To assess the prognostic relevance of mutations in the NPM1 gene encoding a nucleocytoplasmic shuttle protein in younger adults with acute myeloid leukemia (AML) and normal cytogenetics, sequencing of NPM1 exon 12 was performed in diagnostic samples from 300 patients entered into 2 consecutive multicenter trials of the AML Study Group (AMLSG). Treatment included intensive double-induction therapy and consolidation therapy with high cumulative doses of high-dose cytarabine. NPM1 mutations were identified in 48% of the patients including 12 novel sequence variants, all leading to a frameshift in the C-terminus of the nucleophosmin 1 (NPM1) protein. Mutant NPM1 was associated with specific clinical, phenotypical, and genetic features. Statistical analysis revealed a significant interaction of NPM1 and FLT3 internal tandem duplications (ITDs). NPM1 mutations predicted for better response to induction therapy and for favorable overall survival (OS) only in the absence of FLT3 ITD. Multivariable analysis for OS revealed combined NPM1-mutated/FLT3 ITD–negative status, CEBPA mutation status, availability of a human leukocyte antigen (HLA)–compatible donor, secondary AML, and lactate dehydrogenase (LDH) as prognostic factors. In conclusion, NPM1 mutations in the absence of FLT3 ITD define a distinct molecular and prognostic subclass of young-adult AML patients with normal cytogenetics.
Introduction
Acute myeloid leukemia (AML) is a phenotypically and genetically heterogeneous disease. The genetic diversity results mainly from mutant genes that are causally implicated in the pathogenesis of AML.1 To date, karyotype at diagnosis provides the most important prognostic information in adult AML, and cytogenetic data are used to assign patients to distinct prognostic groups within risk-adapted treatment protocols to improve treatment outcome and to minimize toxicity of therapies.2-5
However, by conventional chromosome banding analysis approximately 50% of the AML patients lack clonal chromosome aberrations,3,4 and discrimination between prognostically different subsets of patients within this intermediate-risk group by using molecular genetic approaches is of major importance.6-13 Nucleophosmin (NPM1, also known as nucleolar phosphoprotein B23 or numatrin) is a ubiquitously expressed nucleolar phosphoprotein that continuously shuttles between the nucleus and cytoplasm14 with prominent nucleolar localization.15 It has been implicated in ribosomal protein assembly and transport,16 and also as a molecular chaperone that prevents protein aggregation in the nucleolus.17 NPM1 regulates the stability and transcriptional activity of p53 after different types of stress,18 and in a more recent study, NPM1 was identified as a nucleolar binding partner of the alternate-reading-frame protein (ARF) that uses NPM1 for p53-independent cell cycle regulation.19 In addition, NPM1 is involved in the initiation of centrosome duplication via cyclin E/cyclin-dependent kinase 2 (cdk2) phosphorylation.20
The NPM1 gene is involved in several leukemia- and lymphoma-associated chromosome translocations that result in fusion proteins retaining the amino terminus of NPM1, including NPM1–anaplastic lymphoma kinase (NPM-ALK),21 NPM1–retinoic acid receptor α (NPM-RARα),22 and NPM1–myeloid leukemia factor 1 (NPM-MLF1).23 The NPM1 component itself seems not to have transforming potential but is able to activate the oncogenic potential of the fused protein partner.24 Translocations involving the NPM1 gene cause cytoplasmic dislocation of the protein, a mechanism that might be critical in malignant transformation.15
In a recent study, Falini et al25 investigated the subcellular localization of NPM1 in bone marrow biopsy specimens from 591 adult patients with primary AML, 135 patients with secondary AML, and 980 hematopoietic or extrahematopoietic malignancies other than AML using immunohistochemical methods. Cytoplasmic NPM1 (NPM1c+) was exclusively detected in primary AML with an incidence of 35.2%. It was associated with a broad spectrum of morphologic AML subtypes with highest frequency in monocytic leukemias, the absence of the hematopoietic stem cell markers CD34 and CD133, a normal karyotype, and responsiveness to induction chemotherapy. In NPM1c+ cases, internal tandem duplication (ITD) of the FLT3 gene was twice as frequent compared with cases negative for cytoplasmic NPM1 (NPM1c–). Sequencing of the NPM1 coding region identified exon 12 mutations in 51 of the 52 NPM1c+ cases that were analyzed, including 48 cases with normal karyotype. Six sequence variants (named A, B, C, D, E, and F) were detected, all leading to a frameshift in the C-terminal region of the NPM1 protein that is necessary for nucleolar localization of NPM1.26 Transfection of mutated NPM1 into NIH-3T3 cells confirmed that exon 12 mutations result in delocalization of NPM1.25
Based on these findings, mutations in NPM1 exon 12 and the resulting shift of NPM1 into the cytoplasm are the most frequent events that have been identified in adult AML with normal karyotype to date.
The objective of this study was to assess the incidence and prognostic relevance of NPM1 mutations in a large series of homogeneously treated adults 16 to 60 years of age with AML and normal cytogenetics who had been entered into 2 consecutive multicenter trials of the AML Study Group (AMLSG). In a search for associated gene mutations and to provide meaningful data from multivariable analysis, we have analyzed all our patients for the presence of activating FLT3 mutations, CEBPA mutations, and partial tandem duplications of the mixed-lineage leukemia gene (MLL PTDs).
Patients, materials, and methods
Patients
Diagnostic bone marrow (BM) or peripheral blood (PB) samples were analyzed from 300 adult patients (16 to 60 years) with AML diagnosed according to French-American-British (FAB) Cooperative Group criteria27 and normal cytogenetics who had been entered into the multicenter treatment trials AML HD93 (77 patients; total study population, n = 223; August 1993 to January 1998)5 and AML HD98-A (223 patients; total study population, n = 958; February 1998 to December 2004)28 of the AMLSG. AML HD93 enrolled patients with de novo AML and patients with secondary AML after a primary malignancy (t-AML). AML HD98-A trial also included patients with refractory anemia with excess of blasts in transformation (RAEBt) and patients with secondary AML following myelodysplastic syndrome (s-AML). De novo AML was diagnosed in 257 of the 300 patients, 24 patients had t-AML/s-AML, and in 19 patients the information was missing. For this molecular study, the only criterion used to include or exclude patients was the availability of a BM or PB sample from diagnosis for mutation analysis of the NPM1 gene. Approval was obtained from the institutional review boards of the participating AMLSG institutions. All patients gave informed consent for both treatment and cryopreservation of BM and PB according to the Declaration of Helsinki.
Therapy of patients with normal cytogenetics
All patients entered into the AML HD93 and AML HD98-A trials received intensive, response-adapted double-induction and consolidation therapy. Double-induction therapy consisted of a course of ICE (12 mg/m2 idarubicin on days 1, 3, and 5; cytarabine 100 mg/m2 continuously on days 1 through 7; and 100 mg/m2 etoposide on days 1 through 3), followed by a second course of ICE started between days 21 and 28 in patients with a response to the first course of induction therapy, or by a course of a HAM (3 g/m2 cytarabine every 12 hours on days 1 through 3; 12 mg/m2 mitoxantrone on days 2 and 3)–based regimen in patients with ICE-refractory disease. First consolidation therapy consisted of a course of HAM.
Second consolidation therapy of patients with normal cytogenetics differed between the 2 trials: in the AML HD93 trial, patients 16 to 54 years of age were assigned to receive a course according to the S-HAM protocol (3 g/m2 cytarabine every 12 hours on days 1, 2, 8, and 9; 10 mg/m2 mitoxantrone on days 3, 4, 10, and 11); patients 55 to 60 years of age received the less-intensive HAM regimen. In the AML HD98-A trial, patients were randomized to either a second course of HAM or myeloablative therapy (total body irradiation/cyclophosphamide or busulfan/cyclophosphamide), followed by autologous stem cell transplantation (SCT). In both trials, patients were assigned to allogeneic SCT if a human leukocyte antigen (HLA)–compatible family donor was available. In 280 patients, information about the availability status of an HLA-compatible family donor was present at the time of diagnosis. In 63 (22.5%) of the 280 patients and in 53 (24%) of 217 patients achieving a complete remission (CR) after induction therapy, an HLA-compatible family donor was available.
Cytogenetic and molecular genetic analysis
Pretreatment samples from all patients were studied centrally by G-banding analysis and fluorescence in situ hybridization (FISH). Conventional cytogenetic studies were performed using standard techniques, and chromosomal abnormalities were described according to the International System for Human Cytogenetic Nomenclature.29 To improve the accuracy of cytogenetic diagnosis, all specimens were also analyzed by FISH using a comprehensive DNA probe set allowing for the detection of the most relevant AML-associated genomic aberrations.30 In addition, diagnostic samples from all patients were analyzed for mutations in the FLT3 ([ITDs] and activation loop mutations at D835 and I836), CEBPA, and MLL genes (MLL, PTDs).6-8
Analysis of NPM1 exon 12 mutations
Genomic DNA was isolated from mononuclear cell preparations stored at –70°C using the DNAzol reagent (GibcoBRL, Eggenstein, Germany) according to the manufacturer's recommendations. Polymerase chain reaction (PCR) amplification of NPM1 exon 12 was carried out using primers NPM1–F (5′-TTAACTCTCTGGTGGTAGAATGAA-3′) and NPM1–R (5′-CAAGACTATTTGCCATTCCTAAC-3′), as previously described.25
The total reaction volume of 50 μL contained approximately 100 ng DNA, 10 pmol of each primer, deoxynucleoside triphosphates (dNTPs, 10 mM each), 2.5 U Gold Taq polymerase, and supplied buffer (Applied Biosystems, Darmstadt, Germany). Samples were amplified using the following PCR conditions: 95°C for 5 minutes; 40 cycles of 94°C for 30 seconds; 55°C for 1 minute; 72°C for 1 minute. PCR products were purified by standard methods and directly sequenced with primer NPM1-R2 (5′-GGCATTTT-GGACAACACA-3′) using the ABI Ready Reaction Dye Terminator Cycle Sequencing Kit (Applied Biosystems).
Statistical analyses
The median follow-up for survival was calculated according to the method of Korn.31 The definition of CR followed the recommended criteria.32 Overall survival (OS) end points, measured from entry into one of the prospective studies, were death (failure) and alive at last follow-up (censored).32 Relapse-free survival (RFS) end points, measured from the date of documented CR, were relapse (failure), death in CR (failure), and alive in CR at last follow-up (censored).32 Pairwise comparisons between patient characteristics (covariates) were performed by Mann-Whitney test for continuous variables and by Fisher exact test for categoric variables. A multivariable logistic model was used to analyze associations between presenting features and response to induction therapy. The Kaplan-Meier method was used to estimate the distribution of RFS and OS. Confidence interval estimation for the survival curves was based on the cumulative hazard function using Greenwood's formula for the standard error estimation.33 A Cox model was used to identify prognostic variables.34 Missing data were estimated using a multiple-imputation technique using predictive mean matching with n = 100 for multiple imputations.35 A limited backward-selection procedure was used to exclude redundant or unnecessary variables.35 To provide quantitative information on the relevance of results, 95% confidence intervals (95% CIs) of odds ratios (ORs) and hazard ratios (HRs) were computed. The statistical analyses were performed with the statistical software package R, version 1.9.0,36 together with the Design software library.35
Results
NPM1 exon 12 polymorphisms
A silent nucleotide change (del1146A) located in the 3′ untranslated region (UTR) of the gene that would not affect the predicted amino acid sequence was identified in 177 (59%) of the 300 AML samples and in all 6 peripheral blood samples from healthy volunteers. This polymorphism was not correlated with mutant NPM1 using Fisher exact test (P = .51).
NPM1 exon 12 mutations
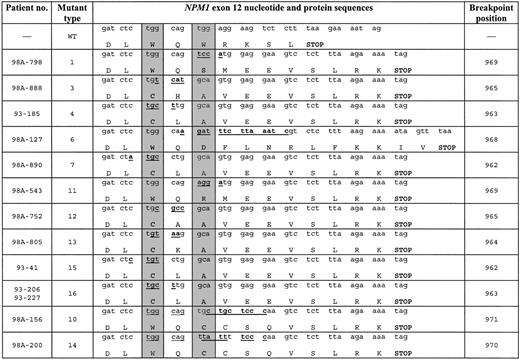
Heterozygous NPM1 mutations were identified in 145 (48%) of the 300 AML patients with normal cytogenetics. Of 145 patients, 131 had de novo AML and 14 presented with t-AML/s-AML. The most frequent mutation type A was present in 110 (76%) of the cases, followed by type B (8%) and type D (7%). In contrast to the previously published studies in adult and childhood AML,25,37 we did not observe type C, E, F, G, and H mutations but identified 12 novel sequence variations (here named as mutations 1, 3, 4, 6, 7, 10, 11, 12, 13, 14, 15, and 16) (Figure 1). In accordance with the findings from Falini et al,25 all but 3 mutations consisted of a 4-nucleotide insertion at positions 962 through 969. Variant number 6 was characterized by a 9–base pair (bp) deletion and a 14-bp insertion with breakpoint at position 968. The same mechanism was detected for variant number 14 that showed a deletion of 5 nucleotides and an insertion of 9 nucleotides at position 970. In variant number 10, an insertion of 2 tetranucleotides was detected with breakpoint at position 971. Independent of the mutation types, all variants caused a frameshift in the region encoding the C-terminal of the NPM1 protein resulting in the replacement of the last 7 amino acids (WQWRKSL) with 11 or 13 residues (mutation type 6). Therefore, all NPM1 mutant proteins showed mutations in at least one of the tryptophan residues at positions 288 and 290.
Novel NPM1 sequence variations in AML patients with normal cytogenetics. Wild-type (WT) sequence and breakpoint positions correspond to GenBank accession number NM_002520. Novel mutation types were named provisionally by numbers. Nucleotide insertions are underlined and boldfaced. The predicted protein is shown for each mutation type; gray columns indicate the positions of the 2 C-terminal tryptophan residues. — indicates information not applicable.
Novel NPM1 sequence variations in AML patients with normal cytogenetics. Wild-type (WT) sequence and breakpoint positions correspond to GenBank accession number NM_002520. Novel mutation types were named provisionally by numbers. Nucleotide insertions are underlined and boldfaced. The predicted protein is shown for each mutation type; gray columns indicate the positions of the 2 C-terminal tryptophan residues. — indicates information not applicable.
Pie chart based on 246 patients with complete data sets indicating the mutation status coexisting in individual patients for the NPM1, FLT3 (ITD and D835), CEBPA, and MLL (PTD) genes. WT indicates wild type.
Pie chart based on 246 patients with complete data sets indicating the mutation status coexisting in individual patients for the NPM1, FLT3 (ITD and D835), CEBPA, and MLL (PTD) genes. WT indicates wild type.
Associated gene mutations
Patients were analyzed for mutations in the FLT3, CEBPA, and MLL genes (Figure 2). In the NPM1-mutated group, the FLT3 ITD and FLT3 tyrosine kinase domain (TKD) mutations were significantly more frequent (41% and 15%, respectively) compared with the NPM1-negative group (25% and 5%, respectively) (P = .003 and P = .01). MLL PTD was more frequent in the NPM1-unmutated group (P < .001). For CEBPA mutations, there were no differences between the NPM1-mutated and NPM1-unmutated groups (P = .3) (Table 1).
FLT3 (ITD and TKD [D835]), CEBPA, and MLL gene mutations according to the NPM1 mutation status
. | NPM1 mutated, n = 145 . | . | NPM1 unmutated, n = 155 . | . | ||
|---|---|---|---|---|---|---|
| Mutation . | No. of patients . | % . | No. of patients . | % . | ||
| FLT3 ITD | 59 | 41 | 38 | 25 | ||
| Missing | 0 | 0 | 0 | 0 | ||
| FLT3 D835 | 21 | 15 | 8 | 5 | ||
| Missing | 4 | 3 | 7 | 5 | ||
| CEBPA | 16 | 13 | 23 | 17 | ||
| Missing | 19 | 13 | 23 | 15 | ||
| MLL PTD | 2 | 1 | 26 | 17 | ||
| Missing | 0 | 0 | 2 | 1 | ||
. | NPM1 mutated, n = 145 . | . | NPM1 unmutated, n = 155 . | . | ||
|---|---|---|---|---|---|---|
| Mutation . | No. of patients . | % . | No. of patients . | % . | ||
| FLT3 ITD | 59 | 41 | 38 | 25 | ||
| Missing | 0 | 0 | 0 | 0 | ||
| FLT3 D835 | 21 | 15 | 8 | 5 | ||
| Missing | 4 | 3 | 7 | 5 | ||
| CEBPA | 16 | 13 | 23 | 17 | ||
| Missing | 19 | 13 | 23 | 15 | ||
| MLL PTD | 2 | 1 | 26 | 17 | ||
| Missing | 0 | 0 | 2 | 1 | ||
Numbers in “Missing” rows represent the number of patients for whom the relevant data were not available.
Patient characteristics
Mutant NPM1 was associated with myelomonocytic or monocytic morphology (M4/M5 subtypes versus other FAB subtypes, P < .001), extramedullary involvement (P < .001), lymphadenopathy (P = .04), female sex (P = .01), higher white blood cell (WBC) counts (P < .001), higher platelet counts (P = .005), higher BM blast counts (P = .02), higher lactate dehydrogenase (LDH) (P < .001), and lower CD34-antigen expression (P < .001). The higher frequency of extramedullary involvement in the NPM1-mutated group was due to a 4-fold higher rate of gingival hyperplasia. There were no other significant differences in presenting clinical characteristics between patients with or without NPM1 mutation, including age (P = .91) and the frequency of secondary AML (P = .28).
Due to a significant interaction between NPM1 and FLT3 ITD mutations (Table 2), we next categorized patient characteristics into 4 groups (NPM1 mutated/FLT3 ITD negative, NPM1 unmutated/FLT3 ITD negative, NPM1 mutated/FLT3 ITD positive, NPM1 unmutated/FLT3 ITD positive) (Table 2). WBC, LDH, PB, and BM blast counts showed highest values in the NPM1-mutated/FLT3 ITD–positive group, followed by the 2 groups with only one mutation, and lowest values in the group without mutation.
Patient characteristics according to the NPM1/FLT3 ITD mutation status
. | FLT3 ITD negative, n = 203 . | . | FLT3 ITD positive, n = 97 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristics . | NPM1 mutated, n = 86 . | NPM1 unmutated, n = 117 . | NPM1 mutated, n = 59 . | NPM1 unmutated, n = 38 . | P . | ||
| Sex, no. | .03 | ||||||
| Female | 51 | 57 | 42 | 19 | |||
| Male | 35 | 60 | 17 | 19 | |||
| Type AML, no. | .50 | ||||||
| De novo | 78 | 95 | 53 | 31 | |||
| s-AML | 3 | 11 | 2 | 2 | |||
| t-AML | 3 | 2 | 1 | 0 | |||
| Missing | 2 | 9 | 3 | 5 | |||
| Median age, y (range) | 49 (25-60) | 49 (16-60) | 47 (18-60) | 43 (19-60) | .55 | ||
| Median hemoglobin level, g/L (range) | 90 (52-149) | 96 (48-176) | 93 (58-140) | 85 (54-119) | .10 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median platelet count, × 109/L (range) | 68 (12-746) | 49 (8-435) | 61 (16-248) | 64 (12-249) | .03 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median WBC count, × 109/L (range) | 19.8 (0.2-328) | 9.69 (0.41-369) | 43.5 (0.7-345) | 12.4 (0.7-235) | < .001 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median PB blasts, % (range) | 40 (0-99) | 32 (0-98) | 50 (0-100) | 48 (0-96) | .05 | ||
| Missing, no. | 5 | 12 | 5 | 3 | |||
| Median BM blasts, % (range) | 80 (0-100) | 70 (0-98) | 85 (0-100) | 83.5 (0-95) | < .001 | ||
| Missing, no. | 8 | 13 | 8 | 4 | |||
| Median percentage of CD34+ cells (range) | 1 (0-90) | 37 (0-100) | 4.5 (0-50) | 48.5 (0-95) | < .001 | ||
| Missing, no. | 29 | 40 | 25 | 8 | |||
| Median LDH, U/L (range) | 461.5 (89-1825) | 313 (121-1620) | 704 (203-4091) | 472 (122-2992) | < .001 | ||
| Missing, no. | 6 | 8 | 5 | 6 | |||
| Lymphadenopathy, no. (%) | 20 (24) | 13 (12) | 14 (25) | 8 (24) | .07 | ||
| Missing, no. | 5 | 9 | 3 | 5 | |||
| Extramedullary involvement, no. (%) | 15 (18) | 8 (7) | 10 (18) | 2 (5) | .05 | ||
| Missing, no. | 3 | 8 | 3 | 4 | |||
| FAB subtype, no. | ND | ||||||
| M0 | 1 | 10 | 0 | 2 | |||
| M1 | 8 | 18 | 6 | 9 | |||
| M2 | 19 | 33 | 15 | 10 | |||
| M4 | 29 | 20 | 23 | 8 | |||
| M5 | 16 | 9 | 9 | 2 | |||
| M6 | 5 | 1 | 0 | 0 | |||
| Missing | 8 | 26 | 6 | 7 | |||
| Splenomegaly,*no. (%) | 37 (43) | 38 (34) | 18 (31) | 12 (34) | .48 | ||
| Missing, no. | 0 | 6 | 2 | 3 | |||
. | FLT3 ITD negative, n = 203 . | . | FLT3 ITD positive, n = 97 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristics . | NPM1 mutated, n = 86 . | NPM1 unmutated, n = 117 . | NPM1 mutated, n = 59 . | NPM1 unmutated, n = 38 . | P . | ||
| Sex, no. | .03 | ||||||
| Female | 51 | 57 | 42 | 19 | |||
| Male | 35 | 60 | 17 | 19 | |||
| Type AML, no. | .50 | ||||||
| De novo | 78 | 95 | 53 | 31 | |||
| s-AML | 3 | 11 | 2 | 2 | |||
| t-AML | 3 | 2 | 1 | 0 | |||
| Missing | 2 | 9 | 3 | 5 | |||
| Median age, y (range) | 49 (25-60) | 49 (16-60) | 47 (18-60) | 43 (19-60) | .55 | ||
| Median hemoglobin level, g/L (range) | 90 (52-149) | 96 (48-176) | 93 (58-140) | 85 (54-119) | .10 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median platelet count, × 109/L (range) | 68 (12-746) | 49 (8-435) | 61 (16-248) | 64 (12-249) | .03 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median WBC count, × 109/L (range) | 19.8 (0.2-328) | 9.69 (0.41-369) | 43.5 (0.7-345) | 12.4 (0.7-235) | < .001 | ||
| Missing, no. | 3 | 8 | 3 | 3 | |||
| Median PB blasts, % (range) | 40 (0-99) | 32 (0-98) | 50 (0-100) | 48 (0-96) | .05 | ||
| Missing, no. | 5 | 12 | 5 | 3 | |||
| Median BM blasts, % (range) | 80 (0-100) | 70 (0-98) | 85 (0-100) | 83.5 (0-95) | < .001 | ||
| Missing, no. | 8 | 13 | 8 | 4 | |||
| Median percentage of CD34+ cells (range) | 1 (0-90) | 37 (0-100) | 4.5 (0-50) | 48.5 (0-95) | < .001 | ||
| Missing, no. | 29 | 40 | 25 | 8 | |||
| Median LDH, U/L (range) | 461.5 (89-1825) | 313 (121-1620) | 704 (203-4091) | 472 (122-2992) | < .001 | ||
| Missing, no. | 6 | 8 | 5 | 6 | |||
| Lymphadenopathy, no. (%) | 20 (24) | 13 (12) | 14 (25) | 8 (24) | .07 | ||
| Missing, no. | 5 | 9 | 3 | 5 | |||
| Extramedullary involvement, no. (%) | 15 (18) | 8 (7) | 10 (18) | 2 (5) | .05 | ||
| Missing, no. | 3 | 8 | 3 | 4 | |||
| FAB subtype, no. | ND | ||||||
| M0 | 1 | 10 | 0 | 2 | |||
| M1 | 8 | 18 | 6 | 9 | |||
| M2 | 19 | 33 | 15 | 10 | |||
| M4 | 29 | 20 | 23 | 8 | |||
| M5 | 16 | 9 | 9 | 2 | |||
| M6 | 5 | 1 | 0 | 0 | |||
| Missing | 8 | 26 | 6 | 7 | |||
| Splenomegaly,*no. (%) | 37 (43) | 38 (34) | 18 (31) | 12 (34) | .48 | ||
| Missing, no. | 0 | 6 | 2 | 3 | |||
WBC indicates white blood cell; PB, peripheral blood; BM, bone marrow; LDH, lactate dehydrogenase, normal value less than 240 U/L; no., number; and ND, not determined.
Defined as more than 13 cm as measured by ultrasonography.
Response to induction therapy
There was a statistically significant difference in response rates after induction therapy according to the NPM1 and FLT3 ITD mutation status (Table 3). The highest remission rate was achieved in the NPM1-mutated/FLT3 ITD–negative group (86%), followed by the NPM1-unmutated/FLT3 ITD–positive group (76%) and the group without mutations (68.5%); the lowest response rate (63%) was achieved in the NPM1-mutated/FLT3 ITD–positive group (P < .001). Multivariable analysis revealed secondary AML (subsuming t-AML and s-AML; P = .008), logarithm of LDH (P = .03), and the NPM1-mutated/FLT3 ITD–negative status (P = .004) as prognostic markers for the achievement of CR (Table 4). Both NPM1 and FLT3 ITD mutations did not appear as independent prognostic markers when tested as single variables.
Achievement of complete remission according to the NPM1/FLT3 ITD mutation status
. | FLT3 ITD negative, n = 203 . | . | FLT3 ITD positive, n = 97 . | . | ||
|---|---|---|---|---|---|---|
| Remission . | NPM1 mutated, n = 86; no. of patients (%) . | NPM1 unmutated, n = 117; no. of patients (%) . | NPM1 mutated, n = 59; no. of patients (%) . | NPM1 unmutated, n = 38; no. of patients (%) . | ||
| CR | 74 (86) | 78 (68.5) | 37 (63) | 28 (76) | ||
| RD | 2 (2) | 23 (20) | 14 (24) | 7 (19) | ||
| ED/HD | 10 (12) | 13 (11.5) | 8 (13) | 2 (5) | ||
| Missing | 0 | 3 | 0 | 1 | ||
. | FLT3 ITD negative, n = 203 . | . | FLT3 ITD positive, n = 97 . | . | ||
|---|---|---|---|---|---|---|
| Remission . | NPM1 mutated, n = 86; no. of patients (%) . | NPM1 unmutated, n = 117; no. of patients (%) . | NPM1 mutated, n = 59; no. of patients (%) . | NPM1 unmutated, n = 38; no. of patients (%) . | ||
| CR | 74 (86) | 78 (68.5) | 37 (63) | 28 (76) | ||
| RD | 2 (2) | 23 (20) | 14 (24) | 7 (19) | ||
| ED/HD | 10 (12) | 13 (11.5) | 8 (13) | 2 (5) | ||
| Missing | 0 | 3 | 0 | 1 | ||
CR indicates complete remission; RD, resistant disease; and ED/HD, early/hypoplastic death.
Results of logistic regression analysis for achievement of CR after induction therapy
Variable . | Odds ratio . | 95% CI . |
|---|---|---|
| NPM1 mutated/FLT3 ITD negative | 2.81 | 1.39-5.69 |
| Secondary AML | 0.30 | 0.12-0.73 |
| Logarithm of LDH | 0.63 | 0.42-0.95 |
Variable . | Odds ratio . | 95% CI . |
|---|---|---|
| NPM1 mutated/FLT3 ITD negative | 2.81 | 1.39-5.69 |
| Secondary AML | 0.30 | 0.12-0.73 |
| Logarithm of LDH | 0.63 | 0.42-0.95 |
Survival analysis
The median follow-up time for survival was 46 months. In univariate analysis, NPM1 mutation status predicted RFS (P = .002) and in trend OS (P = .09) (Figure 3). The difference in RFS was due mainly to the favorable outcome of patients with NPM1-mutated/FLT3 ITD–negative mutation status. Univariate analysis according to the combined NPM1/FLT3 mutation status revealed a significantly better RFS (P < .001) and OS (P = .001) for the NPM1-mutated/FLT3 ITD–negative group, whereas there was no difference between the 3 other groups (Figure 4).
We also included the availability of an HLA-compatible family donor at diagnosis as a variable in our multivariable analysis of RFS and OS, since allogeneic transplantation was intended for these patients in both treatment protocols. For RFS, multivariable analysis revealed the NPM1-mutated/FLT3 ITD–negative status (P < .001) and the availability of an HLA-compatible family donor (P < .001) as favorable prognostic factors. Based on these results, we analyzed the differential effect of an HLA-compatible family donor in the NPM1-mutated/FLT3 ITD–negative group versus all other patients. In the NPM1-mutated/FLT3 ITD–negative group, there was no difference in RFS independent of the availability of an HLA-compatible family donor (P = .57), whereas for all other patients there was a significant difference in RFS in favor of patients with an HLA-compatible family donor (P = .001) (Figure 5). An allogeneic SCT in first CR was performed in 14 of 16 patients of the NPM1-mutated/FLT3 ITD–negative group and in 34 of 36 patients of all other groups with an HLA-compatible family donor.
Multivariable analysis for OS revealed NPM1-mutated/FLT3 ITD–negative mutation status (P < .001), mutated CEBPA (P = .05), and availability of an HLA-compatible family donor (P = .01) as favorable prognostic factors, whereas secondary AML (subsuming s-AML and t-AML) (P = .04) and higher levels of LDH at diagnosis (P = .01) were unfavorable prognostic factors (Table 5).
Results of Cox regression analysis of overall survival
Variable . | Hazard ratio . | 95% CI . |
|---|---|---|
| NPM1 mutated/FLT3 ITD negative | 0.44 | 0.30-0.66 |
| Secondary AML | 1.70 | 1.02-2.84 |
| HLA-compatible family donor | 0.57 | 0.37-0.89 |
| Mutated CEBPA | 0.61 | 0.36-1.00 |
| Logarithm of LDH | 1.35 | 1.07-1.71 |
Variable . | Hazard ratio . | 95% CI . |
|---|---|---|
| NPM1 mutated/FLT3 ITD negative | 0.44 | 0.30-0.66 |
| Secondary AML | 1.70 | 1.02-2.84 |
| HLA-compatible family donor | 0.57 | 0.37-0.89 |
| Mutated CEBPA | 0.61 | 0.36-1.00 |
| Logarithm of LDH | 1.35 | 1.07-1.71 |
Discussion
We evaluated the prevalence and prognostic impact of NPM1 mutations in a well-defined cohort of 300 uniformly treated AML patients with normal cytogenetics. NPM1 mutations were detected in 145 patients (48%) and therefore represent the most frequent molecular events that have been identified in AML with normal karyotype to date. The prevalence was slightly lower compared with that reported in the pivotal study by Falini et al.25 In accordance with their findings, in our study mutant NPM1 was associated with myelomonocytic or monocytic morphology, lower percentage or absence of CD34 expression, and frequent FLT3 mutations. In addition, we found a correlation of mutated NPM1 with extramedullary involvement (mainly gingival hyperplasia), lymphadenopathy, female sex, higher WBC and platelet counts, higher percentage of BM blasts, and higher LDH serum levels. In contrast to the Italian study,25 in our series, NPM1 mutations were not restricted to de novo AML. Moreover, we did not observe an age-dependent increase of NPM1 mutations as previously described in childhood AML37 or in the study from Falini et al.25
To take into account the possible cooperation effects between various gene mutations, we also analyzed our cases for the most important mutations that have previously been identified in AML with normal cytogenetics, notably mutations in FLT3, CEBPA, and MLL.6-13 In their entire cohort of patients, Falini et al25 found a higher frequency of FLT3 mutations in cases with mutant NPM1, indicating a possible pathogenic link between these 2 gene mutations. Consistent with those data, we also found an association of mutant NPM1 with activating FLT3 mutations in our series. Of interest, statistical analysis revealed a significant interaction between NPM1 and FLT3 ITD mutations with regard to presenting clinical features, response to induction therapy, and survival. The highest CR rate (86%) was observed in the NPM1-mutated/FLT3 ITD–negative group, whereas the lowest CR rate (63%) was seen in NPM1-mutated patients, but in those who had simultaneous FLT3 ITD mutations. For response to induction therapy, multivariable analysis revealed secondary AML and logarithm of LDH as negative prognostic markers, whereas NPM1-mutated/FLT3 ITD negativity was significantly associated with induction success. Thus, in contrast to the finding by Falini et al25 in a smaller cohort of patients, in our study NPM1 mutation status per se did not appear as an independent predictor for responsiveness to chemotherapy.
Treatment results according to the NPM1 mutation status. (A) Relapse-free survival. (B) Overall survival.
Treatment results according to the NPM1 mutation status. (A) Relapse-free survival. (B) Overall survival.
Treatment results according to the combined NPM1 and FLT3 ITD mutation status. (A) Relapse-free survival. (B) Overall survival.
Treatment results according to the combined NPM1 and FLT3 ITD mutation status. (A) Relapse-free survival. (B) Overall survival.
Likewise, this interaction effect was seen in survival analysis. In univariate analysis, patients with mutant NPM1 had a significantly better RFS and in trend a better OS, however, this effect was due to the favorable outcome of NPM1-mutated/FLT3 ITD–negative patients (Figure 4). There was no difference in RFS or OS between the remaining 3 groups. Thus, NPM1 mutations predicted favorable outcome in AML with normal cytogenetics only in the absence of FLT3 ITD mutations. Besides NPM1-mutated/FLT3 ITD–negative status, multivariable analysis revealed mutant CEBPA and availability of an HLA-compatible family donor as favorable prognostic factors, whereas secondary AML and LDH were unfavorable prognostic factors for overall survival (Table 5).
In fact, NPM1-mutated/FLT3 ITD–negative patients had a survival probability after 5 years of approximately 60%, comparable with long-term results of patients with either core-binding factor (CBF) AML28,38,39 or normal karyotype AML with CEBPA mutations.8,10,12 In a donor versus no-donor comparison, NPM1-mutated/FLT3 ITD–negative patients did not benefit from allogeneic SCT, raising the question whether these patients should be exempted from this treatment modality in first-line therapy, as it is currently being done in many multicenter treatment trials for patients with CBF AML.
Donor versus no-donor analysis on relapse-free survival according the combined NPM1 and FLT3 ITD mutation status. (A) NPM1-mutated/FLT3 ITD–negative patients. (B) All other patients.
Donor versus no-donor analysis on relapse-free survival according the combined NPM1 and FLT3 ITD mutation status. (A) NPM1-mutated/FLT3 ITD–negative patients. (B) All other patients.
So far, the causal relation between NPM1 mutations and leukemogenesis remains elusive.40 Recent studies demonstrated that NPM1 mutations disrupt the NPM1 nucleolar localization signal causing accumulation of NPM1 in the cytoplasm, possibly a critical step in malignant transfomation.25 However, the mutations might also interrupt other NPM1 functions that have been implicated within its C-terminal region such as nucleic acid binding, adenosine triphosphate (ATP) binding, and stimulation of DNA polymerase α activity.41-43 Due to its multiple functions that occur in distinct subcellular compartments, different or additional mechanisms other than NPM1 mutations have to be implicated in the pathogenic role of NPM1 in malignant transformation.40 Recently, Alcalay et al44 reported on the gene expression profiles of 78 de novo AMLs characterized for the subcellular localization and mutation status of NPM1. Unsupervised clustering clearly separated NPM1c+ from NPM1c– AMLs, supporting the concept that NPM1c+ AML represents a distinct entity. In this study, the molecular signature of NPM1c+ AML was characterized by up-regulation of genes that are putatively involved in hematopoietic development, including HOX and TALE.
A mechanistic link between FLT3 ITD and NPM1 mutations has also not been established yet. It is intriguing that in the gene expression study by Alcalay et al44 a gene signature was identified for the NPMc+ group that was irrespective of the FLT3 mutation status. Nevertheless, based on the clinical data from our study one might speculate that possible synergistic effects are of importance. Both proteins FMS-like tyrosine kinase 3 (FLT3) as well as NPM1 are required for proliferation.19,45 Indeed, in comparison with patients with no or either NPM1 or FLT3 mutation, patients with both NPM1 and FLT3 ITD mutation in our series had significantly higher median WBC counts, higher median PB and BM blasts counts, as well as higher median LDH values, indicative of a high proliferative activity of the leukemic blasts.
In summary, in this large series of young-adult AML patients with normal karyotype we demonstrate that mutations in NPM1, FLT3, and CEBPA are among the most important predictors for outcome. Mutant NPM1 appears to predict favorable prognosis only in the absence of FLT3 ITD. Our results further emphasize the value of comprehensive molecular genetic screening for the dissection of this heterogeneous subgroup of patients that may ultimately lead to improved risk stratification.
Appendix
We thank the members of the AML Study Group (AMLSG) for providing leukemia specimens: Universitätsklinikum Bonn, Germany, A. Glasmacher; Universitätsklinikum Düsseldorf, Germany, U. Germing; Universitätsklinikum Giessen, Germany, H. Pralle; Universitätsklinikum Göttingen, Germany, D. Haase; Allgemeines Krankenhaus Altona, Hamburg, Germany, H. Salwender; Universitätskliniken des Saarlandes, Homburg, Germany, F. Hartmann; Universitätsklinikum Innsbruck, Austria, A. Petzer; Städtisches Klinikum Karlsruhe, Germany, M. Bentz; Universitätsklinikum Kiel, Germany, M. Kneba; Klinikum rechts der Isar der Technischen Universität München, Germany, K. Götze; Städtisches Krankenhaus München-Schwabing, Germany, C. Nerl; Städtische Kliniken Oldenburg, Germany, F. del Valle; Caritasklinik St Theresia Saarbrücken, Germany, J. Preiß; Klinikum Stuttgart, Germany, H. G. Mergenthaler; Krankenhaus der Barmherzigen Brüder, Trier, Germany, H. Kirchen; and Hanusch-Krankenhaus, Wien, Austria, E. Koller.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-05-2164.
Supported by grant 01GI9981 from the Bundesministerium für Bildung und Forschung (Kompetenznetz “Akute und chronische Leukämien”), Germany.
A complete list of the members of the AMLSG appears in “Appendix.”
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal