Abstract
Background: Although myeloablative SCT is curative for a number of pediatric malignancies, it is associated with significant acute toxicity and late effects. Non-myeloablative SCT can result in successful engraftment of adults with diminished toxicity, but there is limited experience in pediatric pts where increased thymic function might confer a greater risk of graft rejection or mixed chimerism. Prolonged periods of mixed chimerism might in turn impair the graft-vs-tumor response and increase the risk of relapse in highly proliferative pediatric cancers.
Methods: Twenty-one pediatric pts (age 7–21, median 14 years) with high-risk malignancies (Hodgkins, ALL, AML, CML, rhabdomyosarcoma, Ewings sarcoma) were enrolled. One to three cycles (C#) of fludarabine-based induction chemotherapy were administered for disease control and host immune depletion (targeted CD4 count reduction). Pre-transplant conditioning consisted of cyclophosphamide (1,200 mg/m2/day) and fludarabine (30 mg/m2/day) x 4 days plus melphalan (100 mg/m2 x 1 dose in sarcoma pts). Unmodified, G-CSF mobilized peripheral blood stem cells collected from 5–6/6 HLA-matched first degree relatives were infused (median CD34 dose 11.4 x 106/kg, range 4.44–19.12; median CD3 dose 414 x 106/kg, range 228–815). Cyclosporine was used as GVHD prophylaxis.
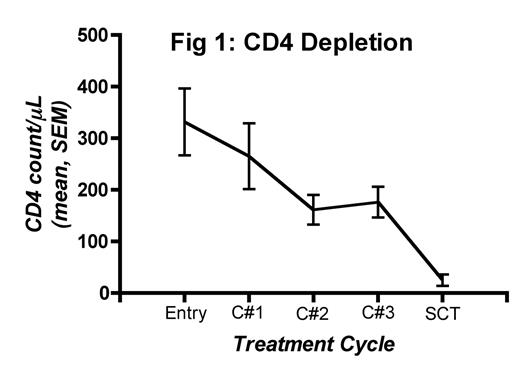
Results: CD4 counts decreased from 332 +/− 65 at entry to 176 +/− 30 (mean +/− SEM/μL) after induction (Fig 1). Treatment was well tolerated and there was no transplant-related mortality. Median time to ANC > 500 cells/μL was post-transplant day (D+) 9 (range 7–11). Complete donor lymphoid chimerism (> 95% by VNTR-PCR on CD3 sorted peripheral blood) was achieved in all pts (18/22 by D+14 and 21/22 by D+28) (Fig 2). Acute GVHD developed in 21/22 patients, but was low grade (18/21 grades 1–2, 3/21 grade 3) and responsive to immunosuppression.
Conclusions: Rapid, complete donor engraftment and chimerism can be achieved in pediatric pts with cancer using targeted pre-transplant immune depletion and a non-myeloablative preparative regimen. This approach may reduce regimen-related toxicity, and thus has the potential to improve the long-term outlook for childhood cancer survivors.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal