The homing receptors L-selectin and α4β7 integrin facilitate entry of T cells into the gut-associated organized lymphoid tissues such as the mesenteric lymph nodes and Peyer patches. We studied the impact of inactivation of genes encoding these receptors on the ability of purified donor CD4+ T cells to induce acute lethal graft-versus-host disease (GVHD) associated with severe colitis in irradiated major histocompatibility complex (MHC)–mismatched mice. Whereas lack of expression of a single receptor had no significant impact on the severity of colitis and GVHD, the lack of expression of both receptors markedly ameliorated colitis and early deaths observed with wild-type (WT) T cells. The changes in colitis and GVHD were reflected in a marked reduction in the early accumulation of donor T cells in the mesenteric lymph nodes and subsequently in the colon. The purified WT donor CD4+ T cells did not accumulate early in the Peyer patches and failed to induce acute injury to the small intestine. In conclusion, the combination of CD62L and β7 integrin is required to induce acute colitis and facilitate entry of CD4+ donor T cells in the mesenteric nodes associated with lethal GVHD in allogeneic hosts.
Introduction
Homing of T cells to peripheral lymph nodes requires the interaction between the T-cell surface receptor L-selectin (CD62L) and the peripheral node addressin (PNAd) ligand expressed on high endothelial venules (HEVs).1 T cells derived from CD62L-/- gene–deficient mice fail to accumulate in the cervical, axillary, and inguinal peripheral nodes of syngeneic recipients after intravenous transfer.2 There is a partial but not complete failure of the CD62L-/- T cells to migrate to mucosal-associated organized lymphoid tissues such as the mesenteric lymph nodes and Peyer Patches because homing to these tissues is facilitated by both CD62L and another T-cell surface receptor, α4β7 integrin, that interacts with an additional HEV homing ligand, the mucosal addressin cell adhesion molecule (MAdCAM).2-4 Since mucosal lymphoid tissue HEVs express both PNAd and MAdCAM, T cells derived from CD62L-/-/β7-/- double gene–deficient donors show a more complete failure to accumulate in the mucosal organized lymphoid tissues of syngeneic hosts compared with T cells derived from either CD62L-/- or β7-/- donors.2,3
Previous reports have suggested that acute graft-versus-host disease (GVHD) requires the migration of allogeneic donor T cells to mucosal organized lymphoid tissues via the interactions between the T-cell surface selectins and integrins (CD62L and α4β7 integrin) and their ligands by using monoclonal antibodies to block these interactions or by using monoclonal antibodies to deplete donor T cells expressing the selectin and integrin homing receptors.5-7 However, the ability of combined anti-CD62L and anti-α4 integrin monoclonal antibody injections to improve survival from acute GVHD was modest (about 10 days) compared with untreated controls when fully major histocompatibility complex (MHC)–mismatched hosts were conditioned with total body irradiation before bone marrow transplantation.6 Similarly, depletion of donor T cells that express β7 integrin by cell sorting improved survival from acute GVHD in MHC-mismatched irradiated hosts by only 20 to 25 days.7
It is of interest that considerably greater improvement in survival of experimental groups was observed when hosts were either nonirradiated immunodeficient severe combined immunodeficiency (SCID) mice or when hosts differed only at minor histocompatibility gene loci with the donor.5,7 Studies of donor T-cell homing in the induction of acute GVHD have shown that the requirements for T-cell homing receptors can be markedly different in irradiated versus nonirradiated hosts.8 Homing of donor T cells to the host Peyer Patches via the chemokine receptor CCR5 was required for the induction of lethal GVHD in nonirradiated hosts but was not required in irradiated hosts.8,9
In the current study, we used irradiated hosts to study the requirements for purified CD4+ donor T cells to express the homing receptors L-selectin and β7 integrin in the induction of acute lethal GVHD. This model more closely reflects the use of total body irradiation conditioning in human bone marrow transplantation. We used donors that had either L-selectin and/or β7 integrin genes inactivated instead of using monoclonal antibodies to block these receptors or to select donor cells based on the expression of the receptors. The selective gene-deficiency approach avoided difficulties in interpretation of results with monoclonal antibodies, including catabolism of the monoclonal antibodies in vivo, binding of the antibodies to both host and donor homing receptors, interference with the immune function of donor T cells by antibody cross-linking of receptors, and the possibility that sorted donor T-cell subsets expressing different patterns of homing receptors may have different immune functions that are separate from the homing functions. For example, sorted T-cell subsets based on the expression of L-selectin distinguish effector memory cells from naive and central memory T cells.10
The current experimental results show that although CD62L-/- donor CD4+ T cells completely failed to home to the peripheral lymph nodes of the irradiated MHC-mismatched hosts, there was no significant impact on the rapidity and severity of acute GVHD. Similarly, the lack of β7 integrin on CD4+ donor T cells did not have a significant impact on the rapidity of lethal GVHD. However, the lack of both CD62L and β7 on T cells from CD62L-/-/β7-/- double gene–deficient donors markedly ameliorated the acute colitis from GVHD and markedly improved survival. The prevention of the acute colitic phase of GVHD was associated with a marked reduction in early donor T-cell homing first to the mesenteric lymph nodes and subsequently to the colon.
Materials and methods
Animals
Wild-type C57BL/6 (H-2Kb) male mice 6 to 8 weeks old and male BALB/c (H-2Kd) mice 8 to 10 weeks old were purchased from the breeding facility of the Department of Comparative Medicine, Stanford University. β7-/- C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). CD62L-/- C57BL/6 mice were provided by Dr Richard Flavell (Xu et al11 ). CD62L-/-/β7-/- C57BL/6 mice were provided by Dr Thomas Tedder (Wagner et al2 ). Both CD62L-/- mice and CD62L-/-/β7-/- mice were obtained through material transfer agreements with Yale University and Duke University, respectively. All mice were housed in a specific pathogen-free facility. Care of all experimental animals was in accordance with institutional guidelines.
Antibodies and flow cytometric analysis (FACS)
The following reagents were used for flow cytometric analysis: unconjugated anti-CD16/32 (2.4G2), anti-CD4 fluorescein isothiocyanate (FITC; RM4-5), anti–T-cell receptor β (anti-TCRβ) allophycocyanin (APC; H57-597), anti-CD62L biotin (Mel-14), anti-β7 phycoerythrin (PE; M293), and anti–H-2Kb FITC (AF6-88.5) monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA), and streptavidin–Texas Red was from Molecular Probes (Eugene, OR). All stainings were performed in phosphate-buffered saline (PBS)/1% calf serum in the presence of purified anti-CD16/32 at saturation to block unspecific staining via FcRII/III. Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude dead cells. All analyses were done on a modified dual laser FACS Vantage (Becton Dickinson, Mountain View, CA) in the Shared FACS Facility (Center for Molecular and Genetic Medicine at Stanford University), using FlowJo software (TreeStar, Ashland, OR) for data analysis.
Cell preparations
Single-cell suspensions were prepared from spleens, washed twice, and filtered through a fine nitex membrane. The samples were then enriched for CD4+ cells with anti-CD4 magnetic microbeads using the MidiMACS system (Miltenyi Biotech, Auburn, CA). Enriched cells were stained with anti-TCRαβ APC and anti-CD4 FITC mAbs to check for purity, and preparations were uniformly at least 95% pure. For preparation of T-cell–depleted bone marrow (TCD BM), bone marrow cells were obtained from the femur and tibia, and single-cell suspensions were prepared, filtered through nitex membrane, stained with anti-Thy1.2 biotin (5a-8; Caltag, Burlingame, CA) and streptavidin-magnetic beads (Miltenyi Biotech), and passed over 2 consecutive magnetic-activated cell separation (MACS) LS-separation columns (Miltenyi Biotech). TCD BM contained less than 0.01% T cells, as determined by staining with anti-TCRβ APC. Thy1.2-depleted splenocytes were used as allogeneic stimulator cells.
GVHD model
Acute GVHD was induced as described previously.12 In brief, BALB/c hosts were lethally irradiated (800 cGy) from a 200-Kv X-ray source and injected with donor cells via tail vein within 24 hours. All host mice received 2 × 106 TCD BM cells for reconstitution with or without CD4+ T cells as indicated in the text and figures. Host mice were kept on antibiotic water (25 μg/mL neomycin/0.3 U/mL polymyxin B; Sigma Aldrich, St Louis, MO) for the first 28 days. Survival and the signs of GVHD (hair loss, hunched back, swollen faces, and diarrhea) were monitored daily and body weight was measured weekly.
Histopathology
Histopathologic specimens from the small and large intestines of hosts were obtained at 7 days after transplantation and fixed in formalin before embedding into paraffin blocks. Tissue sections of 4- to 5-μm thickness were stained with hematoxylin and eosin using standard protocols. Microscopic images were obtained using an Eclipse E1000M microscope (Nikon, Melville, NY) with SPOT RT digital camera and acquisition software (Diagnostic Instruments, Sterling Heights, MI) with a final magnification of × 300 (objective, × 20/0.45 numerical aperture) for all images. Image processing was performed with Photoshop CS (Adobe Systems, San Jose, CA) with standard adjustments of brightness, contrast, and color balance to the entire image.
Cell distribution studies
Cell preparation and acute GVHD induction were performed as described elsewhere under “Materials and methods.” For day-6 analysis lymphocytes from tissues of individual mice and for day-2 biodistribution studies lymphocytes from 3 mice from the same experimental group were pooled before flow cytometric analysis. Single-cell suspensions from mesenteric lymph nodes, peripheral lymph nodes (bilateral axillary and inguinal nodes were pooled), and spleen were filtered through fine nitex membrane to remove aggregates. Mononuclear cells from the liver were isolated according to the method by Lan et al.13 Lamina propria (LP) lymphocytes were purified as described.14
Mixed lymphocyte reactions and cytokine assay
Cultures were set up in triplicates in 96-well round bottom plates (BD Biosciences, Franklin Lakes, NJ) as described previously.15 CD4+ T-responder cells were cultured with irradiated (3000 cGy) allogeneic stimulator cells (1 × 105 cells each). Proliferation was assessed after 3, 4, and 5 days by pulsing the cells with 1 μCi/well (0.037 MBq/well) [3H]-thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) for the last 16 hours of the indicated days. Cells were harvested onto filter membranes using a Wallac harvester (PerkinElmer Life Sciences, Gaithersburg, MD), and the amount of incorporated [3H]-thymidine was measured with a Wallac Betaplate counter (PerkinElmer Life Sciences).
Cytokine production after 60 hours of allogeneic stimulation was assessed in supernatants of cultures of CD4+ T-responder cells (2 × 105 cells) and irradiated (3000 cGy) allogeneic stimulator cells (5 × 105 cells). Five different cytokines were analyzed in a multiplex assay system using fluorescently labeled microsphere beads (Beadlyte Mouse Multi Cytokine Detection system; Upstate Biotechnology, Lake Placid, NY), and cytokine levels were quantitated using the Luminex 100 system (Luminex, Austin, TX).
Statistical analysis
Kaplan-Meier survival curves were made using Prism (GraphPad Software, San Diego, CA). Statistical differences in animal survival were analyzed by log-rank test. Differences in donor type T-cell recovery in tissues of hosts and chemokine receptor expression, proliferation, and cytokine production were analyzed using the 2 tailed Student t test. For all tests, P of .05 or less was considered significant.
Results
Lack of both CD62L and β7 on donor T cells markedly improves acute GVHD and associated colitis
In initial experiments spleen cells were stained with mAbs directed against CD62L and β7, and flow cytometric analyses confirmed the absence of the appropriate receptors on gated CD4+ T cells from CD62L-/-, β7-/-, and CD62L-/-/β7-/- mice (data not shown). In subsequent experiments, graded doses (0.0625 × 106, 0.125 × 106, and 0.25 × 106) of purified CD4+ T cells from the spleen of wild-type (WT) donors were added to a constant number (2 × 106) of T-cell–depleted (TCD) bone marrow cells from WT donors and injected into lethally irradiated (800 cGy) BALB/c (H-2d) recipients. Figure 1A shows that control irradiated recipients that received only TCD bone marrow cells intravenously all survived for 100 days. Although the mean body weight of the latter group decreased transiently during the first week after irradiation, there was a recovery to baseline during the third week and stabilization thereafter. These mice did not show typical clinical features of GVHD including diarrhea, hunched back, ruffled fur, hair loss, and facial swelling. In contrast, all hosts given TCD bone marrow cells and 0.25 × 106 WT donor CD4+ cells developed severe diarrhea and progressive weight loss, which resulted in uniform death within 2 weeks (Figure 1A,C). Reduction of the WT donor CD4+ T-cell dose to 0.125 × 106 cells resulted in severe diarrhea and early death of about 60% of recipients, with the remainder dying by day 52 (Figure 1A). A further reduction of the dose of WT donor CD4+ T cells to 0.0625 × 106 cells resulted in less severe diarrhea with a marked delay in deaths and survival of about 30% of recipients for 100 days. Almost all of these mice had mild to moderate clinical signs of GVHD with associated weight loss (Figure 1A,C).
Induction of GVHD by purified CD4+ T cells from WT and gene-deficient C57BL/6 donors. Lethally irradiated BALB/c host mice were given intravenous injections of 2 × 106 TCD bone marrow cells from WT C57BL/6 donors with or without CD4+ T cells from WT, CD62L-/-, β7-/-, or CD62L-/-/β7-/- donors. There were 10 hosts in each group. (A) Survival of irradiated hosts given TCD bone marrow cells alone or with 0.25 × 106, 0.125 × 106, or 0.0625 × 106 WT CD4+ T cells. (B) Survival of irradiated hosts given TCD bone marrow cells and 0.125 × 106 CD4+ T cells from WT or gene-deficient donors. (C) Mean body weights of host mice given TCD bone marrow and CD4+ T cells as in panel A. Brackets show standard errors of the mean. Analysis was stopped for a given group when there were 2 hosts remaining (+). (D) Mean body weights of host mice given cells as in panel B.
Induction of GVHD by purified CD4+ T cells from WT and gene-deficient C57BL/6 donors. Lethally irradiated BALB/c host mice were given intravenous injections of 2 × 106 TCD bone marrow cells from WT C57BL/6 donors with or without CD4+ T cells from WT, CD62L-/-, β7-/-, or CD62L-/-/β7-/- donors. There were 10 hosts in each group. (A) Survival of irradiated hosts given TCD bone marrow cells alone or with 0.25 × 106, 0.125 × 106, or 0.0625 × 106 WT CD4+ T cells. (B) Survival of irradiated hosts given TCD bone marrow cells and 0.125 × 106 CD4+ T cells from WT or gene-deficient donors. (C) Mean body weights of host mice given TCD bone marrow and CD4+ T cells as in panel A. Brackets show standard errors of the mean. Analysis was stopped for a given group when there were 2 hosts remaining (+). (D) Mean body weights of host mice given cells as in panel B.
We compared 0.125 × 106 CD4+ T cells from WT, CD62L-/-, β7-/-, or CD62L-/-/β7-/- donors added to a constant number of TCD bone marrow cells for the ability to induce lethal GVHD in irradiated BALB/c hosts. Approximately 30% of hosts given the CD62L-/- donor T cells and 70% of the hosts given the β7-/- donor T cells developed severe diarrhea and died within 2 weeks of the cell infusion (Figure 1B). All of the hosts given the CD62L-/- donor T cells died by 55 days, at about the same time as all hosts given WT T cells. There was no statistically significant difference in the survival of the WT versus CD62L-/- T-cell recipients (P > .07) as judged by the log-rank test. Although 20% of recipients given β7-/- T cells survived at least 100 days, the overall survival of the groups given WT versus β7-/- T cells was also not statistically significantly different (P > .5).
Irradiated hosts given 0.125 × 106 CD4+ T cells from CD62L-/-/β7-/- donors all survived at least 40 days and none developed severe diarrhea during the first month after cell infusion. About 50% of these hosts developed progressive weight loss, low-grade diarrhea, and skin changes from GVHD during the second to third months. The latter mice died by 100 days and the remaining 50% survived with stable body weights (Figure 1B,D). The survival of the mice given the CD62L-/-/β7-/- T cells was significantly improved compared with that of the WT T-cell recipients (P < .01). However, the mean body weights of the survivors were significantly reduced compared with the TCD bone marrow controls at all time points tested after 30 days (P < .01).
The development of severe diarrhea and death in the first few weeks in the groups given WT, CD62L-/-, and β7-/- donor T cells was reflected in the development of histopathologic evidence of moderate to severe colitis at day 7. Figure 2B shows that the colonic crypts of hosts given 0.125 × 106 WT T cells were markedly abnormal compared with hosts given only TCD bone marrow cells (Figure 2A). The WT T-cell hosts (3 of 3 examined) had crypt loss, loss of mucin-containing goblet cells within the crypts, increased apoptosis of epithelial cells at the crypt bases, and a severe infiltrate of inflammatory cells between the crypt walls and in the lamina propria. Similar moderate to severe crypt abnormalities were observed in all of 3 hosts given CD62L-/- or β7-/- donor T cells (Figure 2C-D). In contrast, only mild crypt changes were observed in 3 of 3 hosts given CD62L-/-/β7-/- T cells. Mucin-containing cells were retained and there was little crypt dropout, minimal infiltration of inflammatory cells between crypts, and minimal increase in crypt apoptosis (Figure 2E). It is of interest that the small intestines of 3 of 3 hosts given WT T cells showed only mild changes with no blunting or inflammation of the villi and no dropout of villi (Figure 2F). However, there was a mild infiltrate in the areas below the bases of the villi. Histopathologic analysis of the liver of hosts given WT T cells also showed a mild infiltrate in the portal triads without liver tissue injury (data not shown). Thus, early intestinal GVHD in this CD4+ T-cell transfer model is predominantly targeted to the colon.
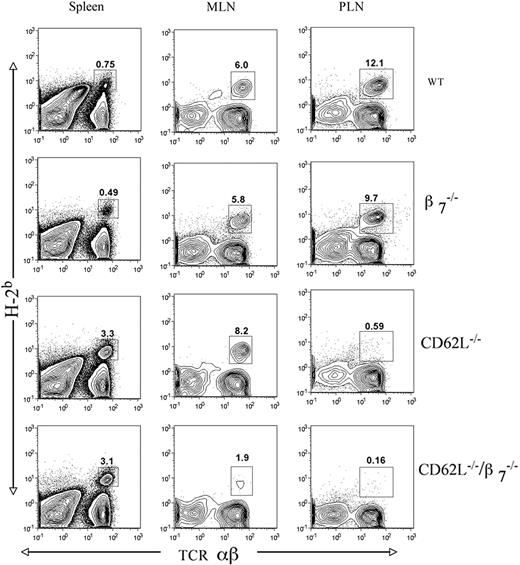
Impact of CD62L and β7 receptor deficiency on donor CD4+ T-cell accumulation in the tissues of allogeneic irradiated hosts
Whereas the impact of CD62L and β7 receptor loss alone or in combination on T-cell accumulation in the lymphoid tissues has been studied previously in nonirradiated syngeneic recipients,2,3 we studied the impact on purified donor CD4+ T-cell accumulation in irradiated allogeneic recipients given TCD WT bone marrow cells. The percentages and absolute numbers of the donor T cells in the host tissues at 2 days or 6 days after intravenous injection were analyzed by flow cytometric analysis of single-cell suspensions. Figure 3 shows representative staining patterns of H-2b versus TCRαβ in the host spleen, mesenteric lymph nodes, and peripheral lymph nodes at day 2 after injection of 2.5 × 106 donor T cells. In order to harvest sufficient donor T cells from the host lymphoid tissues for analysis on day 2, the number of injected donor T cells had to be increased from the maximum dose used in Figure 1 (0.25 × 106) to 2.5 × 106 cells. H-2b+ TCRαβ+ donor-type T cells are enclosed in boxes, and percentages in each tissue are shown. The CD62L-/- and CD62L-/-/β7-/- donor-type T cells had markedly reduced percentages in the host peripheral nodes (<1%) compared with that of the WT (∼12%) and β7-/- (∼10%) donor T cells. In contrast, the percentage of CD62L-/- and CD62L-/-/β7-/- donor T cells was increased in the spleen (∼3%) compared with WT and β7-/- T cells (<1%). The percentages of WT, β7-/-, and CD62L-/- donor T cells in the mesenteric lymph nodes were similar, and in the range of 6% to 8%, and the percentage of donor T cells was reduced about 3-fold (2%) when CD62L-/-/β7-/- cells were used (Figure 3). The pattern of relative accumulation of WT versus gene-deficient donor T cells in the host lymphoid tissues on day 6 after injection was similar to that observed on day 2 (data not shown).
Histopathologic changes in the colon and small intestine of lethally irradiated host 7 days after the injection of donor cells. All hosts received 0.125 × 106 CD4 + T cells and/or 2 × 106 TCD bone marrow cells. (A) Colon of a control host given only TCD bone marrow cells. Arrows show mucin-containing goblet cells. (B) Colon of a host given WT CD4+ T cells and 2 × 106 TCD bone marrow cells. An asterisk indicates infiltrate in area of crypt drop-out; and arrow, crypt abscess. (C) Colon of a host given CD62L-/- CD4+ T cells and TCD bone marrow. An asterisk indicates inflammatory infiltrate and crypt drop-out. (D) Colon of a host given β7-/- CD4+ T cells. Inflammatory infiltrate and crypt drop-out are present. (E) Colon of a host given CD62L-/-/β7-/- CD4+ T cells. Goblet cells are retained in crypt walls (arrow), and crypt drop-outs with infiltrate are minimal. (F) Small intestine of a host given WT CD4+ T cells. Villi are intact and infiltrate is minimal. Tissue sections were stained with hematoxylin and eosin; original magnification × 300. Each panel is representative of each of 3 hosts examined.
Histopathologic changes in the colon and small intestine of lethally irradiated host 7 days after the injection of donor cells. All hosts received 0.125 × 106 CD4 + T cells and/or 2 × 106 TCD bone marrow cells. (A) Colon of a control host given only TCD bone marrow cells. Arrows show mucin-containing goblet cells. (B) Colon of a host given WT CD4+ T cells and 2 × 106 TCD bone marrow cells. An asterisk indicates infiltrate in area of crypt drop-out; and arrow, crypt abscess. (C) Colon of a host given CD62L-/- CD4+ T cells and TCD bone marrow. An asterisk indicates inflammatory infiltrate and crypt drop-out. (D) Colon of a host given β7-/- CD4+ T cells. Inflammatory infiltrate and crypt drop-out are present. (E) Colon of a host given CD62L-/-/β7-/- CD4+ T cells. Goblet cells are retained in crypt walls (arrow), and crypt drop-outs with infiltrate are minimal. (F) Small intestine of a host given WT CD4+ T cells. Villi are intact and infiltrate is minimal. Tissue sections were stained with hematoxylin and eosin; original magnification × 300. Each panel is representative of each of 3 hosts examined.
Figure 4 shows a comparison of the absolute numbers of the different donor T cells in the spleen, mesenteric lymph nodes, liver, and colon that accumulated on day 2 after injection of 2.5 × 106 donor T cells and on day 6 after injection of 0.5 × 106 donor T cells along with 2 × 106 TCD bone marrow cells. Results are shown on a logarithmic scale, and the limit of detection was 0.001 × 106 donor cells (<0.1% of donor cells detected by flow cytometry). At day 2, the mean number of donor T cells in the spleen was not significantly different for WT, CD62L-/-, β7-/-, and CD62L-/-/β7-/- donors (P > .5), and mean values were in the range of 0.02 × 106 to 0.05 × 106 cells. This represented a marked increase in accumulation (10- to 50-fold) of the allogeneic T cells in the spleen compared with the injection of an equal number of syngeneic CD4+ T cells and TCD bone marrow cells, since the syngeneic T cells (BALB/c Thy1.1 donors and BALB/c Thy1.2 hosts) were less than 0.001 × 106 cells in all tissues analyzed at day 2 (data not shown). Although, allogeneic donor T cells were easily detected in the spleen, they were below the limits of detection in the liver and colon on day 2 (Figure 4). The mean number of allogeneic T cells was in the range of 0.1 × 106 to 0.2 × 106 cells in the peripheral lymph nodes on day 2 for WT and β7-/- donor cells but fell below the limit of detection for CD62L-/- and CD62L-/-/β7-/- donor cells. The latter cells were also not detected in the peripheral nodes on day 6. These results demonstrated the requirement for the CD62L receptor for homing of allogeneic T cells to the peripheral nodes as was the case for the homing of syngeneic T cells.2,3
Percentage of donor T cells in the spleen, mesenteric lymph nodes (MLNs), and peripheral lymph nodes (PLNs) of hosts given 2 × 106 TCD bone marrow and 2.5 × 106 CD4+ T cells from WT and gene-deficient donors. Staining was performed on tissues harvested 2 days after donor cell injection, and panels show H-2b versus TCRαβ. Boxes enclose donor T cells (H-2Kb+ TCRαβ+) and percentages enclosed are shown. Representative patterns are from 1 of 3 experiments.
Percentage of donor T cells in the spleen, mesenteric lymph nodes (MLNs), and peripheral lymph nodes (PLNs) of hosts given 2 × 106 TCD bone marrow and 2.5 × 106 CD4+ T cells from WT and gene-deficient donors. Staining was performed on tissues harvested 2 days after donor cell injection, and panels show H-2b versus TCRαβ. Boxes enclose donor T cells (H-2Kb+ TCRαβ+) and percentages enclosed are shown. Representative patterns are from 1 of 3 experiments.
Absolute number of donor T cells in the spleen, mesenteric lymph nodes, peripheral lymph nodes, liver, and colon of irradiated hosts day 2 or day 6 after the injection of 2.5 × 106 (day 2) CD4+ T cells or 0.5 × 106 (day 6) CD4+ T cells. Bars show the means of the absolute number of donor T cells, and brackets show standard errors of groups of mice given T cells from WT or gene-deficient donors. There were 3 separate experiments with 6 to 9 mice in each experiment. # indicates that the absolute number of donor T cells was less than 0.001 × 106; *, statistically significant difference between WT and gene-deficient group (P ≤ .05). All hosts received 2 × 106 WT TCD bone marrow cells.
Absolute number of donor T cells in the spleen, mesenteric lymph nodes, peripheral lymph nodes, liver, and colon of irradiated hosts day 2 or day 6 after the injection of 2.5 × 106 (day 2) CD4+ T cells or 0.5 × 106 (day 6) CD4+ T cells. Bars show the means of the absolute number of donor T cells, and brackets show standard errors of groups of mice given T cells from WT or gene-deficient donors. There were 3 separate experiments with 6 to 9 mice in each experiment. # indicates that the absolute number of donor T cells was less than 0.001 × 106; *, statistically significant difference between WT and gene-deficient group (P ≤ .05). All hosts received 2 × 106 WT TCD bone marrow cells.
The mean absolute numbers of allogeneic WT, CD62L-/-, and β7-/- cells in the mesenteric lymph nodes on day 2 were not significantly different (P > .1) and were in the range of 0.1 × 106 to 0.3 × 106 cells. On the other hand, the mean absolute number of CD62L-/-/β7-/- was 10-fold lower than these values (P < .01) and was just above the level of detection (Figure 4). Thus, the loss of the CD62L receptor alone had little impact on the accumulation of donor cells in the mesenteric nodes, despite the profound impact on accumulation in the peripheral nodes. On the other hand, the loss of both the CD62L and β7 receptors had a profound impact on both mesenteric and peripheral nodes on day 2.
On day 6, the mean numbers of all types of allogeneic donor cells in the spleen had increased about 10- to 50-fold compared with the levels on day 2, despite the reduction of the donor T-cell dose from day 2 to day 6. The accumulation of allogeneic cells was about 100-fold increased compared with an equal number of syngeneic donor CD4+ T cells, since the mean values of the syngeneic cells were less than 0.01 × 106 cells in all tissues examined on day 6 (data not shown). Although the absolute numbers of allogeneic WT donor T cells were undetectable in the liver and colon on day 2, they increased about 500- to 1000-fold by day 6 and were in the same range (0.1 × 106 to 1 × 106 cells) as the day-6 accumulation in the organized lymphoid tissues (Figure 4). The mean absolute number of CD62L-/- and CD62L-/-/β7-/- cells was significantly lower (P < .02) than the WT cells in the liver, and the mean number of CD62L-/-/β7-/- cells was significantly lower than WT cells in the colon (P < .01) at day 6. The reduction of the mean number of CD62L-/-/β7-/- donor cells compared with WT cells was even more profound in the mesenteric lymph nodes (P < .001). A significant but smaller reduction in the latter tissue was observed with CD62L-/- donor cells (P < .02).
On the whole, the results show that donor T cells accumulate in the organized lymphoid tissue before they accumulate in the liver and colon. This is followed by a massive accumulation of donor T cells in the later 2 tissues 4 days later. We analyzed the Peyer patches and the small intestine for the accumulation of WT allogeneic donor T cells at day 6, but the absolute number of cells was less than 0.001 × 106 (data not shown).
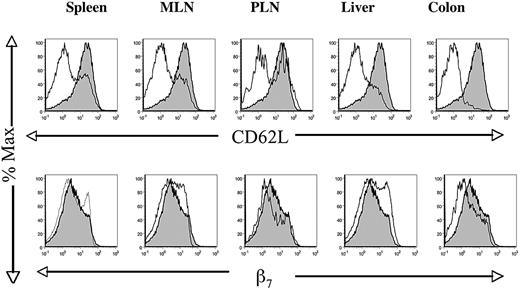
Changes in the expression of CD62L and β7 on the surface of donor T cells harvested from host tissues on day 6. Hosts received WT TCD bone marrow and 0.125 × 106 WT CD4+ donor T cells, and tissues were analyzed first for H-2Kb versus TCRαβ. H-2Kb+ TCRαβ+–gated cells (donor T cells) were analyzed subsequently for CD62L or β7 (thin lines) and compared with similar analysis of CD62L and β7 at the time of donor T-cell injection (shaded area). Profiles are representative of at least 6 hosts.
Changes in the expression of CD62L and β7 on the surface of donor T cells harvested from host tissues on day 6. Hosts received WT TCD bone marrow and 0.125 × 106 WT CD4+ donor T cells, and tissues were analyzed first for H-2Kb versus TCRαβ. H-2Kb+ TCRαβ+–gated cells (donor T cells) were analyzed subsequently for CD62L or β7 (thin lines) and compared with similar analysis of CD62L and β7 at the time of donor T-cell injection (shaded area). Profiles are representative of at least 6 hosts.
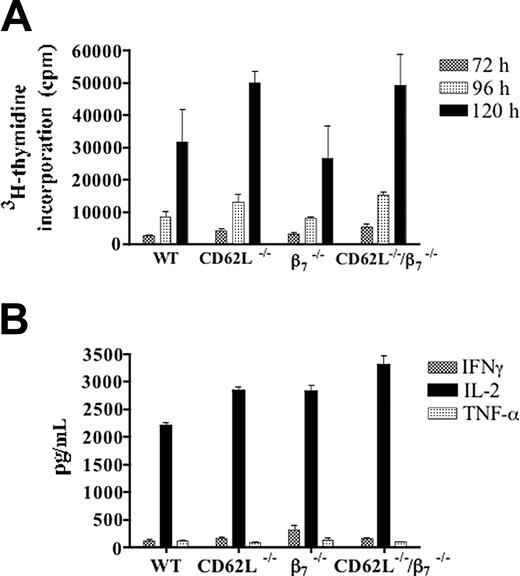
Responses of CD4+ T cells from WT or gene-deficient donors to host stimulator cells in the MLR. Panel A shows means ± SEM of 3H-thymidine incorporation of triplicate cultures of donor T cells at 72 (▥), 96 (▦), and 120 hours (▪). Panel B shows mean ± SEM concentrations of IFNγ (▥), IL-2 (▪), and TNF-α (▦) in the supernatants of triplicate MLR cultures at 60 hours. Background 3H-thymidine incorporation was less than 3000 cpm with syngeneic stimulator cells, and background cytokine concentrations were less than 100 pg/mL IL-2. Results are representative of at least 3 MLR cultures.
Responses of CD4+ T cells from WT or gene-deficient donors to host stimulator cells in the MLR. Panel A shows means ± SEM of 3H-thymidine incorporation of triplicate cultures of donor T cells at 72 (▥), 96 (▦), and 120 hours (▪). Panel B shows mean ± SEM concentrations of IFNγ (▥), IL-2 (▪), and TNF-α (▦) in the supernatants of triplicate MLR cultures at 60 hours. Background 3H-thymidine incorporation was less than 3000 cpm with syngeneic stimulator cells, and background cytokine concentrations were less than 100 pg/mL IL-2. Results are representative of at least 3 MLR cultures.
In addition to the studies of accumulation, we analyzed the changes in the expression of CD62L and β7 receptors on allogeneic WT CD4+ T cells in the different host tissues on day 2 and day 6. Figure 5 shows that the expression of CD62L was markedly down-regulated on the donor T cells in all the host tissues on day 6 compared with the level at the time of injection of the donor cells. In some tissues such as the colon, CD62L on donor T cells was barely detectable, whereas in the spleen, mesenteric lymph nodes, and peripheral nodes, a minority of cells expressed high levels of the receptor with a bimodal distribution. In contrast, on day 2 the levels of expression of CD62L on donor cells in the nodes and spleen were no different than those of the injected cells (data not shown). Although the CD62L staining intensity was decreased on donor T cells on day 6, the β7 integrin staining intensity on day 6 was similar to that of the injected cells in all host tissues examined (Figure 5). There was a slight increase in staining in the mesenteric lymph nodes and liver and a slight decrease in the peripheral nodes and colon. Staining on day 2 was almost completely overlapping (data not shown).
Lack of CD62L and β7 receptors did not alter responses of donor CD4+ T cells to alloantigens in vitro
Purified CD4+ T cells from WT, CD62L-/-, β7-/-, and CD62L-/-/β7-/- C57BL/6 donors were stimulated with irradiated spleen cells from BALB/c mice in the mixed leukocyte reaction (MLR), and proliferation and cytokine secretion were measured. As shown in Figure 6A, the mean proliferative responses of the CD62L-/- and CD62L-/-/β7-/- T cells were higher than the WT responses at 72, 96, and 120 hours. The increases were significantly different only at 72 hours (P < .05 to P < .01). The β7-/- responses were slightly lower than WT responses at all time points, but differences were not significant (P > .1). Background proliferation using syngeneic stimulator cells was less than 3000 counts per minute (cpm) at 72 hours.
All 4 responder cell populations vigorously secreted interleukin 2 (IL-2) in the range of about 3000 pg/mL in supernatants harvested at 60 hours (Figure 6B), and no significant differences were observed (P > .1). Secretion of interferon γ (IFNγ) and tumor necrosis factor α (TNF-α) was at least 10-fold lower than IL-2 and similar for all cell populations (Figure 6B). Thus, the loss of the CD62L or β7 receptors did not reduce the ability of the donor T cells to respond to host alloantigens in the in vitro assays. Background secretion of IL-2 was less than 100 pg/mL when syngeneic stimulator cells were used.
Discussion
The current studies used a purified CD4+ subset of T cells from WT and receptor gene–deficient donors to induce acute GVHD in lethally irradiated MHC-mismatched hosts. The proportion of naive and memory CD4+ T cells in all donors was similar as judged by CD44 receptor expression and by real-time polymerase chain reaction (PCR) analysis of CCR7 mRNA levels (data not shown). In this model of bone marrow transplantation, rapid death of hosts occurred within 2 weeks with 0.25 × 106 WT T cells injected due to severe colitis that was documented by histopathologic changes on day 7. There was little histopathologic evidence of injury to the small intestine or the liver at this early time point, indicating that CD4+ T cells mainly attacked the colon. The predominance of colon injury was reflected in the marked early accumulation of donor CD4+ T cells in the mesenteric lymph nodes at day 2 and 6 with little accumulation in the Peyer patches. A recent study in hosts given no radiation or chemical conditioning showed the importance of Peyer patches in small intestinal GVHD and observed that CD8+ T cells migrate early to these areas.9 Thus, CD8+ T cells may play a predominant role in GVHD injury to the small intestine, which was not observed in the current model due to the use of purified CD4+ donor T cells. In addition, conditioning with radiation may injure the colon and increase its susceptibility to become a predominant target organ of GVHD. Preparative regimens using chemical or other biologic reagents may result in less colonic injury than conditioning with radiation and reduce the likelihood of severe early GVHD colitis. Thus, this mouse model is most relevant to radiation-based clinical regimens.
It is of interest that WT donor CD4+ T cells accumulated in the spleen, mesenteric nodes, and peripheral nodes about 10- to 50-fold more than syngeneic CD4+ T cells at day 2, but no detectable accumulation occurred in the liver and colon at this early time point. However, between day 2 and day 6, the accumulation of WT T cells increased about 500- to 1000-fold in the liver and colon. This increase could not be accounted for by expansion of a small number of donor T cells that migrated directly to the liver and colon by day 2 and subsequently had doubling times of every 12 hours. The most likely explanation is that donor T cells that migrated directly to the organized lymphoid tissues at day 2 expanded there and subsequently migrated to the liver and colon between day 2 and day 6. Analysis of donor T cells in the lymphoid tissues and colon suggests that only the subset that down-regulates CD62L migrates to the colon.
Whereas acute colitis and deaths were observed early with WT, CD62L-/-, and β7-/- donor cells, mild colitis without deaths for the first 40 days was observed when CD62L-/-/β7-/- donor cells were used. The reduced severity of colitis with the latter cells was associated with a marked reduction (>10-fold) in the accumulation of donor T cells in the mesenteric lymph nodes at days 2 and 6 and a 3- to 5-fold reduction in the accumulation in the colon at day 6. Although the reduction in the colon was not profound, the associated reduction in microscopic colonic injury, diarrhea, and death was considerable. The results suggest that a threshold of donor T-cell accumulation in the colon is required for severe colonic injury. The results also indicate that therapeutic agents that block only the interaction between CD62L and its ligands, or only the interaction between α4β7 and its ligands, are unlikely to be effective in reducing colonic GVHD.
Early accumulation of donor T cells in the nodes may be markedly influenced by colonic antigens recognized in the context of antigen-presenting cells. Whereas the organized lymphoid tissues such as the mesenteric lymph nodes have activated antigen-presenting dendritic cells that present colonic microflora antigens associated with host MHC class II receptors to donor T cells, the colonic lamina propria is not a major site for dendritic cell interactions with T cells for presentation of colonic microflora antigens.16,17 The mesenteric lymph nodes constitute a specialized lymphoid tissue for T-cell interactions with colonic antigens that does not occur in the spleen, peripheral nodes, or Peyer patches. Colonic microflora antigens are an important source of CD4+ T-cell activation in autoimmune colitis17,18 and may also be important in GVHD-associated colitis. The marked amelioration of GVHD in germ-free compared with conventionally raised host mice indicates the importance of gut microflora in the induction of intestinal GVHD.19 In addition, reduction of gut microflora with antibiotics ameliorates gut GVHD in patients receiving allogeneic bone marrow transplants.20
In conclusion, alterations in cell trafficking to the mesenteric nodes due to homing receptor deficiencies is the most likely mechanism by which amelioration of GVHD-associated colitis was achieved. The study points out the specialized role of mesenteric lymph nodes in CD4+ T-cell–induced immune injury to the colon and emphasizes the important role that colonic antigens presented to T cells in the mesenteric nodes in these models may play in this disease.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-06-2339.
Supported in part by grants from the National Institutes of Health (HL-58250, HL-57743, CA-49604) and a Stanford Vice Provost Undergraduate Education (VPUE) Faculty Grant.
S.D. designed research, performed research, and analyzed data; J.E. and D.T. performed research and analyzed data; Y.P.L. performed research; T.I.G. performed pathologic review of slides; C.G.F. designed research; and S.S. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Thomas F. Tedder (Duke University) for provision of CD62L-/-/β7-/- gene–deficient mice, Dr Richard Flavell (Yale University) and Drs Irving Weissman and Holger Karsunky (Stanford University) for CD62L-/- mice, Caroline Tudor for digital artwork, and Mary Hansen for preparation of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal