Abstract
Chromosomal rearrangements affecting RUNX1 and CBFB are common in acute leukemias. These mutations result in the expression of fusion proteins that act dominant-negatively to suppress the normal function of the Runt-related transcription factor 1 (RUNX)/core binding factor β (CBFβ) complexes. In addition, loss-of-function mutations in Runt-related transcription factor 1 (RUNX1) have been identified in sporadic cases of acute myeloid leukemia (AML) and in association with the familial platelet disorder with propensity to develop AML (FPD/AML). In order to examine the hypothesis that decreased gene dosage of RUNX1 may be a critical event in the development of leukemia, we treated chimeric mice generated from Runx1lacZ/lacZ embryonic stem (ES) cells that have homozygous disruption of the Runx1 gene with N-ethyl-N-nitrosourea (ENU). We observed an increased incidence of T-lymphoblastic lymphoma in Runx1lacZ/lacZ compared with wild-type chimeras and confirmed that the tumors were of ES-cell origin. Our results therefore suggest that deficiency of Runx1 can indeed predispose mice to hematopoietic malignancies.
Introduction
AML1ETO, the fusion gene resulting from t(8;21) in acute myeloid leukemia (AML) subtype M2, and CBFB-MYH11, the fusion gene resulting from inv(16) in AML subtype M4 Eo, are both capable of blocking hematopoiesis in mouse models.1,2 Runx1+/ETO and Cbfb+/MYH11 heterozygous embryos die at embryonic day 12.5 (E12.5) to E13.5 from hemorrhages and defective hematopoiesis, similar to Runx1-/- and Cbfb-/- embryos.3-5 In vitro studies have demonstrated that the proteins encoded by these 2 fusion genes are capable of repressing the expression of Runt-related transcription factor 1 (Runx1)/core binding factor β (Cbfβ) target genes.6,7 These observations have led to the hypothesis that the fusion genes block hematopoietic differentiation and induce leukemia by inhibiting Runx/Cbfb activity in a dominant-negative manner. More recently, sporadic loss-of-function mutations, both monoallelic and biallelic, have been found in patients with AML.8,9 In addition, the disease familial platelet disorder with propensity to develop AML (FPD/AML) has been linked to heterozygous mutations in RUNX1.10,11 Together these studies have led to the hypothesis that inhibition of Runx1/Cbfb function is a critical step in the development of hematopoietic malignancies.
In this study, we used a mouse model to determine whether disruption of Runx1 expression predisposes mice to the development of hematopoietic malignancies. Chimeras generated with Runx1lacZ/lacZ embryonic stem (ES) cells developed lymphoma at increased incidence after treatment with N-ethyl-N-nitrosourea (ENU) to induce additional genetic changes. This finding provides strong evidence that Runx1-deficient cells are susceptible to malignant transformation.
Study design
Transgenic mice
Runx1lacZ/lacZ ES cells were generated from Runx1+/lacZ ES cells12 (Runx1tm2Spe/+) by growth in 4.0 mg/mL G418.13 Runx1lacZ/lacZ (LZD) and wild-type 129 (TC1) ES cells were used to generate chimeric mice using standard protocols. One-month-old mice were given a single dose of ENU (100 mg/kg) by intraperitoneal injection.14
Immunohistochemistry
Liver sections were stained with an anti-CD3 antibody and a peroxidase staining kit (DAKO, Carpinteria, CA). Spleen sections were stained with a rabbit anti–β-galactosidase antibody and a diaminobenzidine (DAB) substrate kit for peroxidase (Vector Laboratories, Burlingame, CA).
Pictures of the stained tissue sections were obtained using a Nikon Eclipse E800 microscope equipped with a Plan-Apo 40 ×/0.95 objective lens (Nikon, Melville, NY) and a Photometrics CoolSnap HQ camera (Roper Scientific, Tucson, AZ). Pictures were acquired through IPLab 3.5.5 software (Scanalytics, Fairfax, VA) and were organized for publication with Adobe Photoshop 6.0.1 (Adobe, San Jose, CA).
Flow cytometry
Thymocytes were stained with phycoerythrin (PE)–conjugated anti-CD4 and Cy-chrome–conjugated anti-CD8 (BD Pharmingen, San Diego, CA) and analyzed using FACSCalibur (BD Biosciences, San Diego, CA).
Southern blot analysis
Southern blot analysis was performed on DNA isolated from bone marrow cells, using a 400–base pair (bp) Runx1 intron 8 probe as described previously.12
Genomic PCR analysis
Thymocytes were stained with a PE-conjugated anti-CD4 and a fluorescein isothiocyanate (FITC)–conjugated anti-CD8 antibody (BD Pharmingen) and sorted with FACS Aria (Becton Dickinson, San Jose, CA). Bone marrow cells were isolated and sorted after staining with an FITC-conjugated anti–c-kit antibody and PE-conjugated antibodies against CD3, B220, Mac-1, Gr-1, and TER119 (BD Pharmingen). The DNA was extracted from the subpopulations. Polymerase chain reaction (PCR) was performed using 200 to 400 ng DNA and the following PCR conditions: 94°C for 4 minutes; 40 cycles of 94°C for 1 minute, 56°C for 1 minute, and 72°C for 2 minutes; 72°C for 4 minutes. The PCR primers used were LacZ forward (ACTGGCAGATGCACGGTTAC) and LacZ reverse (GTGGCAACATGGAAATCGCTG). Spleen sections effaced by lymphoma were treated with zylene and methanol to remove paraffin, and the genomic DNA was isolated using QIAamp DNA mini kit (QIAGEN, Valencia, CA). Primers for markers D7Mit44 and D11Mit188 were used for PCR genotyping to distinguish 129 from C57BL/6J cells.15
Results and discussion
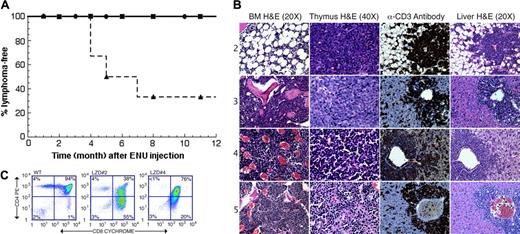
We generated chimeric mice using Runx1lacZ/lacZ ES cells and monitored for leukemia development. None of the mice developed malignancy (Figure 1A). We then treated them with a single injection of 100 mg/kg ENU. Chimeras generated with wild-type ES cells were used as controls. Due to technical difficulties, only a relatively small number of chimeras (8 for the test and 9 for the control groups, respectively) became available for the study. Four of the Runx1lacZ/lacZ chimeras developed lymphoblastic lymphoma within the first 12 months after ENU treatment. These 4 lymphomas were all derived from the ES cells (see below). Two mice died from unknown causes at 4 and 8 months after injection and were excluded from statistical calculation. During the same time period, one chimera generated with wild-type ES cells developed lymphoma. However, genotyping by PCR indicated that the lymphoma was derived from the C57BL/6J cells (data not shown). In addition, 2 other chimeras generated with wild-type ES cells died from unknown causes at 7 and 8 months after ENU injection. These 3 mice were excluded from the statistical analysis. Therefore, 4 of the 6 Runx1lacZ/lacZ chimeras developed lymphoma of ES-cell origin, whereas none of the 6 wild-type ES-cell chimeras did (Figure 1A). This represents a significant increase (P = .03, by Fisher exact test, one-sided) in the incidence of lymphoma in the Runx1lacZ/lacZ chimeras compared with wild-type chimeras.
Runx1lacZ/lacZ chimeras are more susceptible to developing T-lymphoblastic lymphoma than wild-type chimeras. (A) Kaplan-Meier survival curves (lymphoma-free) are shown for untreated Runx1lacZ/lacZ (n = 6) and Runx1lacZ/lacZ (n = 6) and wild-type (n = 6) chimeras treated with 100 mg/kg ENU. • indicates untreated Runx1lacZ/lacZ chimeras; ▪, wild-type ES-cell chimeras treated with ENU; ▴, Runx1lacZ/lacZ chimeras treated with ENU. (B) Hematoxylin and eosin (H&E) staining of thymus, bone marrow (BM), and liver from LZD nos. 2 through 5. Immunohistochemistry using an anti-CD3 antibody demonstrates that the malignant cells in the livers of these animals stained positively for CD3. (C) Flow cytometry of thymocytes from LZD no. 2 and LZD no. 4 using antibodies against CD4 and CD8 demonstrates that the majority of cells have an abnormal CD4+/CD8+ phenotype. WT indicates wild type. Numbers in each quadrant indicate the percentage of total thymocytes in that quadrant.
Runx1lacZ/lacZ chimeras are more susceptible to developing T-lymphoblastic lymphoma than wild-type chimeras. (A) Kaplan-Meier survival curves (lymphoma-free) are shown for untreated Runx1lacZ/lacZ (n = 6) and Runx1lacZ/lacZ (n = 6) and wild-type (n = 6) chimeras treated with 100 mg/kg ENU. • indicates untreated Runx1lacZ/lacZ chimeras; ▪, wild-type ES-cell chimeras treated with ENU; ▴, Runx1lacZ/lacZ chimeras treated with ENU. (B) Hematoxylin and eosin (H&E) staining of thymus, bone marrow (BM), and liver from LZD nos. 2 through 5. Immunohistochemistry using an anti-CD3 antibody demonstrates that the malignant cells in the livers of these animals stained positively for CD3. (C) Flow cytometry of thymocytes from LZD no. 2 and LZD no. 4 using antibodies against CD4 and CD8 demonstrates that the majority of cells have an abnormal CD4+/CD8+ phenotype. WT indicates wild type. Numbers in each quadrant indicate the percentage of total thymocytes in that quadrant.
Runx1lacZ/lacZ chimeras that developed lymphoblastic lymphoma (LZD nos. 2, 3, 4, and 5) had enlarged thymuses that were completely effaced by the malignant cells (Figure 1B). There was neoplastic infiltration in the lungs, liver (Figure 1B), kidney, spleen (Figure 2A), and bone marrow (Figure 1B). Staining of liver sections with anti-CD3 antibody revealed that the lymphoma was of T-cell origin (Figure 1B). Flow cytometric analysis revealed coexpression of CD4 and CD8 (Figure 1C), a common phenotype for T-lymphoblastic lymphoma.16
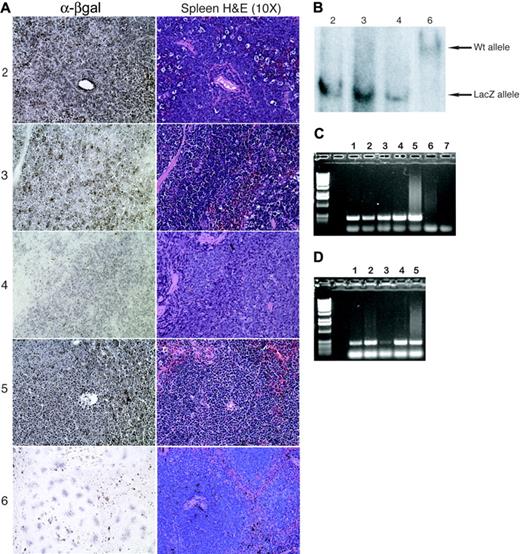
To verify the ES-cell origin of the lymphoblastic lymphomas that developed in the Runx1lacZ/lacZ chimeras, we stained spleen sections for β-galactosidase and performed Southern blot hybridization with DNA isolated from bone marrow cells. The spleens of Runx1lacZ/lacZ chimeras that developed lymphoma were completely effaced by β-galactosidase–positive tumor cells (Figure 2A), indicating their ES-cell origin. In LZD nos. 2, 3, and 4, the bone marrow was completely effaced by lymphoma and only the targeted LacZ allele was detected by Southern blot hybridization (Figure 2B), confirming that the malignant cells were derived from ES cells. On the other hand, only the wild-type allele was detected in the bone marrow of LZD no. 6 (unaffected; Figure 2B). These results suggest that the 4 T-lymphoblastic lymphomas that developed within 7 months were of ES-cell origin.
T-lymphoblastic lymphoma is of ES-cell origin in Runx1lacZ/lacZ chimeras. (A) Immunohistochemistry using an anti–β-galactosidase antibody demonstrates that the malignant cells in the spleens of LZD nos. 2 through 5 express β-galactosidase and are therefore derived from Runx1lacZ/lacZ ES cells. The normal splenocytes from LZD no. 6 (which did not develop lymphoma) serve as negative controls. (B) Southern blot analysis detects presence of only the Runx1-LacZ knock-in allele in lymphoma-effaced bone marrow from LZD nos. 2 through 4 but only wild-type Runx1 allele from normal bone marrow of LZD no. 6. (C) Genomic PCR using LacZ gene primers and DNA from sorted thymic cells of a Runx1lacZ/lacZ chimera. Lane 1, CD8+; lane 2, CD8+/CD4+; lane 3, CD4-/CD8-; lane 4, CD4+; lane 5, total thymus; lane 6, total thymus from a wild-type mouse; lane 7, no DNA. (D) Genomic PCR using LacZ gene primers and DNA from sorted bone marrow cells of a Runx1lacZ/lacZ chimera. Lane 1, c-kit+/lin-; lane 2, c-kit+/lin+; lane 3, c-kit-/lin+; lane 4, c-kit-/lin-; lane 5, total bone marrow.
T-lymphoblastic lymphoma is of ES-cell origin in Runx1lacZ/lacZ chimeras. (A) Immunohistochemistry using an anti–β-galactosidase antibody demonstrates that the malignant cells in the spleens of LZD nos. 2 through 5 express β-galactosidase and are therefore derived from Runx1lacZ/lacZ ES cells. The normal splenocytes from LZD no. 6 (which did not develop lymphoma) serve as negative controls. (B) Southern blot analysis detects presence of only the Runx1-LacZ knock-in allele in lymphoma-effaced bone marrow from LZD nos. 2 through 4 but only wild-type Runx1 allele from normal bone marrow of LZD no. 6. (C) Genomic PCR using LacZ gene primers and DNA from sorted thymic cells of a Runx1lacZ/lacZ chimera. Lane 1, CD8+; lane 2, CD8+/CD4+; lane 3, CD4-/CD8-; lane 4, CD4+; lane 5, total thymus; lane 6, total thymus from a wild-type mouse; lane 7, no DNA. (D) Genomic PCR using LacZ gene primers and DNA from sorted bone marrow cells of a Runx1lacZ/lacZ chimera. Lane 1, c-kit+/lin-; lane 2, c-kit+/lin+; lane 3, c-kit-/lin+; lane 4, c-kit-/lin-; lane 5, total bone marrow.
Since there is an early block in hematopoiesis in Runx1lacZ/- embryos,12 it is somewhat puzzling that there are hematopoietic Runx1lacZ/lacZ cells in the adult chimeras that can give rise to lymphoma. It is likely that similar to Cbfb+/MYH11 chimeras,14 some Runx1lacZ/lacZ precursors are able to survive in the context of a chimeric animal. To address this possibility, we analyzed the contribution of Runx1lacZ/lacZ ES cells to the hematopoietic compartments in untreated healthy Runx1lacZ/lacZ chimeras. By genomic DNA PCR we were able to detect the presence of ES cell–derived cells in all populations from the thymus (Figure 2C). ES cell–derived cells were also detected by PCR in the c-kit+/lin- and c-kit+/lin+ subpopulations in the bone marrow but were barely or not at all detectable in the c-kit-/lin+ population (Figure 2D). However, the overall hematopoietic contribution from the ES cells must be small, since by Southern blot we can only detect the wild-type allele in LZD no. 6, an ENU-treated Runx1lacZ/lacZ chimera that did not develop neoplasia (Figure 2B). In addition, there was no significant β-galactosidase staining of the spleen sections from LZD no. 6 (Figure 2A) and 2 untreated healthy Runx1lacZ/lacZ chimeras (data not shown). The results suggest that Runx1lacZ/lacZ ES cells gave rise to hematopoietic stem cells and early progenitors, but later stages of differentiation were blocked.
It is somewhat unexpected that T-lymphoblastic lymphoma was induced in the Runx1lacZ/lacZ chimeras, since RUNX1 deficiency is more commonly associated with AML. However, there is one report of a translocation involving RUNX1 in a 12-year-old boy with T-cell acute lymphoblastic leukemia,17 suggesting that defects in RUNX1 expression can be associated with T-cell malignancies in humans as well as in mice. While our results may reflect some of the biologic differences between humans and mice, it is also possible that Runx1 deficiency is capable of inducing both myeloid and lymphoid malignancies, and myeloid leukemia would have been observed with a larger set of mice or higher dose of ENU. In one recent report, a transgenic mouse model was used to determine if expression of AML1-ETO can induce leukemia in mice.18 The AML1-ETO transgenic mice developed both AML and T-cell lymphoma (55% and 45%, respectively) after treatment of ENU at a relatively high dose (300 mg/kg), whereas 100% of the wild-type controls developed lymphoma.
Our results suggest that Runx1lacZ/lacZ -derived cells are more susceptible than wild-type cells to developing lymphoblastic lymphoma after chemical mutagenesis. Although we cannot exclude the possibility that the Runx1-lacZ fusion gene exerts some novel activity, the phenotype of Runx1lacZ/- embryos is identical to that of Runx1-/- embryos with embryonic lethality between E12.5 and E13.5 from hemorrhages and absence of definitive hematopoiesis.12 Therefore, our results suggest that Runx1 deficiency can predispose to development of hematologic malignancies in mice. Additional studies in Runx1-/- chimeras or Runx1 conditional knockout mice will be needed to confirm the findings reported here.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-04-1447.
M.K. is a recipient of a Damon-Runyon Cancer Research Fund Fellowship Award.
M.K. designed and performed the experiments, analyzed the data, and wrote the manuscript; S.C. performed the experiments and participated in analyzing the data; L.G.-B. performed the experiments; T.S. generated and contributed the Runx1lacZ/lacZ ES cell line; M.F.S. and M.E. analyzed the data; N.A.S. contributed the ES cell line and participated in writing the manuscript; and P.P.L. designed and performed the experiments, analyzed the data, and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Shelley Hoogstraten-Miller and Eugene Elliott for assistance with veterinary issues and technical support with the ENU injections and Joan Bailey-Wilson for statistical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal