitumor effects of celecoxib has come from the use of its close structural analog, dimethyl-celecoxib (DMC), which lacks COX-2 inhibitory function, yet was shown to faithfully mimic the antitumor effects of celecoxib on prostate carcinoma and Burkitt lymphoma in vitro and in vivo.10,11
Lack of protection from DMC by IL-6 and IGF-1. RPMI8226 and 8226/Dox40 MM cells were cultured in regular growth medium with or without exogenously added human IL-6 (25 ng/mL) and IGF-1 (200 ng/mL). DMC at various concentrations was added for 72 hours, and MTT assays were performed as described in “Materials and methods.” Shown are the mean values of n equal to 8, plus or minus SE.
Lack of protection from DMC by IL-6 and IGF-1. RPMI8226 and 8226/Dox40 MM cells were cultured in regular growth medium with or without exogenously added human IL-6 (25 ng/mL) and IGF-1 (200 ng/mL). DMC at various concentrations was added for 72 hours, and MTT assays were performed as described in “Materials and methods.” Shown are the mean values of n equal to 8, plus or minus SE.
In the present study, we provide evidence that both compounds, celecoxib and DMC, similarly block the proliferation and survival of multiple myeloma cell lines. Amazingly, these growth-inhibitory effects take place even in cells that otherwise are highly resistant to the inhibitory effects of various anticancer drugs that are commonly used in the clinic for the treatment of cancer patients. This is even more astonishing in light of the diverse mechanisms that underlie the manifestation of drug resistance in these cell lines. For instance, multidrug resistance of 8226/Dox cells is associated with overexpression of P-glycoprotein (P-gp, mdr1, ABCB1),63 a membrane-bound efflux pump that transports a wide variety of functionally and structurally diverse cytotoxic drugs out of tumor cells.64 Resistance of 8226/MR20 cells to mitoxantrone has been linked to increased expression of BCRP (breast cancer resistance protein, ABCG2),65 another transport protein that is able to efflux a spectrum of anticancer drugs.64 Resistance of 8226/LR5 to melphalan is associated with increased levels of glutathione,66 a tripeptide involved in antioxidation and detoxification of xenobiotics.67 And finally, resistance of MM1.R cells to dexamethasone was shown to be due to greatly diminished levels of glucocorticoid receptor,34 a situation that is commonly observed in drug-resistant tumor cells that emerge in MM patients after glucocorticoid therapy.68
We demonstrate that all of the described drug-resistant cells are as effectively growth inhibited by celecoxib and DMC as are their drug-sensitive counterparts. Thus, celecoxib and DMC are able to bypass the block to growth inhibition conferred by several well-described resistance mechanisms and indiscriminately induce cell death in all of these cell lines. As has been consistently observed in earlier studies that used these 2 drugs side by side,10,11,23,42 growth inhibition and induction of apoptosis by DMC is noticeably more potent than what is observed with celecoxib (ie, roughly 20%-50% less DMC is required to faithfully mimic the antitumor effects of celecoxib). This increased effectiveness of DMC might be relevant with regard to the potential clinical usefulness of this drug. In contrast to celecoxib, DMC has not yet been studied in humans and is not currently used for patient therapy. However, preliminary pharmacokinetic studies in animals have demonstrated a profile that is similar to celecoxib.11 It is therefore tempting to speculate that DMC might be superior to celecoxib with regard to anticancer therapy of at least some types of tumors.
In addition to its higher potency, DMC might have an additional advantage: because it does not inhibit COX-2, its use in human patients might not cause the serious cardiovascular side effects that recently emerged with the prolonged use of coxibs such as celecoxib, rofecoxib, and valdecoxib.26-28 However, although our present study provides the necessary basic in vitro studies of molecular and cellular DMC effects in MM cells, additional extensive in vivo studies are required to substantiate these speculations. For instance, although the known side effects of coxibs have been attributed to an imbalance of prostanoid levels due to the selective inhibition of COX-2,69,70 one could hypothesize that some of the other, non-COX-2-related mechanisms—which are retained in DMC—might potentially contribute to the unwanted side effects as well. However unlikely, such a possibility needs to be kept in mind. In addition, it should be emphasized that our study does not call into question a large number of reports (see, for example, Hawk et al,24 Dannenberg and Subbaramaiah,51 and Howe and Dannenberg71 for review) documenting that inhibition of COX-2 has anticancer effects. Rather, celecoxib and DMC appear to posess an additional, COX-2-independent function, which by itself seems to exert potent antitumor properties as well. Considering that MM is strongly regulated by its stromal microenvironment, which includes the presence of elevated levels of prostaglandins, it will be important to determine whether the COX-2 independent function alone (ie, without the simultaneous inhibition of COX-2) will be able to reduce MM growth in vivo. In any case, while DMC certainly requires further study before it can be used clinically, celecoxib, which is being used in several anticancer clinical trials, might be worth considering for inclusion in trials aimed at the therapeutic management of relapsed, drug-resistant MM, similar to some of the novel biologically based treatment strategies that have shown promise in preclinical and clinical studies.72-74
A general criticism that has been put forward relates to the observation that many of the antiproliferative effects of celecoxib observed in cell culture were achieved at drug concentrations that were generally higher (10-100 μM) than those measured in the serum of patients or animals (3-10 μM).75-77 This discrepancy has led to the suggestion78 that in vitro effects of celecoxib (and perhaps DMC) might be an artifact and not reflective of the mechanisms taking place in vivo. In this regard, however, recent reports have indeed verified that certain effects observed in vitro do also take place in vivo in tumor-bearing animals. For example, inhibition of cyclin A and cyclin B expression as well as the down-regulation of the PDK1/Akt pathway, events which have been observed first in in vitro studies with elevated concentrations of these drugs, later on were documented also in tumors from animals treated with celecoxib or DMC.10,11 Although it remains to be established whether this will also hold true for the several additional intracellular targets analyzed in this present study, the previous findings10,11 have set an encouraging precedent. In addition, at least in the case of down-regulation of cyclin A and cyclin B by DMC in 8226/Dox40 cells, clear inhibitory effects of this drug can be seen at concentrations as low as 5 μM (Figure 4A, right panels). This same concentration has also been shown to inhibit the migration and invasion of human lung adenocarcinoma cells in vitro.79
It has become generally accepted that efficient cancer treatment regimens will require multimodal therapies and combination drug treatments, and multiple pathways in tumor cells should be targeted in order to achieve therapeutic success. In this regard, our observation that celecoxib and DMC are able to simultaneously block several critical growth and survival pathways within the same tumor cell type is quite intriguing. For instance, our results demonstrate that DMC down-regulates critical components of the cell-cycle machinery (cyclins A and B; Figures 3A, 4); blocks the activity of important mitogenic and survival pathways (MEK, NF-κB, STAT3, survivin; Figures 3B-C, 4, 5); and leads to increased caspase activity (Figures 3C, 4). In this context, it is interesting to note that others have demonstrated that the combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of MM cells in the presence of bone marrow stromal cells (ie, under conditions that resemble the microenvironment in vivo). As we show that DMC is able to potently block both of these pathways, this might provide an explanation as to the extensive amount of apoptosis that follows (Figure 2). However, we also observe that survivin is efficiently down-regulated by DMC (and celecoxib; Figures 3C, 4). Because this protein is an important antiapoptotic component that is frequently elevated in tumor cells,45 it is possible that the robust decline in survivin expression might contribute to drug-induced apoptosis in our experiments. In any case, while some molecular details of the observed processes remain to be established, our results clearly demonstrate that the multifaceted actions of DMC and celecoxib lead to efficient growth arrest and apoptosis—even in highly drug-resistant MM cells.
Finally, it should be emphasized that DMC appears to mimic all of the multitarget effects of celecoxib—except inhibition of COX-2. In all studies so far, including the present report (which newly identifies MEK, survivin, NF-κB, and caspase-3 as components of DMC-initiated effects), DMC has been able to potently mimic celecoxib with regard to inhibition of various intracellular signaling pathways, blockage of cell-cycle components, and induction of apoptosis.10,18,19 In addition, previous studies have demonstrated that both drugs are able to inhibit angiogenesis in the chicken chorioallantoic membrane (CAM) model,23 and tumor growth in vivo in different animal models.10,11 Thus, at least for the cell types and tumor models investigated so far, no COX-2 inhibitory function appears to be required to mediate the antitumor properties of these 2 drugs. In addition, as we have demonstrated in this report, these COX-2-independent antiproliferative and proapoptotic effects also take place in several MM cell lines, whether or not they display various highly drug-resistant phenotypes.
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-07-2819.
Supported by funding from the James H. Zumberge Faculty Research and Innovation Fund (N.A.P., T.C.C., and A.H.S.) and by funding from the Margaret E. Early Medical Research Trust (A.H.S.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Nancy L. Krett (Northwestern University, Chicago, IL) and the laboratory of Dr William S. Dalton (Moffit Cancer Center, Tampa, FL) for providing the various drug-resistant multiple myeloma cell lines.

![Figure 3. Expression levels and activity of proteins involved in cell cycle, signaling, and apoptosis in response to treatment with celecoxib or DMC. RPMI8226 and 8226/Dox40 cells were cultured in the presence of 30 and 50 μM celecoxib (CXB) or 20 and 40 μM DMC for 48 hours as indicated. In parallel, some cell cultures received 0.5 μM doxorubicin (Dox). Total cellular lysates were prepared and analyzed by Western blotting with specific antibodies or by in vitro kinase assay. (A) Analysis of the cell-cycle regulatory proteins cyclin A (cycA) and cyclin B (cycB), and the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \({\otimes}\) \end{document} indicates that RPMI8226 cells were treated with doxorubicin as well; however, due to efficient killing of these drug-sensitive cells, the recovered amount of protein was insufficient for analysis. (B) Analysis of the activity of representatives of 3 major signaling pathways, the mitogenic MAP kinase pathway, the Akt/PKB survival pathway, and the antiapoptotic NF-κB pathway. The activity of MAP kinase kinases 1 and 2 (MEK1 and MEK2) is determined with the use of antibodies that specifically recognize the phosphorylated (ie, active) forms of MEK1 and MEK2 (p-MEK1/2). The activity of Akt is determined with the use of antibodies that recognize Akt phosphorylated on either threonine-308 (p-Akt, thr-308) or on serine-473 (p-Akt, ser-473). In comparison, the total amount of Akt (active and inactive) is shown in the panel labeled Akt. The activity of NF-κB signaling is indicated by in vitro kinase assays measuring the activity of IκB kinase (IKK), which acts as an activator of NF-κB via the phosphorylation (and thus inactivation) of the NF-κB inhibitor, IκB. The panel labeled 32P-IκBα is an autoradiograph reflective of IKK enzymatic activity, whereas the panel labeled IκBα shows Coomassie blue staining of the same gel to confirm that equal amounts of substrate (IκBα) were used in each reaction. (C) Analysis of proteins involved in apoptosis. Activation of caspase-3 is revealed via the detection of the active caspase p17 fragment, and via the emergence of cleaved PARP, which is a substrate of caspase-3. Also shown is survivin, an antiapoptotic protein that is often found elevated in tumor cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/13/10.1182_blood-2005-07-2819/2/m_zh80240588300003.jpeg?Expires=1767753185&Signature=ppM3Z377mEIwdlbbqorJsYde4xZckVWloO-bABhFagHftgX9KLPGYHdFqiLZpOrADSugo70equX0hIMakBTm8cVzoZXsIZXxVc19F8Bc7VkV2Mk4ILaHHoAmAAPV9VDH6m2EUf4cXw1EfAhwkbWfP3mkSmGZPNcxqo3IEAKzCvCX0HoZZZYxedgXdl9bpDZNbr73F3jFp8~aTpHzSinbF3EXPYJ5vv2ZMIKwmIpLCt9jMp~XmX4ttp0tL4Q5ARYXW1ohf4bjsP4FybDoOWMheOndZgBNdKCFlHfBHHEDSssDe4VQAqnGYAzWaPzTn3ZMLhCDc9D7w3207-fedoCt6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal