Abstract
Peripheral destruction of sickled erythrocytes is a cardinal feature of sickle cell disease (SCD). Less well established is the potential contribution of ineffective erythropoiesis to the pathophysiology of this hemoglobinopathy. Since patients with SCD frequently develop mixed hematopoietic chimerism after allogeneic nonmyeloablative stem cell transplantation, we used this opportunity to directly compare the differentiation and survival of SCD and donor-derived erythropoiesis in vivo. Donor and recipient erythropoiesis was compared in 4 patients with SCD and 4 without SCD who developed stable mixed hematopoietic chimerism following transplant. Molecular analysis of chimerism in peripheral blood and bone marrow demonstrated higher expression of donor-derived β-globin RNA relative to the level of donor-derived genomic DNA in patients with SCD. Analysis of chimerism in immature (glycophorin A–positive [GYPA+], CD71hi) and mature (GYPA+, CD71neg) erythroblasts confirmed the intramedullary loss of SS erythroblasts with progressive maturation. In patients with SCD, relative enrichment of donor erythroid precursors began to appear at the onset of hemoglobinization. Ineffective erythropoiesis of homozygous hemoglobin S (SS) progenitors thus provides a maturation advantage for homozygous hemoglobin A (AA) or heterozygous hemoglobin S/hemoglobin A (SA) donor erythroid precursor cells that results in greater donor contribution to overall erythropoiesis following stem-cell transplantation and improvement of clinical disease.
Introduction
Sickle cell disease (SCD) is a common and severe disorder in which a missense mutation in the β-globin gene results in polymerization of hemoglobin S under deoxygenating conditions, leading to chronic peripheral hemolysis and intravascular vaso-occlusion. Less fully appreciated is the extent to which sickle hemoglobin in committed erythroid precursor cells might also affect earlier events in erythropoiesis. Improved understanding of the impact of SCD on intramedullary erythropoiesis is relevant to the development of new therapeutic strategies for this chronic debilitating disease.
One approach to directly assess the contribution of ineffective erythropoiesis to the pathophysiology of sickle cell disease is to analyze erythropoiesis in sickle cell disease patients who have undergone matched related donor allogeneic stem cell transplantation (SCT) following a nonmyeloablative (NMA) conditioning regimen. A variety of nonmyeloablative conditioning regimens have been developed to facilitate engraftment of donor stem cells and reduce the treatment-related toxicity of hematopoietic SCT.1-4 In this nonmyeloablative setting, host hematopoiesis frequently persists following transplantation and coexists with engrafted donor cells within the marrow. Depending on the underlying disease and the intensity of the conditioning regimen, stable mixed hematopoietic chimerism occurs frequently and can persist for long intervals. Mixed hematopoietic chimerism has been well documented after allogeneic hematopoietic SCT for SCD, and this provides a unique opportunity to perform a direct side-by-side comparison of normal and sickle erythropoiesis in vivo.5,6
To distinguish between donor and host erythropoiesis, we previously developed methods to specifically measure erythroid lineage chimerism in both nucleated and non-nucleated erythroid elements. Although molecular methods for measurement of donor chimerism are well established, they are based on analysis of genomic DNA, and thus measure nucleated-cell chimerism, which is predominantly leukocyte derived in peripheral blood.7-9 Therefore, in addition to measuring overall engraftment by analysis of genomic DNA, the present studies also use two alternate methods to distinguish between donor and host-derived erythropoiesis. One method relies on direct enumeration of donor-derived erythroid precursors on paraffin-imbedded marrow sections following staining with a donor-specific marker, such as red blood cell (RBC) isohemagglutinin antigens in an ABO disparate setting. A second method uses a novel molecular assay, RNA β-globin pyrosequencing, which directly measures erythroid lineage-specific chimerism from total RNA by quantifying the amount of RBC-specific RNA transcripts that bear a donor or host-specific single nucleotide polymorphism (SNP) or mutation.6 In addition to using the sickle mutation as an informative β-globin SNP, we also previously identified an alternate β-globin SNP, 3H3, that occurs at high minor allele frequency.6 Since β-globin RNA is expressed only within erythroid progenitors, analysis of chimerism within this transcript in peripheral blood and bone marrow is directly informative of the level of chimerism within red-cell precursors.
In the present study, we use these methods to directly compare recipient hemoglobin S (SS) and donor heterozygous hemoglobin S/hemoglobin A (SA) erythropoiesis in vivo in SCD patients who have developed mixed hematopoietic chimerism after allogeneic SCT. Our study, for the first time, directly presents evidence of ineffective erythropoiesis in patients with SCD. We propose that ineffective erythropoiesis is a potentially important mechanism of disease pathophysiology in SCD, and its presence in SCD underscores the efficacy of stem-cell–based therapies for the treatment of SCD.
Patients, materials, and methods
Patient samples and cell preparation
The clinical characteristics of 8 patients who received transplants are shown in Table 1. Patient 1 is a 34-year-old woman with history of frequent and severe vaso-occlusive crises. Patient 2 is a 52-year-old man who experienced exacerbation of SCD symptoms after also developing multiple myeloma. Patient 3 is a 5-year-old boy who had a history of stroke and chronic transfusion therapy. Patient 4 is an 8-year-old boy who had a history of repeated episodes of acute chest syndrome. Patients 5 through 8 had homozygous A (AA) hemoglobin and underwent NMA-SCT for treatment of chronic lymphocytic leukemia (CLL), Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL). The sibling donors for 3 of 4 SCD patients were male, 3 with sickle trait (SA hemoglobin) and 1 with β-thalassemia trait. Patients 1 and 4 through 8 and their donors were sex mismatched. Patients 1, 2, 5, 6, and 7 were ABO mismatched with their respective donors. Patients 1, 2, 5, 7, and 8 received peripheral-blood stem cells after receiving a conditioning regimen of fludarabine (30 mg/m2/d × 4 days) and busulfex (0.8 mg/kg/d × 4 days). Patient 6 received an identical preparative regimen with the addition of antithymocyte globulin (ATG) prior to receiving unrelated umbilical cord blood stem cells. Patients 3 and 4 received bone marrow stem cells following a nonmyeloablative conditioning regimen consisting of fludarabine, busulfan, total lymphoid irradiation (TLI), and ATG.
Patient clinical characteristics
. | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . |
|---|---|---|---|---|---|---|---|---|
| Patient diagnosis | SCD | SCD | SCD | SCD | CLL | HL | CLL | NHL |
| Patient age/sex | 34/F | 52/M | 5/M | 8/M | 64/M | 36/F | 51/M | 50/M |
| Donor sickle genotype/sex | Sickle trait/M | Sickle trait/M | Sickle trait/M | β-thalassemia trait/M | —/F | —/M | —/F | —/F |
| Pt/Donor ABO type | A/O | O/B | O/O | B/AB | B/O | A/O | A/O | O/O |
| Transplant conditioning regimen | Flu/Bu* | Flu/Bu* | Flu/Bu/ATG/TLI† | Flu/Bu/ATG/TLI† | Flu/Bu* | Flu/Bu/ATG* | Flu/Bu* | Flu/Bu* |
| Stem-cell source | PBSC | PBSC | BM | BM | PBSC | UCB | PBSC | PBSC |
| CD34+ cells/kg infused | 3.5 × 106 | 7.3 × 106 | 3.5 × 106 | 4.72 × 106 | 9.8 × 106 | 0.38 × 106 | 3.4 × 106 | 12.9 × 106 |
| GVHD prophylaxis | Tac/Pred | Tac/Pred | CsA/MMF | CsA/MMF | Tac/MTX | Tac/Rapa | Tac/MTX | Tac/Rapa |
. | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . |
|---|---|---|---|---|---|---|---|---|
| Patient diagnosis | SCD | SCD | SCD | SCD | CLL | HL | CLL | NHL |
| Patient age/sex | 34/F | 52/M | 5/M | 8/M | 64/M | 36/F | 51/M | 50/M |
| Donor sickle genotype/sex | Sickle trait/M | Sickle trait/M | Sickle trait/M | β-thalassemia trait/M | —/F | —/M | —/F | —/F |
| Pt/Donor ABO type | A/O | O/B | O/O | B/AB | B/O | A/O | A/O | O/O |
| Transplant conditioning regimen | Flu/Bu* | Flu/Bu* | Flu/Bu/ATG/TLI† | Flu/Bu/ATG/TLI† | Flu/Bu* | Flu/Bu/ATG* | Flu/Bu* | Flu/Bu* |
| Stem-cell source | PBSC | PBSC | BM | BM | PBSC | UCB | PBSC | PBSC |
| CD34+ cells/kg infused | 3.5 × 106 | 7.3 × 106 | 3.5 × 106 | 4.72 × 106 | 9.8 × 106 | 0.38 × 106 | 3.4 × 106 | 12.9 × 106 |
| GVHD prophylaxis | Tac/Pred | Tac/Pred | CsA/MMF | CsA/MMF | Tac/MTX | Tac/Rapa | Tac/MTX | Tac/Rapa |
Pred indicates prednisone; CsA, cyclosporine; MMF, mycophenylate mofetil; MTX, methotrexate; Rapa, rapamycin; Tac, tacrolimus; PBSC, peripheral-blood–derived stem cells; BM, bone marrow; and UCB, umbilical cord blood.
Fludarabine, 30 mg/m2 × 4 days; busulfex, 3.2 mg/kg
Fludarabine, 30 mg/m2 × 4 days; busulfan, 6.4 mg/kg; antithymocyte globulin, 30 mg/kg/dose IV daily × 5 days; and total lymphoid irradiation, 500 cGy with shielding of the liver, lungs, heart, and gonads
Heparinized blood and bone marrow aspirate samples from the patients were obtained prior to and at various times after transplantation, upon enrollment into institutional review board (IRB)–approved research protocols at the Dana-Farber Cancer Institute (Boston, MA) and the Children's Hospital (Pittsburgh, PA). Peripheral-blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) from healthy donors and patients were isolated by Ficoll/Hypaque density gradient centrifugation, cryopreserved with 10% DMSO (dimethyl sulfoxide), and stored in vapor phase liquid nitrogen until the time of analysis. Heparinized blood and bone marrow aspirate samples from the patients were obtained prior to and at various times after transplantation, upon enrollment in the IRB-approved research protocols at Dana-Farber Cancer Institute and Children's Hospital of Pittsburgh. Informed consent was provided according to the Declaration of Helsinki.
RNA extraction and reverse transcription
RNA was extracted from 20 × 106 mononuclear cells by the single-step acid guanidinium thiocyanate/phenol/chloroform method (Trizol) according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). First-strand cDNA was generated from 2 μg of total RNA using random hexanucleotides (Pharmacia LKB Biotechnology, Picscataway, NJ) and reverse transcriptase (Superscript; GIBCO BRL, Gaithersburg, MD).
Assessment of chimerism in genomic DNA
Genomic DNA was extracted from 1 to 3 × 106 PBMCs or BMMCs according to the manufacturer's protocol (Wizard Genomic DNA Purification Kit; Promega, Madison, WI). Prior to amplification, all DNA samples were quantified by ultraviolet (UV) spectrophotometry and diluted to working concentrations. Chimerism analysis of genomic DNA was performed at the Brigham and Women's Hospital Tissue Typing Laboratory by analysis of short tandem repeats (STR) (AmpFlSTR Profiler Plus Kit; Applied Biosystems, Foster City, CA).10,11 Alternatively, genomic DNA was concurrently measured using beta-globin pyrosequencing, with genomic DNA-specific polymerase chain reaction (PCR) primers.6
β-globin RNA pyrosequencing assay
Erythroid lineage-specific chimerism was monitored by β-globin RNA pyrosequencing as previously described.6 Briefly, PCR was performed using primers specific for β-globin cDNA (reverse transcribed from RNA), such that the amplicon encompassed the β-globin locus polymorphism (H3H) or mutation (sickle) of interest. For each set of primers, one of the primers was biotinylated for the pyrosequencing reaction. Each 50-μL reaction mixture contained 3 μL cDNA and the following concentrations of other components: 1 X Taq Gold buffer (Applied Biosystems), 3 mM MgCl2, 400 nmol each primer, 200 nmol dATP, dCTP, dGTP, and dTTP, and 2 units AmpliTaq Gold DNA polymerase (Applied Biosystems). One cycle of denaturation (95°C for 10 minutes) was followed by 46 cycles of PCR (94°C for 30 seconds, 62°C for 30 seconds, 72°C for 30 seconds), and finally, extension at 72°C for 10 minutes.
For pyrosequencing, biotinylated single-strand DNA fragments were generated by mixing the PCR product with streptavidin-coated paramagnetic beads (Dynalbeads M280; Dynal, Oslo, Norway) and processed according to the manufacturer's instructions (Pyrosequencing AB, Uppsala, Sweden). Throughout the sample preparation steps, the immobilized fragments coupled to the beads were moved using a magnetic tool (PSQ 96 Sample Preparation Tool; Pyrosequencing AB). An automated pyrosequencing instrument, PSQ96 (Pyrosequencing AB, Uppsala, Sweden), was used to determine cDNA sequence, following analysis using Pyrosequencing software (Pyrosequencing AB).
Flow cytometric analysis and sorting of immature and mature erythroblast populations
Mononuclear cells were isolated from fresh bone marrow aspirate by Ficoll-Hypaque density centrifugation and stained with fluorophore-conjugated monoclonal antibodies specific for glycophorin A (anti-GYPA-PE, Immunotech, Fullerton, CA) and CD71 (anti–CD71-FITC; Becton Dickinson, Franklin Lakes, NJ). Isolation of GYPA+, CD71+ cells and GYPA+ and CD71- cells was performed by cell sorting (EPICS Altra, Beckman Coulter, Miami, FL) and confirmed to be more than 91% pure. Genomic DNA was generated from the isolated cells and submitted for chimerism analysis.
Quantitation of erythroid precursor engraftment
Immunohistochemistry was performed on paraffin-embedded bone marrow trephine biopsies with anti–A- or B-antigen monoclonal antibodies (DAKO, Carpinteria, CA) after heat-induced epitope retrieval of sections in 10 mM citrate buffer. Positive staining was visualized with horseradish peroxidase–conjugated antimurine antibodies (Envision plus kit; DAKO) and diamino-benzidine (DAB) chromogen (DAKO). Nuclei were counterstained with Harris hematoxylin. Immature and more mature nucleated erythroid populations were enumerated and scored for the presence or absence of membrane staining under direct visual inspection by a hematopathologist (J.L.K.). At least 200 cells were scored from multiple high-power fields in this analysis.
Results
Stable mixed hematopoietic chimerism detected by analysis of genomic DNA
Mixed hematopoietic chimerism is frequently observed following NMA-SCT, and this was observed in 4 SCD and 4 non-SCD patients (Table 1). As shown in Figure 1A-B (dotted lines), conventional assessment of peripheral-blood genomic DNA (gDNA) using conventional STR-based assays demonstrated engraftment of donor stem cells and relatively stable levels of donor chimerism during the period of analysis. The level of donor engraftment in genomic DNA was assessed to be 25%, 50%, 50%, and 85% in SCD patients 1 to 4, respectively. The level of donor engraftment in non-SCD patients 5 to 8 was 50%, 18%, 51% to 66%, and 20% to 44%, respectively. The maximal level of engraftment in all 4 SCD patients was established by approximately day 30 and was maintained at a stable level during the duration of graft-versus-host disease (GVHD) prophylactic immunosuppressive therapy.
Higher expression of donor-derived genomic DNA in peripheral blood from SCD patients. Peripheral blood genomic DNA and beta-globin RNA donor chimerism were compared between patients with SCD (A) and without SCD (B). ▪ indicates genomic DNA chimerism;  , Beta-globin RNA chimerism.
, Beta-globin RNA chimerism.
Higher expression of donor-derived genomic DNA in peripheral blood from SCD patients. Peripheral blood genomic DNA and beta-globin RNA donor chimerism were compared between patients with SCD (A) and without SCD (B). ▪ indicates genomic DNA chimerism;  , Beta-globin RNA chimerism.
, Beta-globin RNA chimerism.
Higher expression of donor-derived β-globin RNA compared with donor-derived genomic DNA in peripheral blood from SCD patients
Despite only partial engraftment of donor cells after transplantation in SCD patients, we observed significant improvement of sickle cell disease–related clinical symptoms. This was accompanied by a decrease in the percentage of hemoglobin S (% Hb S) on serial measurements by hemoglobin electrophoresis. As shown in Table 2, all 4 donors had SA hemoglobin and Hb S values ranged from 0% to 40%. Prior to transplantation, the Hb S levels of patients 1, 2, and 4 were more than 75%, consistent with their diagnosis of SS. Hb S levels in patient 3 were only approximately 40% because of chronic transfusion therapy prior to transplantation. By day 100, Hb S levels for all 4 patients were similar to donor levels, suggesting the near-complete replacement of the patient's mature erythrocytes with RBCs of donor origin. None of the SCD patients received red blood cell transfusions after day 30.
Change in hemoglobin electrophoresis profile following transplantation
Patient . | Donor Hb S . | Pretransplant Hb S . | Posttransplant Hb S (day 100) . |
|---|---|---|---|
| SCD patient 1 | 37.5* | 78 | 29.8 |
| SCD patient 2 | 35.2 | 96.6 | 33.4 |
| SCD patient 3 | 40.3 | 40.1 | 32.4 |
| SCD patient 4 | 0 | 76.4 | 3.8 |
Patient . | Donor Hb S . | Pretransplant Hb S . | Posttransplant Hb S (day 100) . |
|---|---|---|---|
| SCD patient 1 | 37.5* | 78 | 29.8 |
| SCD patient 2 | 35.2 | 96.6 | 33.4 |
| SCD patient 3 | 40.3 | 40.1 | 32.4 |
| SCD patient 4 | 0 | 76.4 | 3.8 |
%Hb S determined by hemoglobin electrophoresis
Marked improvements measured by hemoglobin electrophoresis were consistent with assessment of erythroid-lineage chimerism shown in Figure 1A. We previously reported that β-globin RNA pyrosequencing can be used to monitor erythroid lineage-specific engraftment.6 This method quantitatively sequences coding region polymorphisms in β-globin RNA that distinguish recipient from donor RNA. By β-globin pyrosequencing of peripheral blood, we consistently observed up to 2-fold higher expression of donor-derived β-globin RNA than the level of genomic DNA chimerism in SCD patients. Thus, as shown in Figure 1A, patient 1 developed 25% overall donor engraftment at day 30, however, 60% to 70% of peripheral-blood β-globin RNA was donor derived. Similarly, patients 2 and 3 both developed 50% donor genomic DNA chimerism in peripheral-blood mononuclear cells, which was associated with 100% donor chimerism in peripheral-blood β-globin RNA. Likewise, patient 4 expressed 100% donor β-globin RNA when total donor peripheral-blood mononuclear-cell engraftment was 85%. These results confirm predominant to full replacement of peripheral-blood erythrocytes with donor cells in all 4 SCD patients and explain their significant clinical improvement despite only partial donor engraftment.
The chimerism results in SCD patients contrasted with 4 non-SCD patients who underwent similar conditioning regimens and also developed mixed hematopoietic chimerism. Patients 5 to 8 were disparate for the β-globin SNP 3H3 compared to their donors, and thus erythroid lineage-specific chimerism could be assessed despite the absence of the sickle mutation. Unlike patients with SCD, patients 5 through 8 developed levels of erythroid-lineage chimerism that were very similar to the levels of overall genomic chimerism. As shown in Figure 1B, patient 5 developed approximately 50% donor gDNA and β-globin RNA chimerism by day 30, which was stable up to day 100. Likewise, patient 5 developed 10% to 20% donor gDNA and β-globin RNA chimerism between days 30 and 60. Patients 7 and 8 developed gradually rising levels of donor genomic DNA chimerism between days 30 and 90 that were mirrored by similar rises in the level of β-globin RNA chimerism. Since large differences between overall genomic and RBC-specific chimerism were consistently observed only in the SCD patients, our observations support the notion that the observed disparity in chimerism is a SCD-specific effect.
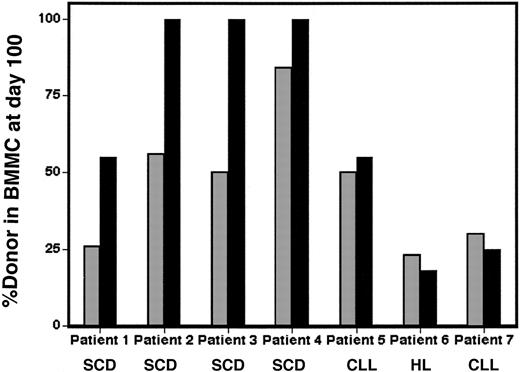
Recipient SS erythroblasts are selectively lost within the intramedullary space. Genomic DNA (▦) and β-globin RNA (▪) donor chimerism derived from bone marrow mononuclear cells (BMMCs) were compared between patients with SCD (patients 1-4) or other diseases (patients 5-8) at day 100 following allogeneic stem cell transplantation.
Recipient SS erythroblasts are selectively lost within the intramedullary space. Genomic DNA (▦) and β-globin RNA (▪) donor chimerism derived from bone marrow mononuclear cells (BMMCs) were compared between patients with SCD (patients 1-4) or other diseases (patients 5-8) at day 100 following allogeneic stem cell transplantation.
Analysis of marrow RBC chimerism reveals ineffective erythropoiesis in SCD
While the disparity between the posttransplant levels of erythroid lineage-specific RNAand peripheral-blood nucleated-cell–derived DNA in SCD patients could be explained by the known short half-life of sickle erythrocytes in the peripheral circulation,12,13 our analysis of marrow chimerism revealed the contribution of a pathogenic mechanism that occurred earlier in erythroid differentiation. When DNA chimerism was measured in bone marrow at multiple time points in 7 of 8 patients by conventional STR analysis, this was found to be consistently similar to overall peripheral-blood chimerism. As shown in Figure 2, the percentage of donor bone marrow mononuclear chimerism (gray bars) was 23%, 55%, 52%, and 85% for patients 1 through 4, respectively, and 50%, 18%, and 30% for patients 5 through 7, respectively. As in peripheral blood, levels of donor-derived β-globin RNA in marrow relative to the level of donor-derived gDNA differed markedly between non-SCD and SCD patients. As shown in Figure 2, β-globin RNA pyrosequencing of marrow samples derived from non-SCD patients 5 through 7 revealed equivalent levels of donor-derived β-globin RNA compared to donor-derived gDNA. However, in the SCD patients, marrow donor β-globin RNA expression approached the elevated levels observed in peripheral blood. For example, only 25% of marrow mononuclear-cell DNA in patient 1 was donor derived, but 55% of marrow β-globin RNA was of donor origin. Similarly, for patients 2 through 4, 100% of marrow β-globin RNA was of donor origin, despite having only 50% (patients 2 and 3) or 85% (patient 4) donor gDNA. These results suggest that recipient SS erythroblasts are selectively lost within the intramedullary space as well as in the peripheral circulation.
Loss of SS erythroblasts occurs early in the erythroid maturation sequence
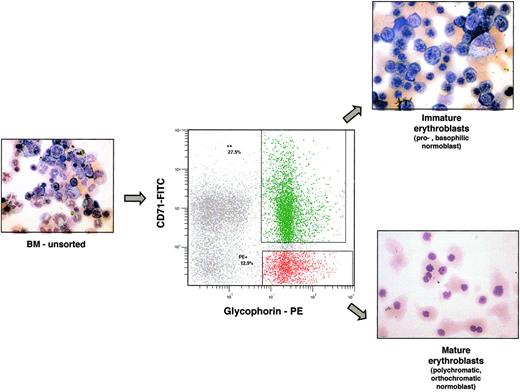
To determine the stage of erythrocyte maturation at which sickle erythroblasts are lost, flow cytometric cell sorting of marrow samples was used to identify and separate erythroblasts at different levels of maturation. This approach has been previously developed in murine systems14 using the phenotypic markers Ter 119 and CD71. We adapted this method for analysis of human marrow. Bone marrow mononuclear cells were stained with monoclonal antibodies specific for glycophorin A (GYPA) and transferrin receptor (CD71). As erythroblasts mature, GYPA increases and CD71 decreases. Thus, the earliest erythroblasts (pronormoblasts) are CD71 positive but glycophorin A dim, more mature erythroblasts (basophilic normoblasts) stain doubly positive for CD71 and GYPA, and the most differentiated erythroblasts (polychromatophilic and orthochromatophilic normoblasts) are GYPA-positive but CD71 negative. We confirmed that these “immature” and “mature” erythroblast populations can be separated using 2-color flow cytometry by direct light microscopic inspection of May-Grunwald Giemsa–stained cell-sorted populations. As shown in Figure 3, the double-positive GYPA and CD71 populations contain basophilic normoblasts and some pronormoblasts, while the GYPA single-positive cells consist entirely of more mature normoblasts.
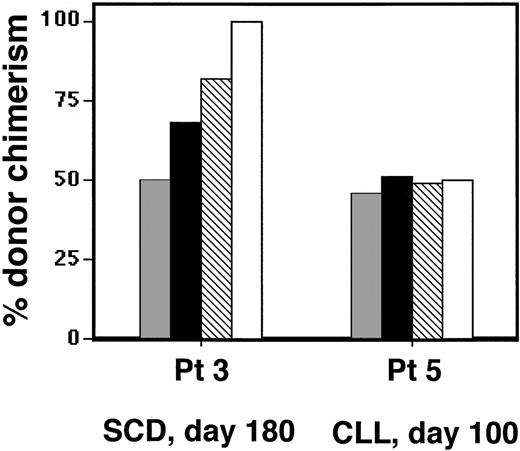
When immature and mature erythroblast populations were isolated from marrow of non-SCD patients 5 and 6 at days 60 to 100 after transplant, and chimerism was assessed by genomic analysis, we found no difference in erythroid-lineage donor chimerism between the unsorted, purified immature, and purified mature erythroblasts. As shown in Figure 4, the unsorted, GYPA+/CD71+, and GYPA+/CD71- populations isolated from non-SCD patient 5 all revealed approximately 48% to 52% donor chimerism. Similarly, donor chimerism in unsorted, immature, and mature marrow erythroblasts from patient 6 all were approximately 8% to 14% (data not shown). In contrast, the same analysis performed on patient 3's day 180 marrow demonstrated that erythroblast maturation was associated with increased representation of donor-derived cells. Whereas the unsorted marrow demonstrated 51% donor chimerism, 68% of the immature erythroblasts were donor derived, and 82% of the mature sorted population was donor derived. Circulating nucleated RBCs in peripheral blood obtained at the same time were 100% donor. Similar analysis, performed on immature erythroblasts isolated from patients 1 and 2, revealed that 40% and 74% of cells were donor derived, compared with overall donor marrow chimerism of 25% and 47%, respectively.
The number of donor-derived erythroid-lineage immature and mature populations also were directly enumerated from anti-ABO antigen–stained paraffin sections of marrow from patients 1, 2, 5, and 6 (who had ABO-mismatched donors). As shown in Table 3, immature and mature erythroblast populations in patients 5 and 6 had similar levels of donor chimerism. In contrast, patients 1 and 2 showed increased donor representation as erythroblasts transitioned from immature (54% and 53%, respectively) to mature states (83% and 76%, respectively). Taken together, these results directly demonstrate that loss of SS-derived erythroblasts occurs at the early stages of erythroblast maturation within the marrow milieu, resulting in ineffective erythropoiesis.
Increased donor representation with SS erythroblast maturation, as enumerated by ABO antibody staining of bone marrow biopsies
. | Donor early erythroblasts (%) . | Donor late erythroblasts (%) . |
|---|---|---|
| SCD patient 1 | 52 of 97 (54%) | 130 of 156 (83%) |
| SCD patient 2 | 24 of 45 (53%) | 110 of 145 (76%) |
| Non-SCD patient 5 | 19 of 30 (63%) | 73 of 105 (69%) |
| Non-SCD patient 6 | 23 of 67 (34%) | 40 of 121 (33%) |
. | Donor early erythroblasts (%) . | Donor late erythroblasts (%) . |
|---|---|---|
| SCD patient 1 | 52 of 97 (54%) | 130 of 156 (83%) |
| SCD patient 2 | 24 of 45 (53%) | 110 of 145 (76%) |
| Non-SCD patient 5 | 19 of 30 (63%) | 73 of 105 (69%) |
| Non-SCD patient 6 | 23 of 67 (34%) | 40 of 121 (33%) |
Discussion
Sickle cell disease is a well-characterized RBC genetic disorder that generates tremendous heterogeneity in clinical phenotype. The pathophysiology of SCD has been primarily explained in terms of oxygen-dependent polymerization of sickle hemoglobin followed by sickling of erythrocytes. Because the extent of sickling has been thought to be related to the intracellular concentration of sickle hemoglobin, sickling has been considered to occur primarily in mature erythrocytes, which have a high intracellular hemoglobin concentration.
Early and late erythroblasts can be identified and isolated by flow cytometry. Immunophenotyping of marrow mononuclear cells with glycophorin A-PE and CD71-FITC can identify immature from mature erythroblasts, as confirmed by histochemical staining of the sorted populations (inset photographs). Samples were analyzed using an Olympus BX41 microscope equipped with a UPlanFL 40×/0.75 objective lens (Olympus, Melville, NY). Pictures were taken with Olympus QColor3, and were analyzed with QCapture 2.60 software (QImaging, Burnaby, BC, Canada) and Adobe Photoshop 6.0 software (Adobe, San Jose, CA).
Early and late erythroblasts can be identified and isolated by flow cytometry. Immunophenotyping of marrow mononuclear cells with glycophorin A-PE and CD71-FITC can identify immature from mature erythroblasts, as confirmed by histochemical staining of the sorted populations (inset photographs). Samples were analyzed using an Olympus BX41 microscope equipped with a UPlanFL 40×/0.75 objective lens (Olympus, Melville, NY). Pictures were taken with Olympus QColor3, and were analyzed with QCapture 2.60 software (QImaging, Burnaby, BC, Canada) and Adobe Photoshop 6.0 software (Adobe, San Jose, CA).
In the current study, we present definitive in vivo evidence of ineffective erythropoiesis in patients with severe SCD. We use the development of mixed hematopoietic chimerism in SCD patients following nonmyeloablative transplant as a model system to directly compare SA and SS erythropoiesis. By employing 2 independent methods to assess erythroid-lineage chimerism, the extent to which SS or SA erythroblasts develop and mature within the same marrow milieu was directly measured. Our studies demonstrate that compared to SA erythroblasts, SS erythroblasts are at a competitive disadvantage and that low levels of donor (SA) erythroid engraftment result in predominantly donor representation in maturing erythroblasts and among mature erythrocytes. In support of the notion that this is SCD specific, this effect was observed in all 4 SCD patients studied, irrespective of conditioning regimens, and not in the control patients with hematologic malignancies.
Because our studies are undertaken in the context of allogeneic hematopoietic SCT, it is important to consider whether these observations may be influenced by transplant-related factors. Since all recipients received conditioning therapy prior to SCT, it is theoretically possible that differential sensitivity of SS erythroid precursors to chemotherapy could account for superior maturation and differentiation of donor stem cells. However, if chemotherapy-induced injury were solely responsible for the difference between recipient and donor erythropoiesis, this would be expected to be greatest early following transplant and to diminish over time. Instead, reduced maturation of SS red-cell precursors was relatively stable and persisted for long periods of up to 6 months following transplant. Another potential explanation for reduced levels of recipient erythropoiesis could be selective immune destruction of SS erythroid precursors by the donor immune system. Previous studies have documented that donor antibodies can target recipient RBC antigens, especially in cases of ABO incompatibility, and that this can result in selective hemolysis of recipient RBC and RBC precursors.15,16 GVHD can also target recipient hematopoietic stem cells.17-21 However, none of the patients in this report had evidence of immune-mediated hemolysis despite the presence of ABO incompatibilities in 6 of the 8 patients. Moreover, none of the patients had evidence of GVHD, and it is therefore very unlikely that selective depletion of maturing recipient RBCs was caused by alloimmune responses directed against these cells.
Comparison of donor chimerism. Comparison of donor chimerism between unsorted (▦), immature (▪), and mature (▧) marrow erythroblasts isolated from marrow and peripheral circulating nucleated RBCs (□) between SCD patient 3 and non-SCD patient 5.
Comparison of donor chimerism. Comparison of donor chimerism between unsorted (▦), immature (▪), and mature (▧) marrow erythroblasts isolated from marrow and peripheral circulating nucleated RBCs (□) between SCD patient 3 and non-SCD patient 5.
Still another potential factor that could have impact on extent of host erythropoiesis would be the presence of concurrent defects of hemoglobin synthesis. The alpha globin deletions -3.7 and -4.2 are common in the African-American population, with a prevalence of 20% to 30%. Of the 4 SCD patients analyzed, only 1 was a heterozygote for the -α3.7 mutation (patient 2, by gap PCR, data not shown). While highly prevalent, the α-thalassemia syndromes are generally acknowledged to generate hemolytic anemia and would not be expected to contribute to the ineffective erythropoiesis that we observed in our 4 SCD patients.22 Consistent with this notion, no differences in the patterns of overall versus red-cell–lineage chimerism were observed between patient 2 and patients 1, 3, and 4.
The presence of ineffective erythropoiesis in SCD is supported by previous studies, which have identified structural abnormalities in SS erythroid precursor cells, thus indirectly indicating the increased susceptibility of these cells for clearance and loss. Blouin et al examined erythropoiesis in the SAD mouse model and found significant morphologic alteration in erythroid lineage late precursors (polychromatophilic normoblasts) within the marrow.23 These morphologic studies identified high levels of hemoglobin polymers that were associated with increased cell fragmentation occurring during medullary transendothelial migration of reticulocytes. Older ultrastructural studies of bone marrow aspirates derived from SCD patients identified reticulocytes that contained bundles of hemoglobin S polymers in the absence of intentional deoxygenation, as well as sickling of nucleated erythroblasts and extensive marrow erythrophagocytosis.24,25 In an in vitro system, Hasegawa et al also found that cultured nucleated erythroid precursors (orthochromatophilic normoblasts) can undergo sickling under deoxygenating conditions.26 Older studies of transfused SCD patients have revealed an inappropriately low reticulocyte response that was out of proportion with the degree of erythroid hyperplasia, suggesting compromise in the exiting of sickle RBCs from marrow.27-29 Consistent with these observations, Finch et al performed ferrokinetic measurements of erythropoiesis on a continuously transfused woman with severe SCD, which revealed high radioactive iron uptake in the erythroid marrow, of which a disproportionately large quantity was not subsequently released into circulating RBCs. They hypothesized that in the setting of chronic transfusion, intramedullary erythroid maturation would occur relatively slowly and thereby increase the amount of sickle hemoglobin within the marrow, which would result in a mechanical type of ineffective erythropoiesis.30 Finally, apoptosis, measured by annexin-V staining, is a known mechanism of intramedullary clearance of ineffective erythroblasts in thalassemia,31 and similar studies undertaken to analyze peripheral-blood samples of SCD patients have indicated substantial annexin-V staining in circulating immature erythrocytes in peripheral blood, suggesting some degree of abnormally elevated apoptotic activity in the marrow of SCD patients.32,33 Taken together, these studies have identified that significant abnormalities in SS erythroid precursors exist within the intramedullary space and that cells prone to sickling may be selectively destroyed prior to release from the erythropoietic compartment. Although prior studies have primarily pointed to abnormalities in late erythroid precursors and implicate the degree of hemoglobinization as a critical factor in the susceptibility to damage, our detailed chimerism analysis presents the novel finding that this loss appears to occur even earlier in erythroid development than previously appreciated. Our analysis of immature, basophilic erythroblast–enriched populations by both molecular chimerism analysis and by direct visual inspection of marrow sections stained with a donor-specific RBC marker revealed increased donor representation relative to overall nucleated-cell marrow chimerism. These results suggest that the degree of ineffective erythropoiesis in SCD is more profound than previously appreciated and probably reflects a different mechanism than intramedullary sickling.
Our findings have several important clinical implications. First, our results resolve how even low levels of incomplete donor engraftment of erythroid precursors can be functionally effective. Although all 3 of the SCD patients studied developed partial rather than complete donor engraftment following transplant, all demonstrated marked improvement in the clinical symptoms of sickle cell disease. Older studies from SCD patients treated with myeloablative transplant have suggested that as little as 10% donor chimerism may be sufficient to reduce symptoms associated with severe sickle cell disease.5 In thalassemia, in which ineffective erythropoiesis is appreciated to play a substantial role in its pathophysiology, the curative potential of posttransplant mixed hematopoietic chimerism also has been clearly observed.34,35 While peripheral destruction of SS erythrocytes undoubtedly contributes to the increased donor representation in the peripheral blood that we detected by our erythroid lineage-specific chimerism assays, the presence of host-ineffective erythropoiesis more fully explains why mixed chimerism so effectively corrects the RBC defect in these patients. Since full replacement of mature erythrocytes can be achieved with relatively low amounts of donor erythroid progenitors, these studies strongly support mixed hematopoietic chimerism as a reasonable end point of hematopoietic stem cell transplantation, provided that novel conditioning regimens that can consistently establish stable mixed hematopoietic chimerism can be generated. Moreover, our findings suggest that stem-cell–based approaches to treatment of sickle cell disease, including gene therapy, may have a greater impact on the correction of disease than expected.
Second, SCD is a disease of great clinical heterogeneity, and a central challenge in the clinical care of SCD patients is the early identification of patients at high risk for severe disease who would benefit from earlier institution of aggressive therapy. The biologic basis of this heterogeneity in phenotypic disease expression despite a common genotype is still not well understood and is the active subject of ongoing investigation.36-40 We speculate that ineffective erythropoiesis may contribute to this phenotypic diversity, and prospective studies will be required to both define the mechanism of ineffective erythropoiesis in SCD and to explore the relationship between extent of ineffective erythropoiesis and clinical disease severity in patients with SCD. Some murine studies have demonstrated that despite the detection of intramedullary ineffective erythropoiesis, a compensatory hematopoietic mechanism is present. Blouin et al characterized this as both a bone marrow response, in which there is increased proliferation of granulocyte-erythrocyte-megakaryocyte-macrophage colony-forming unit (CFU-GEMM) and erythroid progenitor cells (erythroid burst-forming unit [BFU-E] and erythroid colony-forming unit [CFU-E]) as well as increase peripheral mobilization of multipotent cells and splenic response.23,41 Kean et al identified peripheral destruction rather than ineffective erythropoiesis as the primary mechanism of anemia in SCD based on RBC biotinylation studies in which erythropoietic demand was compensated through splenic erythroid hyperplasia.41 If similar or alternative compensatory mechanisms likewise operate in human patients, then the ineffective erythropoiesis of SCD may not be clinically relevant outside the context of hematopoietic stem cell transplantation.42 For example, Croizat et al have identified a highly significant inverse correlation between Hb F levels and the number of circulating BFU-E in patients with SCD.38,43 If, however, future studies demonstrate clinically significant ineffective erythropoiesis in SCD, this would have implications on the choice of both supportive treatments for this group of patients, such as therapies aimed at addressing iron use, as well as choice of therapeutic alternatives.
Prepublished online as Blood First Edition Paper, August 9, 2005; DOI 10.1182/blood-2005-04-1376.
Supported by National Institutes of Health grants KO8 HL04293, HL070149, and AI29530; the Ted and Eileen Pasquarello Research Fund; and the Doris Duke Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank David C. Fisher, MD; Michele Walsh, RN; Evan McEwing, and Vincent Ho, MD, for assistance in sample procurement. We thank John Daley for his expert help with flow cytometric analyses. We also thank Vuong Nguyen for skilled technical assistance. We thank David Chui, MD, and Hong-Yuan Luo, MD, for conducting the alpha-thalassemia mutational analysis. Finally, we thank David Golan, MD, Carlo Brugnara, MD, and Frank Bunn, MD, for helpful and insightful discussions. Dr Wu is a recipient of a Doris Duke Clinical Scientist Development Award.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal