Cyclin D1 overexpression is believed to be essential in the pathogenesis of mantle cell lymphoma (MCL). Hence, the existence of cyclin D1-negative MCL has been controversial and difficult to substantiate. Our previous gene expression profiling study identified several cases that lacked cyclin D1 expression, but had a gene expression signature typical of MCL. Herein, we report the clinical, pathologic, and genetic features of 6 cases of cyclin D1-negative MCL. All 6 cases exhibited the characteristic morphologic features and the unique gene expression signature of MCL but lacked the t(11;14)(q13; q32) by fluorescence in situ hybridization (FISH) analysis. The tumor cells also failed to express cyclin D1 protein, but instead expressed either cyclin D2 (2 cases) or cyclin D3 (4 cases). There was good correlation between cyclin D protein expression and the corresponding mRNA expression levels by gene expression analysis. Using interphase FISH, we did not detect chromosomal translocations or amplifications involving CCND2 and CCND3 loci in these cases. Patients with cyclin D1-negative MCL were similar clinically to those with cyclin D1-positive MCL. In conclusion, cases of cyclin D1-negative MCL do exist and are part of the spectrum of MCL. Up-regulation of cyclin D2 or D3 may substitute for cyclin D1 in the pathogenesis of MCL.
Introduction
Mantle cell lymphoma (MCL) is now recognized as an aggressive B-cell lymphoma with various growth patterns (mantle zone, nodular, or diffuse) and a broad range of cytologic features.1-5 Most cases of MCL exhibit a characteristic phenotype (CD20+, CD5+, CD43+, CD3-, CD10-, CD23-) and have the t(11;14)(q13;q32) with overexpression of the cyclin D1 (CCND1) gene on chromosome 11q13.6,7 Cyclin D1, a D-type cyclin that is not expressed in normal B lymphocytes, plays a key role in cell cycle regulation during the G1 to S phase transition by binding to cyclin-dependent kinase 4 (CDK4) and CDK6, resulting in phosphorylation and inactivation of the retinoblastoma protein (RB).8-10 The current World Health Organization guidelines for the diagnosis of MCL rely on morphologic examination and immunophenotyping, with demonstration of cyclin D1 protein overexpression and/or the t(11; 14)(q13;q32) for confirmation.11
The existence of cyclin D1-negative MCL has been controversial and difficult to substantiate since cyclin D1 overexpression is believed to be essential in the pathogenesis of MCL. Most reported cases of cyclin D1-negative MCL have been attributed to suboptimal immunostaining, inadequate genetic or molecular analyses, or misdiagnosis. Nevertheless, in a recent study of 99 lymphomas that were morphologically consistent with MCL, we identified a small group of cases that lacked cyclin D1 mRNA expression by both quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and Lymphochip cDNA microarray analyses.12 However, these cases had the characteristic MCL gene expression signature by cDNA microarray analysis and, therefore, were considered to be cases of cyclin D1-negative MCL. We have further refined the algorithm for diagnosing MCL using gene expression profiling and, herein, we report the clinical, pathologic, and genetic features of 6 cases of cyclin D1-negative MCL.
Patients, materials, and methods
Case selection
The lymph node biopsies from 6 patients without a previous history of malignancy are included in this study. All 6 cases were reviewed by a panel of expert hematopathologists to confirm the diagnosis. Two of these cases were identified in our previous report.12 This study was approved by the institutional review board at the University of Nebraska Medical Center. Informed consent was provided according to the Declaration of Helsinki.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
To measure cyclin D1 mRNA expression, 2.5-ng aliquots of mRNA were analyzed by quantitative RT-PCR using TaqMan reagents and a thermal cycler (Applied Biosystems, Foster City, CA).12 The samples were run in triplicate and the β2-microglobulin transcript was used as a reference. Primers and probes for β2-microglobulin and the coding region of cyclin D1 have been previously described.13
Microarray gene expression profiling
Lymphochip cDNA microarrays containing 12 196 cDNA elements14 were used to profile mRNA expression in the lymphoma samples. A set of MCL signature genes that can be used to distinguish MCL from other lymphoma subtypes has been described previously.12 These cases were further analyzed for gene expression using Affymetrix Human U133 A/B microarrays (Affymetrix, Santa Clara, CA). The gene expression profiles of these cases were compared with those of other B-cell non-Hodgkin lymphomas, including the various subtypes of diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, extranodal marginal zone lymphoma, mucosa-associated lymphoid tissue (MALT) type, and splenic marginal zone lymphoma. The distributions of the Bayesian predictor for each category were used to estimate the probability that any particular case belonged to that category by applying Bayes rule.12,15 Specifically, for each lymphoma category we generated a Bayesian predictor between that category and cyclin D1-positive MCL samples, based on the 50 genes with the largest t-statistics between them. Only those cases for which all pair-wise predictors agreed that there was a more than 90% estimated probability of the case being MCL were considered to be cyclin D1-negative MCL samples. This set of models was applied to our data set in a leave-one-out, cross-validated fashion so that the models tested on a given case were based on a data set that excluded that case. Cases for which there was a less than 90% likelihood of being in any category were termed unclassified.
Histologic and immunologic studies
The lymph node specimens were fixed in 10% neutral buffered formalin and embedded in paraffin, and 4-μm sections were cut and stained with hematoxylin and eosin (H&E) for histologic evaluation. Immunohistochemical stains for cyclin D1 protein were performed on formalin-fixed, paraffin-embedded tissue sections. Briefly, after deparaffinization in xylene and rehydration in graded alcohols, endogenous peroxidase was blocked with hydrogen peroxide. Heat-induced antigen retrieval was performed using citrate buffer, pH 6.0.16 After rinsing in phosphate-buffered saline, mouse anti-cyclin D1 antibody was applied at a dilution of 1:200. The rabbit monoclonal antibody SP4 against cyclin D1 (Neomarkers, Fremont, CA) was also used, using the suggested procedure for antigen retrieval with minor modification (Table 1).17 Antibodies against CD3, CD5, CD20, CD23, CD43, cyclin D2, cyclin D3, cyclin E, RB, and p27kip1 were also used for immunohistochemical stains (Table 1). These stains were performed on a Ventana ES automated immunostainer (Ventana Biotek, Tucson, AZ) with a streptavidin-biotin peroxidase detection system. Positivity for the cyclins, RB and p27kip1 was defined as a strong nuclear staining in more than 50% of the neoplastic cells.
Antibodies and methods for immunohistochemical stains
Antibody . | Clone . | Source . | Dilution . | Retrieval . |
|---|---|---|---|---|
| CD3 | PS1 | Ventana Medical Systems, Tucson, AZ | Neat | A |
| CD5 | 4C7 | Novocastra, Newcastle upon Tyne, United Kingdom | 1:20 | A |
| CD20 | L26 | DAKO, Carpinteria, CA | 1:200 | A |
| CD23 | BU38 | The Binding Site, San Diego, CA | 1:5 | B |
| CD43 | L60 | Ventana Medical Systems | Neat | None |
| Cyclin D1 | DCS-6 | DAKO | 1:200 | A |
| Cyclin D1 | SP4 | Neomarkers | 1:100 | C |
| Cyclin D2 | Polyclonal | Santa Cruz Biotech, Santa Cruz, CA | 1:500 | D |
| Cyclin D3 | DCS-22 | Neomarkers | 1:100 | E |
| Cyclin E | 13A3 | Novocastra | 1:10 | E |
| RB | Rb1 | DAKO | 1:10 | A |
| p27Kip1 | SX53G8 | DAKO | 1:20 | A |
Antibody . | Clone . | Source . | Dilution . | Retrieval . |
|---|---|---|---|---|
| CD3 | PS1 | Ventana Medical Systems, Tucson, AZ | Neat | A |
| CD5 | 4C7 | Novocastra, Newcastle upon Tyne, United Kingdom | 1:20 | A |
| CD20 | L26 | DAKO, Carpinteria, CA | 1:200 | A |
| CD23 | BU38 | The Binding Site, San Diego, CA | 1:5 | B |
| CD43 | L60 | Ventana Medical Systems | Neat | None |
| Cyclin D1 | DCS-6 | DAKO | 1:200 | A |
| Cyclin D1 | SP4 | Neomarkers | 1:100 | C |
| Cyclin D2 | Polyclonal | Santa Cruz Biotech, Santa Cruz, CA | 1:500 | D |
| Cyclin D3 | DCS-22 | Neomarkers | 1:100 | E |
| Cyclin E | 13A3 | Novocastra | 1:10 | E |
| RB | Rb1 | DAKO | 1:10 | A |
| p27Kip1 | SX53G8 | DAKO | 1:20 | A |
A indicates 10 mM citrate buffer, pH 6.0, 30 minutes, water bath (95°C); B, protease I enzymatic digestion, 8 minutes; C, 1 mM EDTA (ethylenediaminetetraacetic acid, pH 8.0), 30 minutes, water bath (95°C); D, 1 mM EDTA, pH 8.0, 60 minutes, water bath (95°C); and E, 10 mM citrate buffer, pH 6.0, 10 minutes, pressure cooker (115°C).
Fluorescence in situ hybridization (FISH) analysis
Interphase FISH analysis was performed on cells left over from prior cytogenetic analyses or on formalin-fixed, paraffin-embedded tissue sections. For detection of the t(11;14)(q13;q32), a commercially available LSI IGH/CCND1 double-color, double-fusion probe was used (Vysis, Downers Grove, IL). For break-apart FISH assays for the CCND1 (11q13), CCND2 (12p13), and CDKN1B/p27KIP1 (12p13) loci, appropriate bacterial artificial chromosome (BAC) clones flanking the respective genes were selected using bioinformatic resources available at the University of California at Santa Cruz (http://genome.ucsc.edu). All BAC clones were derived from the RPCI11 library and were obtained from Invitrogen/Research Genetics (Carlsbad, CA) or the Sanger Center (Hinxton, United Kingdom). The following clones were used: CCND1 (pooled RP11-211G23/RP11-378E8 and pooled RP11-300I6/RP11-626H12), CCND2 (RP11-578L13 and RP11-388F6), and CDKN1B/p27KIP1 (RP11-180M15 and RP11-59H1). For each locus, centromeric and telomeric BAC clones were differentially labeled with Spectrum Orange or Spectrum Green (Vysis) and pooled for break-apart assays. Bacterial culture, BAC DNA isolation and labeling, probe preparation, and FISH on cytogenetic suspensions were performed as previously described.18,19 The CCND3 locus was investigated using a recently described break-apart assay.20 Locus-specific interphase FISH was performed on paraffin-embedded tissue sections according to the manufacturer's instructions (Vysis) or recently described protocols21 with minor modifications. Whenever possible, at least 100 cells were analyzed.
INK4a/ARF locus deletion analysis
To detect genomic loss of INK4a/ARF tumor suppressor locus in the specimens, we performed quantitative real-time PCR assays using genomic DNA as previously described.12 The REL locus on chromosome 2p was chosen as the reference gene, and a cutoff ratio of INK4a/ARF locus copy number relative to REL locus copy number was used to assess tumor DNA for genomic deletions. A tumor DNA sample that yielded an INK4a/ARF-to-REL ratio below the cutoff ratio was considered to have a genomic deletion of the INK4a/ARF locus. The primers and probe sets for the INK4a/ARF and the REL loci have been described previously.22,23
Statistical analysis
The clinical characteristics of the cyclin D1-negative and cyclin D1-positive cases were compared using Fisher exact test. Overall survival was calculated as the time from initial diagnosis to the date of death or last follow-up, and those alive at last follow-up were treated as censored. The Kaplan-Meier method was used to estimate the overall survival distributions of the 2 groups, and the log-rank test was used to compare the distributions.
Results
Case selection
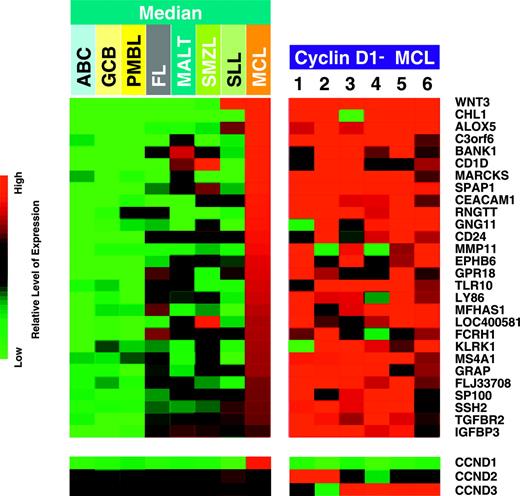
In our previous study,12 we identified 7 cyclin D1-negative cases by quantitative RT-PCR and cDNA microarray analysis among 99 cases of lymphoma with MCL morphology. Subsequent studies revealed that 1 of these 7 cases had the characteristic t(11;14)(q13; q32) by FISH analysis and expressed cyclin D1 protein by immunohistochemical staining (data not shown). Thus, this case was thought to be a false-negative case and was reclassified as a cyclin D1-positive MCL. During the interim, we performed additional gene expression profiling analysis using Affymetrix U133A/B microarrays and refined the algorithm for diagnosing MCL. Using the refined algorithm, 4 of the other 6 original cases were regarded as unclassifiable B-cell lymphomas and, thus, were excluded from the current study. Recently, we identified 4 additional cases of MCL that were negative for cyclin D1 by both immunohistochemistry and quantitative RT-PCR analysis. All 6 cases in the current study (2 from the previous study and 4 newly identified) exhibited the characteristic gene expression signature of MCL, which is distinct from the other common types of B-cell non-Hodgkin lymphoma including the various subtypes of diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, extranodal marginal zone lymphoma, MALT type, and splenic marginal zone lymphoma (Figure 1 and Table S1, which is available on the Blood website; see the Supplemental Materials link at the top of the online article). By applying Bayesian analysis, all 6 cases were predicted as MCL in all pair-wise models with 99.99% confidence. On the other hand, none of the cases from the other categories were predicted as MCL with more than 20% confidence in their respective cross-validated, pair-wise comparisons. Therefore, these 6 cases are considered to be bona fide cases of cyclin D1-negative MCL.
Histologic and immunologic features
All 6 cases exhibited a nodular or diffuse growth pattern and consisted of tumor cells with typical mantle cell cytology (Table 2, Figure 2A, and Figure S1). Immunophenotypic analysis of the tumor cells demonstrated a B-cell phenotype in all cases. Expression of CD5 antigen was noted in all 6 cases. The tumor cells in 1 of the 6 cases were weakly positive for CD23 antigen. Expression of CD43 antigen was noted in 5 of the 6 cases (Table 2).
Pathologic and genetic features of 6 patients with cyclin D1-negative MCL
. | Case no. 1 . | Case no. 2 . | Case no. 3 . | Case no. 4 . | Case no. 5 . | Case no. 6 . |
|---|---|---|---|---|---|---|
| Pathologic features | ||||||
| Growth pattern | Nodular | Diffuse | Nodular | Diffuse | Nodular | Nodular |
| Cytology | Typical | Typical | Typical | Typical | Typical | Typical |
| CD20 | + | + | + | + | + | + |
| CD3 | – | – | – | – | – | – |
| CD5 | + | + | + | + | + | + |
| CD23 | – | – | – | + (w) | – | – |
| CD43 | + (w) | + | – | + | + | + |
| Cyclin D1 | – | – | – | – | – | – |
| Cyclin D2 | + | + | – | – | – | NA |
| Cyclin D3 | – | – | + | + | + | NA |
| Cyclin E | – | – | – | – | – | NA |
| RB | + | + | + | + | + | NA |
| p27Kip1 | – | – | – | – | – | – |
| Genetic features | ||||||
| t(11;14)(q13;q32) | – | – | – | – | – | – |
| 11q13 (cyclin D1) | Normal* | Normal | Normal | Normal | Normal | Normal |
| 12p13 (cyclin D2) | Normal | Normal | Normal | Normal | Normal | Normal |
| 6p21 (cyclin D3) | Normal | Normal | Normal | Normal | Normal | Normal |
| 12p13 (p27kip1) | Normal | Normal | Normal | Normal | Normal | Normal |
. | Case no. 1 . | Case no. 2 . | Case no. 3 . | Case no. 4 . | Case no. 5 . | Case no. 6 . |
|---|---|---|---|---|---|---|
| Pathologic features | ||||||
| Growth pattern | Nodular | Diffuse | Nodular | Diffuse | Nodular | Nodular |
| Cytology | Typical | Typical | Typical | Typical | Typical | Typical |
| CD20 | + | + | + | + | + | + |
| CD3 | – | – | – | – | – | – |
| CD5 | + | + | + | + | + | + |
| CD23 | – | – | – | + (w) | – | – |
| CD43 | + (w) | + | – | + | + | + |
| Cyclin D1 | – | – | – | – | – | – |
| Cyclin D2 | + | + | – | – | – | NA |
| Cyclin D3 | – | – | + | + | + | NA |
| Cyclin E | – | – | – | – | – | NA |
| RB | + | + | + | + | + | NA |
| p27Kip1 | – | – | – | – | – | – |
| Genetic features | ||||||
| t(11;14)(q13;q32) | – | – | – | – | – | – |
| 11q13 (cyclin D1) | Normal* | Normal | Normal | Normal | Normal | Normal |
| 12p13 (cyclin D2) | Normal | Normal | Normal | Normal | Normal | Normal |
| 6p21 (cyclin D3) | Normal | Normal | Normal | Normal | Normal | Normal |
| 12p13 (p27kip1) | Normal | Normal | Normal | Normal | Normal | Normal |
+ indicates positive; –, negative; + (w), weakly positive; and NA, not available.
Normal indicates no split or amplification
Detection of cell cycle regulatory proteins
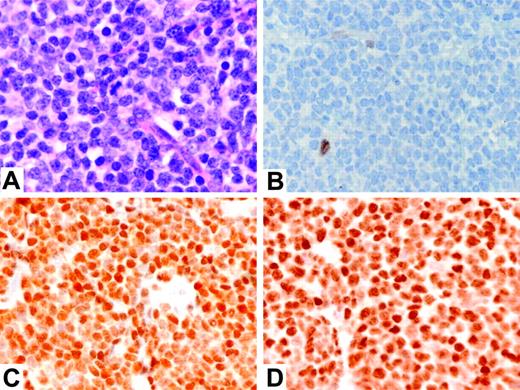
Immunostains for cyclin D1 and p27kip1 were performed on all 6 cases, and stains for cyclins D2, D3, E, and RB were performed on 5 cases with available formalin-fixed, paraffin-embedded tissue (Table 2). All 6 cases were negative for cyclin D1 by using the mouse monoclonal antibody DCS-6 (Figure 2B). Four of the 6 cases were also studied using the newly available rabbit monoclonal antibody SP4, and all 4 cases were negative for cyclin D1 (data not shown). Two cases (cases 1 and 2) demonstrated overexpression of cyclin D2 by immunostaining (Figure 2C), which correlated well with the increased cyclin D2 mRNA levels detected by microarray analysis (Figure 1, lower panel). Both of these cases were negative for cyclin D3. Three cases (cases 3-5) exhibited overexpression of cyclin D3 by immunostaining (Figure 2D), which correlated well with the increased cyclin D3 mRNA levels detected by microarray analysis (Figure 1, lower panel), and these cases were negative for cyclin D2. Case 6 also showed up-regulation of cyclin D3 mRNA by microarray analysis (Figure 1, lower panel), but the tissue block from this case was not available for immunostains.
Expression profiles of mantle cell lymphoma (MCL) signature genes in 6 cases of cyclin D1-negative MCL using Affymetrix U133 A/B arrays. These expression profiles are compared with 22 cases of cyclin D1-positive MCL, 78 cases of activated B-cell-like (ABC), 85 cases of germinal center B-cell-like (GCB), and 33 cases of primary mediastinal (PMBL) variants of diffuse large B-cell lymphoma, 193 cases of follicular lymphoma (FL), 14 cases of extranodal marginal zone lymphoma, MALT type (MALT), 6 cases of splenic marginal zone lymphoma (SMZL), and 14 cases of small lymphocytic lymphoma (SLL) (median expression levels of the MCL signature genes in these entities are shown). In the 6 cases of cyclin D1-negative MCL, each column represents a single lymphoma specimen and each row represents the level of expression of a single gene in the MCL signature. Red squares indicate increased expression and green squares indicate decreased expression relative to the median expression level, according to the color scale shown over a 4-fold range. In the bottom panel, the gene expression levels of the D-type cyclins in the various entities and the 6 cases of cyclin D1-negative MCL are shown according to the color scale over a 16-fold range. For microarray data of all cases, refer to Table S1.
Expression profiles of mantle cell lymphoma (MCL) signature genes in 6 cases of cyclin D1-negative MCL using Affymetrix U133 A/B arrays. These expression profiles are compared with 22 cases of cyclin D1-positive MCL, 78 cases of activated B-cell-like (ABC), 85 cases of germinal center B-cell-like (GCB), and 33 cases of primary mediastinal (PMBL) variants of diffuse large B-cell lymphoma, 193 cases of follicular lymphoma (FL), 14 cases of extranodal marginal zone lymphoma, MALT type (MALT), 6 cases of splenic marginal zone lymphoma (SMZL), and 14 cases of small lymphocytic lymphoma (SLL) (median expression levels of the MCL signature genes in these entities are shown). In the 6 cases of cyclin D1-negative MCL, each column represents a single lymphoma specimen and each row represents the level of expression of a single gene in the MCL signature. Red squares indicate increased expression and green squares indicate decreased expression relative to the median expression level, according to the color scale shown over a 4-fold range. In the bottom panel, the gene expression levels of the D-type cyclins in the various entities and the 6 cases of cyclin D1-negative MCL are shown according to the color scale over a 16-fold range. For microarray data of all cases, refer to Table S1.
All of the cases studied were negative for cyclin E, but showed positive immunostaining for RB (Table 2). The expression levels of RB protein were similar in all of the cases and were comparable with that seen in cases of cyclin D1-positive MCL (data not shown). Down-regulation of p27kip1 protein expression was observed in all 6 cases with the intensity of nuclear staining much weaker than that seen in reactive T lymphocytes (Table 2).
Genetic features
FISH analysis for the t(11;14)(q13;q32) was performed on all 6 cases and none displayed the IGH/CCND1 fusion. FISH studies with a locus-specific probe were also negative for variant translocations or amplifications involving the CCND1 locus at band 11q13 in all of the 6 cases (Table 2). Conventional cytogenetic analysis was also performed on case 6 and did not reveal a chromosomal alteration affecting band 11q13. FISH analysis using break-apart probes for the CCND2 (12p13), CCND3 (6p21), and CDKN1B/p27KIP1 (12p13) loci did not reveal any evidence of chromosomal translocation or amplification in these cases (Table 2). We also used a quantitative PCR assay to evaluate the genomic loss of one or both alleles of the INK4a/ARF locus, which encodes the tumor suppressor proteins p16INK4a and p14ARF. No INK4a/ARF locus deletions were detected in any of the 6 cases of cyclin D1-negative MCL.
Clinical features
The clinical features of the 6 patients are summarized in Table 3. The patients consisted of 5 males and 1 female, with a median age of 61 years (range, 54-77 years). All patients presented with stage IV disease. Lymphadenopathy was the most common presentation and extranodal sites were involved by lymphoma in all 6 patients. Five patients received combination chemotherapy initially, but none of these patients achieved a complete clinical response. One patient (case 1) was not treated initially and developed gastrointestinal involvement 26 months after the initial diagnosis. At the time of last follow-up, one of the patients had died and the other 5 were alive with disease.
Clinical features of 6 patients with cyclin D1-negative MCL
. | Case no. 1 . | Case no. 2 . | Case no. 3 . | Case no. 4 . | Case no. 5 . | Case no. 6 . |
|---|---|---|---|---|---|---|
| Age, y/sex | 54/F | 61/M | 61/M | 60/M | 54/M | 77/M |
| B symptoms | – | + | – | + | – | – |
| Serum LDH level | Normal | High | Normal | High | Normal | Normal |
| Extranodal sites | BM, PB | BM | BM | BM, spleen | BM | BM, lung, GI |
| IPI score | 2 | 3 | 2 | 3 | 2 | 3 |
| Initial therapy | None | R-CHOP | CHOP | COP | CHOP | COP |
| Response | NA | PR | PR | PR | PR | PR |
| Progression | + | – | + | + | + | + |
| Follow-up, mo | 38 | 5 | 88 | 19 | 70 | 30 |
| Status | AWD | AWD | DOD | AWD | AWD | AWD |
. | Case no. 1 . | Case no. 2 . | Case no. 3 . | Case no. 4 . | Case no. 5 . | Case no. 6 . |
|---|---|---|---|---|---|---|
| Age, y/sex | 54/F | 61/M | 61/M | 60/M | 54/M | 77/M |
| B symptoms | – | + | – | + | – | – |
| Serum LDH level | Normal | High | Normal | High | Normal | Normal |
| Extranodal sites | BM, PB | BM | BM | BM, spleen | BM | BM, lung, GI |
| IPI score | 2 | 3 | 2 | 3 | 2 | 3 |
| Initial therapy | None | R-CHOP | CHOP | COP | CHOP | COP |
| Response | NA | PR | PR | PR | PR | PR |
| Progression | + | – | + | + | + | + |
| Follow-up, mo | 38 | 5 | 88 | 19 | 70 | 30 |
| Status | AWD | AWD | DOD | AWD | AWD | AWD |
All patients were at Ann Arbor stage IV.
LDH indicates lactate dehydrogenase; IPI, International Prognostic Index; BM, bone marrow; PB, peripheral blood; GI, gastrointestinal tract; R, Rituxan; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; COP, cyclophosphamide, vincristine, and prednisone; NA, nonapplicable; PR, partial response; AWD, alive with disease; and DOD, dead of disease.
Cytologic features and expression of D-type cyclins in cyclin D1-negative MCL. (A) Typical MCL cytology (case 1) (hematoxylin and eosin stain; original magnification, × 500); (B) cyclin D1 protein, showing only a rare nontumor cell with nuclear staining (case 1); (C) cyclin D2 protein, showing strong nuclear staining of the tumor cells (case 2); (D) cyclin D3 protein, showing strong nuclear staining of the tumor cells (case 4). Panels B-D are immunoperoxidase stains; original magnification, × 400. Figure S1 provides high-power photos of cytomorphology of other 5 cases.
Cytologic features and expression of D-type cyclins in cyclin D1-negative MCL. (A) Typical MCL cytology (case 1) (hematoxylin and eosin stain; original magnification, × 500); (B) cyclin D1 protein, showing only a rare nontumor cell with nuclear staining (case 1); (C) cyclin D2 protein, showing strong nuclear staining of the tumor cells (case 2); (D) cyclin D3 protein, showing strong nuclear staining of the tumor cells (case 4). Panels B-D are immunoperoxidase stains; original magnification, × 400. Figure S1 provides high-power photos of cytomorphology of other 5 cases.
Ninety-three cases (92 cases in the original study plus the false-negative case) of cyclin D1-positive MCL12 were used for comparison. The median follow-up for these patients was 26 months (range, 7-166 months). The clinical features including age and sex distribution, stage, presence of B symptoms, serum lactate dehydrogenase (LDH) levels, extranodal sites, International Prognostic Index (IPI) scores, types of treatment, and clinical responses were similar between the cyclin D1-positive and cyclin D1-negative groups (data not shown). At the time of last follow-up, 65 of 93 patients with cyclin D1-positive MCL had died, with a median overall survival of 31 months. No significant difference in overall survival between the cyclin D1-positive and cyclin D1-negative groups was identified.
Discussion
In the current study, we have confirmed and extended our previous findings in which we identified a new variant of MCL, which we designated cyclin D1-negative MCL.12 All 6 cases in this study were negative for cyclin D1 mRNA expression by both quantitative RT-PCR and gene expression analysis. These cases also lacked the characteristic IGH/CCND1 fusion by FISH analysis and were negative for cyclin D1 protein expression by immunostains. Nevertheless, all of the cases exhibited the characteristic pathologic features of MCL and, more importantly, shared the characteristic MCL gene expression profile by microarray analysis. Therefore, these cases are regarded as bona fide cases of cyclin D1-negative MCL. The existence of such cases sheds new light on the pathobiology of MCL and challenges the idea that cyclin D1 overexpression is essential in the pathogenesis of MCL. We have also demonstrated that patients with cyclin D1-negative MCL have clinical and pathologic features similar to those with cyclin D1-positive MCL. In particular, tumors in both groups had the similar growth patterns and common cytologic and immunohistochemical features. Similar age and sex distribution, stage, serum LDH levels, extranodal sites, IPI scores, response to initial treatment, and overall survival were seen in the cyclin D1-positive and -negative groups.
Yatabe et al24 previously reported 151 cases of lymphoma with the morphologic features of MCL. Among these, they identified 23 cases (15%) that were negative for cyclin D1 protein expression by immunohistochemical staining performed on formalin-fixed, paraffin-embedded tissue. Conventional cytogenetics was performed on only 3 of these 23 cases, and all 3 were negative for the t(11;14)(q13;q32). However, FISH or quantitative RT-PCR analysis was not performed on any of these cases. Compared with their group of cyclin D1-positive MCL (n = 128), the cyclin D1-negative cases had a significantly better overall survival. In another report, Hashimoto et al25 identified 3 cases of apparent cyclin D1-negative MCL by immunostains and compared them with 14 cases of typical cyclin D1-positive MCL. They also suggested that cyclin D1-negative MCL is a more indolent form of MCL. However, both of these studies failed to provide convincing evidence that their cases of cyclin D1-negative MCL were actually true cases of MCL. In fact, Yatabe et al24 noted in their paper that some of their cyclin D1-negative cases might have been marginal zone B-cell lymphomas or atypical small lymphocytic lymphomas. In contrast, we have clearly demonstrated the characteristic MCL gene expression signature in all of our cases of cyclin D1-negative MCL, and we did not find any significant differences in the clinical features between our 2 groups of MCL. However, since there are only 6 patients in our cyclin D1-negative group, with some having relatively short clinical follow-up, additional studies are warranted to confirm our findings.
The pathogenic mechanisms involved in the development of the cyclin D1-negative MCL are currently unknown. Since the oncogenic effect of overexpressed cyclin D1 is considered to be cell cycle dysregulation, we examined other proteins involved in cell cycle control, especially during the G1 to S phase transition. The D-type cyclins, D1, D2, and D3, are all positive promoters of cell cycle progression from the G1 to S phase. The D-type cyclins are similar in structure and biochemical function,26 but are expressed in a lineage-specific manner.27 There is considerable redundancy in the growth-promoting function of the D-type cyclins, since only limited phenotypic consequences due to the absence of either cyclin D1, D2, or D3 are seen in gene knock-out mice.28-31 In nonneoplastic lymph nodes and tonsils, cyclin D2 is found mainly in interfollicular T cells, whereas cyclin D3 is found in centroblasts in lymphoid follicles and in scattered B cells and T cells of the interfollicular areas.32 However, cyclin D1 is not expressed in nonneoplastic T cells or B cells.6,33 In low-grade B-cell malignancies, overexpression of cyclin D2 mRNA was observed by Northern blot analysis in 29 of 34 cases of chronic lymphocytic leukemia and in all 7 cases of lymphoplasmacytic lymphoma, but not in 2 cases of MCL.34 Cyclin D3 appears to be expressed more ubiquitously in B-cell malignancies, including follicular lymphoma, marginal zone lymphoma, and diffuse large B-cell lymphoma,30 but is usually not expressed in lymphoid malignancies with either cyclin D1 or D2 overexpression.35-37 In the current study, overexpression of either cyclin D2 or D3 was observed in all 6 cases of cyclin D1-negative MCL, indicating an important substitute role for these cyclins in the pathogenesis of cyclin D1-negative MCL. However, the mechanism of cyclin D2 or D3 up-regulation in our cases remains unclear. We did not find any chromosomal translocations or gene amplifications involving the cyclin D2 or D3 gene loci by FISH analysis in these cases. Our findings are consistent with several previous studies that suggested that deregulation of cyclin D2 or D3 expression is often due to epigenetic mechanisms.38-40
Dysregulation of other genes or factors important in cell cycle control could also play a role in the pathogenesis of such cases. These may include dysregulation of p27kip1, up-regulation of cyclin E, inactivation of the RB gene, and deletion of the p16INK4a/p14ARF locus, as well as involvement of other genes. The p27kip1 protein regulates cellular progression from G1 into S phase by inhibiting the cyclin E/CDK2 complex.41 Regulation of p27kip1 occurs primarily through posttranscriptional mechanisms, including sequestration by cyclin D1 or cyclin D3,42,43 or proteasomal degradation.44 In a prior study, the expression of p27kip1, as assessed by immunostains, was noted in only 5 of 40 cases of typical MCL, but was found in 8 of 10 cases of blastic MCL.45 In the current study, down-regulation of p27kip1 protein expression was seen in all 6 cases, similar to that seen in typical cyclin D1-positive MCL.
The E-type cyclins, including cyclin E1 and E2, are also important in the G1 phase of the cell cycle. When combined with CDK2, cyclin E promotes the hyperphosphorylation of RB protein and, thereby, facilitates the entry of cells into S phase.46 None of our cases, however, was positive for cyclin E expression, arguing against a role for cyclin E in the pathogenesis of cyclin D1-negative MCL. Inactivation of the RB tumor suppressor gene has been implicated in the development of various types of human malignancy. However, RB protein expression was identified in all of our cases and the expression levels were similar to those seen in cyclin D1-positive MCL. Our findings are consistent with a previous study in which the authors concluded that RB protein appears to be normally regulated in MCL.47 We also investigated whether deletions of the tumor suppressor genes p16INK4a and p14ARF play a role in cyclin D1-negative MCL. p16INK4a regulates the G1/S phase transition by forming binary complexes with CDK4 and CDK6, thereby preventing these subunits from association with D-type cyclins.48 Deletion of p16INK4a or cyclin D1 overexpression may therefore promote the G1/S phase transition by the same mechanism. An important function of p14ARF is to augment p53 function by antagonizing murine double minute 2 (MDM2), and loss of p14ARF function may contribute to the enhanced proliferation in tumor cells.48 As we have shown previously, INK4a/ARF locus deletions occur in up to 21% (18/85) of cases of MCL and are preferentially observed among the more proliferative cases.12 However, deletion of the INK4a/ARF locus was not identified in any of the 6 cases, arguing against a role for p16INK4a/p14ARF in the pathogenesis of cyclin D1-negative MCL.
Careful morphologic examination, with knowledge of the full spectrum of pathology in MCL, is critical in order to suspect a diagnosis of cyclin D1-negative MCL. This diagnosis may be challenging, particularly since some cases are weakly positive for CD23 or fail to express CD43. However, all 6 cases in our study were positive for CD5, which should suggest the diagnosis of MCL. In such cases, positive immunostains for cyclin D2 or D3 are supportive of this diagnosis. Nevertheless, careful examination to rule out other types of low-grade B-cell lymphoma, which may also be positive for cyclin D2 or D3, is essential. In the near future, gene expression profiling, or a panel of immunostains based on the MCL signature, will provide the means to confirm this diagnosis. Gene expression studies may also shed further light on the pathogenesis and biology of cyclin D1-negative MCL.
Appendix
The Lymphoma/Leukemia Molecular Profiling Project is an international consortium of the following institutions: the University of Nebraska Medical Center, the National Cancer Institute, the University of Arizona Health Sciences Center, the University of Oregon Health Sciences Center, the University of Rochester Medical Center, Cleveland Clinic, the British Columbia Cancer Agency, University of Würzburg in Germany, University of Barcelona in Spain, Norwegian Radium Hospital in Oslo, and St. Bartholomew's Hospital in London.
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-04-1753.
Supported in part by the United States Public Health Service grants CA36727 and CA84967 awarded by the National Cancer Institute, the Department of Health and Human Services (W.C.C.), the Lymphoma Research Foundation (mantle cell lymphoma grantee: T.C.G.), and Deutsche Krebshilfe (A.R., R.S., G.O., H.K.M.-H.).
The Lymphoma/Leukemia Molecular Profiling Project's full membership is listed in Appendix.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Gregory Cochran for performing the immunostains, Diane L. Pickering and Adrian Wiestner for technical assistance, James C. Lynch for advice regarding statistical analysis, and other members of the Lymphoma/Leukemia Molecular Profiling Project.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal