Abstract
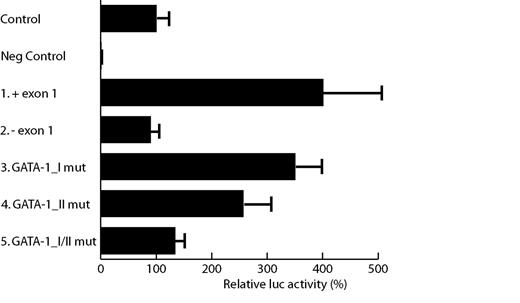
Protoporphyrinogen oxidase (PPOX), the penultimate enzyme in the heme biosynthetic pathway, catalyzes the six-electron oxidation of protoporphyrinogen IX to protoporphyrin IX. Like other heme biosynthetic proteins, PPOX is involved in synthesizing heme for red cells (erythroid-specific expression) and as a cofactor for the respiratory cytochromes (housekeeping expression). To date, little is known about transcriptional regulation of the human PPOX gene (PPOX). We established the molecular basis for erythroid-specific expression of PPOX. Using transient in vitro transfection assays in human erythroleukemic K562 cells we studied tissue-specific expression of PPOX. We found that reporter constructs lacking exon 1 showed a 75% reduction in promoter strength in K562 cells (Figure, no 1 and 2). Hence, in vitro high-level erythroid-specific expression of PPOX is dependent on the presence of exon 1. Examination of erythroid-specific regulatory elements in exon 1 revealed two GATA-1 sites, one consensus (A/T)GATA(A/G) site (GATA-1_II AGATAA) and one non-consensus site, deviating at the first nucleotide (GATA-1_I, CGATAG). To study the relative contribution of these two GATA-1 sites to erythroid-specific transcriptional regulation, we performed in vitro transfections of wild-type and mutant (GATA → GTTA) reporter plasmids in K562 cells. We found that the highest level of transcription depended on the integrity of both sites (Figure, no 5). The consensus GATA-1_II site contributed the most to promoter strength (Figure, no 4). Subsequent electrophoretic mobility shift assay and supershift experiments using K562 nuclear extracts demonstrated that both GATA sites were able to bind GATA-1 in vitro. Our experiments showed that exon 1 was dispensable for PPOX promoter activity in human hepatoma HepG2 cells. Interestingly, in HeLa human cervical carcinoma cells the presence of exon 1 decreased promoter activity. Conclusively, exon 1 of the human PPOX gene contains two GATA-1 binding sites, which are required for high level erythroid-specific expression of PPOX and, in addition, bind GATA-1 in vitro. Our results contribute to a better understanding of the molecular mechanisms involved in differential regulation of the human PPOX promoter in erythroid and non-erythroid cells.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal