Abstract
Hypochromic, microcytic anemias are typically the result of inadequate hemoglobin production because of globin defects or iron deficiency. Here, we describe the phenotypic characteristics and pathogenesis of a new recessive, hypochromic, microcytic anemia mouse mutant, nm1054. Although the mutation nm1054 is pleiotropic, also resulting in sparse hair, male infertility, failure to thrive, and hydrocephaly, the anemia is the focus of this study. Hematologic analysis reveals a moderately severe, congenital, hypochromic, microcytic anemia, with an elevated red cell zinc protoporphyrin, consistent with functional erythroid iron deficiency. However, serum and tissue iron analyses show that nm1054 animals are not systemically iron deficient. From hematopoietic stem cell transplantation and iron uptake studies in nm1054 reticulocytes, we provide evidence that the nm1054 anemia is due to an intrinsic hematopoietic defect resulting in inefficient transferrin-dependent iron uptake by erythroid precursors. Linkage studies demonstrate that nm1054 maps to a genetic locus not previously implicated in microcytic anemia or iron phenotypes.
Introduction
Hypochromic, microcytic anemias are the result of impaired hemoglobin production, typically as a consequence of defects in globin gene expression, as in the thalassemias, or inadequate heme production, as in systemic iron deficiency. Although inherited globin defects are relatively common in humans, inherited hypochromic, microcytic anemias as a result of mutations in genes in the heme biosynthesis and iron acquisition pathways are exceptional. By contrast, several strains of laboratory rodents such as hypotransferrinemic (Trfhpx) mice,1-5 sex-linked anemia (Hephsla) mice,6-9 the Belgrade rat (b/b or Slc11a2b),10-20 and microcytic anemia (mk/mk or Slc11a2mk) mice21-26 have hypochromic, microcytic anemias as a result of inherited defects in iron metabolism. These animals have provided a great deal of insight into iron homeostasis and defined several novel genes important for systemic and intraerythroid iron metabolism (reviewed in Andrews27 ).
More so than any other cell type in the body, erythroid precursors depend on the transferrin (Tf) cycle for delivery of iron sufficient for their needs. As a consequence, defects in the Tf cycle disproportionately affect the erythroid lineage. The profound anemia in mice with spontaneous and targeted mutations in Tf,5 or transferrin receptor-1 (Trfr1) genes,28 respectively, illustrate this uniquely erythroid dependence on the Tf cycle.
In the Tf cycle, diferric Tf (Fe2-Tf) bound to TfR1 on the cell surface is internalized in an endosome, the endosome is acidified to release iron from Tf, iron is transported out of the endosome, and the protein components are recycled to the cell surface.29 Extensive physiologic characterization of iron uptake in b/b rat and mk/mk mouse reticulocytes demonstrated that these strains have a defect in iron export from the endosome.10,11,13-15,18,19,23,30 Consequently, the identification of mutations in Slc11a2, also known as Dmt1, Nramp2, and Dct1,in b/b rats and mk/mk mice conclusively demonstrated that Slc11a2 is the Tf cycle endosomal iron exporter.16,22,31
Although insight into iron metabolism has advanced since positional cloning of sla, mk, and b, our understanding of transferrin-mediated iron uptake is still incomplete. Several mutants that are not yet cloned may provide further insight into this process. Chief among them is the hemoglobin deficit (hbd) mouse, which has a hypochromic microcytic anemia as a result of an intrinsic erythroid defect that is not unlike b/b rats and mk/mk mice, but maps to a different chromosomal location.32-36 In this report, we describe another autosomal recessive mouse mutant, nm1054, with an intrinsic erythroid defect in iron metabolism, physiologically similar to, but genetically distinct from, mk, hbd, and other known mutants or proteins previously implicated in iron metabolism.
Materials and methods
Strain maintenance
The nm1054 mutation arose on CBA/J and was subsequently transferred onto the C57BL/6J background by sequential backcross-intercross matings of heterozygous animals to form the B6.CBA-nm1054 congenic line, which is currently at N19. All hematologic data for nonchimeric animals are from F2 intercross progeny of a B6.CBA-nm/+ (N15F3) male bred to a 129S6/SvEvTac female (abbreviated 129B6F2). Iron uptake data are from N5F1 or N5F2 mutant and control animals on a 129S6/SvEvTac background. Hematologic parameters of the mutant on the 129S6/SvEvTac background are not qualitatively different from the 129B6F2 intercross animals (data not shown). MK/ReJ-Slc11a2mk animals were maintained by mating homozygous males to heterozygous females at F>100. All animal procedures were approved by the Animal Care and Use Committee, Children's Hospital Boston.
Hematologic and iron analyses
May-Grünwald-Giemsa (Sigma, St Louis, MO) stained peripheral blood smears were photographed on a Nikon Eclipse E600 microscope (Garden City, NJ) with a 100 × / 0.30 numeric aperture oil immersion lens and an RT Slider SPOT 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI) using SPOT Advanced software (version 3.5.9). Complete blood counts were determined from blood obtained by retroorbital puncture. Blood (75 μL) was collected using heparinized microcapillary tubes and diluted in 225 μL phosphate buffered saline (PBS) in EDTA (ethylenediaminetetraacetic acid)–anticoagulated Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ), and processed on an ADVIA 120 hematology analyzer (Bayer Diagnostics, Tarytown, NY) equipped with a mouse-specific software patch designed to detect extremely small microcytes (D. Zelmanovic, Bayer, Tarytown, NY). Zinc protoporphyrin to heme (ZnPP/H) ratios were determined by front surface hematofluorometry with the Helena Laboratory ProtoFluro-Z hematofluorometer system (Helena Laboratories, Beaumont, TX), using heparinized whole blood washed 2 times and resuspended in PBS. These tests were performed in the Clinical Core Laboratories at Children's Hospital Boston. Tissue nonheme iron was determined on acid-digested samples as previously described.37 Serum iron parameters were measured on samples collected in Microtainer serum separator tubes, by using the serum iron and unbound iron binding concentration (UIBC) kit from Sigma Diagnostics (St Louis, MO).
Fetal liver cell transplantation
Embryonic day 16.5 (E16.5) fetal livers were harvested from timed matings between B6.CBA-nm/+ parents at N15F3 using aseptic technique. Homozygous mutant embryos of undetermined sex were identified by obvious pallor. Grossly unaffected,?/+, littermates served as controls. Hematopoietic cells were disaggretated by pipetting each liver in 1 mL αMEM (Minimum Essential Media) medium containing 10% fetal calf serum, and the suspensions were strained through 40-μm sterile filter cups (Becton Dickinson, Franklin Lakes, NJ). Nucleated cells (1 × 107) resuspended in PBS were transplanted by tail vein injection into 8-week-old C57BL/6J-Igha Thy1a Gpi1a female mice (Jackson Laboratory, Bar Harbor, ME) that had received a total of 10.5 Gy (1050 Rad) from a Gammacel 40 irradiator (Nordion, Kanata, ON, Canada), in 2 divided doses, separated by 2.5 hours. Animals were maintained on sterilized bedding and food and on acidified (pH 2) water containing 40 μg/mL trimethoprim/sulfamethoxazole for the first 8 weeks following transplantation. Antibiotic prophylaxis was discontinued thereafter. Three 75-μL heparinized microcapillary tubes of blood were obtained at 0, 4, 8, 12, and 16 weeks following transplantation and processed for complete blood count (CBC), ZnPP/H, and chimerism analyses. For the glucose-6-phosphate isomerase 1 (Gpi1) isozyme chimerism assay, the buffy coat was removed from red blood cells by centrifugation in microcapillary tubes. Red blood cell lysates were electrophoretically separated, and Gpi1 activity was measured in situ on cellulose acetate plates, essentially as previously described.38 Red blood cell (RBC) chimerism was quantified by using the ImageQuant software supplied with the BioRad ChemiDoc gel documentation system (Hercules, CA).
Preparation of 55Fe-Tf
Mouse apo–Tf (Sigma-Aldrich, Bedford, MA), was loaded with 55Fe using the nitrilotriacetic acid (NTA) method.18 In brief, 55Fe-NTAwas generated by mixing 55FeCl3 (94.57 mCi/mg [3499.09 MBq]; Perkin-Elmer, Boston, MA) with 100 mM NTA and added to apo-Tf in sodium carbonate buffer (10 mM NaHCO3, 0.25 M Tris [tris(hydroxymethyl)aminomethane] base) at a ratio of 2.5 moles 55FeCl3 to 1 mole apo-Tf and incubated for 1 hour, before exhaustive dialysis against Hanks Balanced Salt Solution (HBSS, without calcium or magnesium; Invtrogen/Gibco, Carlsbad, CA) at 4°C.
Reticulocyte iron uptake assay
129S6/SvEvTac-nm/nm, 129S6/SvEvTac-?/+, MK/ReJ-mk/mk, and MK/ReJ-mk/+ mice were retroorbitally bled 2% of body weight on days 0 and 3. Reticulocyte-rich blood was collected in heparinized tubes on day 7. The buffy coat was removed from pelleted cells, the reticulocyte-rich RBCs were washed 3 times with HBSS with 1% bovine serum albumin (HBSS-BSA), incubated at 37°C for 30 minutes in HBSS-BSA to clear endogenous transferrin, pelleted, and resuspended at a hematocrit of .25 (25%) in HBSS-BSA. For iron uptake, 250 μL RBCs was incubated at 37°C with 5 μM 55Fe-Tf (> 95% saturated) for 30 minutes, washed 3 times with ice-cold HBSS-BSA, and finally lysed in 100 mM HCl. Aliquots of the hemolysate were used to determine total cell associated and heme iron, the latter following extraction into n-butyl acetate. Radioactivity was counted by liquid scintillation. To determine background cell-associated iron, including surface-bound iron, a 250-μL aliquot of each reticulocyte suspension was incubated on ice with 55Fe-Tf for 30 minutes, washed, lysed, extracted, and analyzed in a manner similar to samples incubated at 37°C. Background values were deducted from total iron and heme iron values for the corresponding sample incubated at 37°C. RNA content of the reticulocyte suspension was determined essentially as described.39 Data points represent the average of duplicate assays performed on reticulocytes from individual animals.
Reticulocyte iron egress assay
129S6/SvEvTac-nm/nm and -?/+ reticulocyte-rich RBCs were collected and washed as described in “Reticulocyte iron uptake assay,” incubated with 5 μM 55Fe-Tf for 15 minutes at 37°C, washed 3 times with ice-cold HBSS-BSA, and incubated with 1 mg/mL Pronase for 15 minutes on ice to remove surface-bound iron. Cells were washed 3 times with ice-cold HBSS-BSA, then incubated in serum from?/+ mice at 37°C for 5 minutes, and washed 3 times in HBSS-BSA, and the radioactivity remaining in the pellet was determined by scintillation counting. Efflux was measured by comparing cell-associated iron from cells incubated on ice to those incubated at 37°C.
Chromosomal mapping
An F2 intercross between CBA/J-nm1054 and the genetically highly polymorphic Mus musculus castaneous subspecies (CAST/Ei strain) was generated. nm/nm animals were identified at birth by pallor, and the phenotype was later confirmed by gross examination for hydrocephalus and an elevated blood ZnPP/H. To map the locus, Massachusetts Institute of Technology (MIT) microsatellite repeat marker primer pairs40 distributed at approximately 30 centimorgan (cM) across the genome were used to polymerase chain reaction (PCR) amplify DNA pooled from 25 affected and 25 unaffected animals, and the relative ratio of the CBA/J- and CAST/Ei-specific alleles in each pool was determined for each marker.41 Linked markers were identified by a predominance of the CBA allele. Subsequent genotyping of 23 of the affected animals individually with closely linked markers confirmed localization to chromosome 1, and a linkage map of the region was created using MapManager QTXb20.42 To exclude ferroportin (Slc40a1) as a candidate, this set was also analyzed with a microsatellite marker within the Slc40a1 gene (primers, 5′-CCTAGCATGCAGAAGGCTCT-3′ and 5′-TGTGGTGTGTTGGGAGAAAA-3′).
Results
nm1054, a new pleiotropic mutation
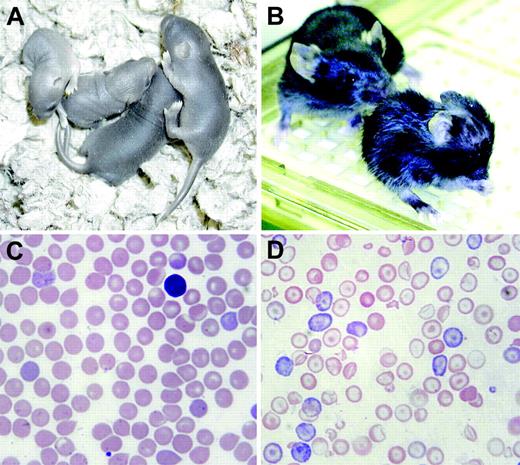
nm1054, for the 1054th new mutation catalogued at the Jackson Laboratory, was first identified as an anemic animal in a litter of CBA/J mice. The trait is autosomal recessive, and the mutation is pleiotropic. Homozygous nm1054 mutants (hereafter abbreviated nm/nm) can be readily identified at birth by their runting and pallor. Figure 1A shows a litter of 2 nm/nm and 2?/+ pups on P4. There is significant prenatal lethality on the C57BL/6J background; only 15 mutant animals were observed in 220 live births (7.3%) in nm/+ × nm/+ crosses in which 25% homozygous mutants are expected (chi-square, P < .001). The vast majority of live-born mutants on the C57BL/6J background die prior to P7, and few have been weaned successfully, particularly at later generations of backcrossing. Figure 1B shows a severely runted nm/nm animal at P19 in the foreground and a wild-type (?/+) littermate in the background. Sparse hair, particularly on the dorsal surfaces, and hydrocephalus are also evident in the mutant.
The nm1054 phenotype. (A) Two nm/nm (left) and 2 ?/+ (right) pups on postnatal day of life 4 (P4). The mutants are easily recognizable by their runting and pallor. (B) A nm/nm animal at P19 in the foreground and a wild-type (?/+) littermate in the background. Runting, abnormal fur, and hydrocephalus are evident in the mutant. (C-D) Peripheral blood smears from adult ?/+ and nm/nm animals, respectively. Hypochromia, microcytosis, anisocytosis, polychromasia, and target cells are prominent features in the mutant smear.
The nm1054 phenotype. (A) Two nm/nm (left) and 2 ?/+ (right) pups on postnatal day of life 4 (P4). The mutants are easily recognizable by their runting and pallor. (B) A nm/nm animal at P19 in the foreground and a wild-type (?/+) littermate in the background. Runting, abnormal fur, and hydrocephalus are evident in the mutant. (C-D) Peripheral blood smears from adult ?/+ and nm/nm animals, respectively. Hypochromia, microcytosis, anisocytosis, polychromasia, and target cells are prominent features in the mutant smear.
By contrast, [129S6/SvEvTac × C57BL/6J]F2-nm/nm animals (129B6F2) are born at the expected Mendelian frequency, and most survive to adulthood. These 129B6F2 mutants are all anemic, but only a rare animal has grossly discernible hydrocephalus, suggesting that the hydrocephaly, not the anemia, is the predominant cause of postnatal death on the C57BL/6J background. Homozygous mutant males, but not females, are uniformly infertile on all genetic backgrounds examined.
nm1054 anemia
Although the mutation is pleiotropic, we were most interested in characterizing the anemia. In comparison to?/+ control animals (Figure 1C), homozygous nm/nm erythrocytes (Figure 1D) are hypochromic, microcytic, and have a moderate degree of aniso-poikilocytosis. Target cells are particularly prominent. There is polychromasia, indicative of a high reticulocyte count. A crystal violet stain of peripheral blood is negative for Heinz bodies, and siderocytes and ringed sideroblasts are not present on iron stains of the blood or bone marrow, respectively (data not shown). Similarly, transmission electron micrographs of red blood cells and spleen do not reveal inclusions typical of globin precipitates or intramitochondrial electron-dense deposits characteristic of sideroblastic anemia (data not shown). Furthermore, hemoglobin electrophoresis on cellulose acetate gels demonstrates a wild-type pattern of globins. No abnormally migrating α- or β-chain is present on acid-urea gel electrophoresis of red cell lysates, and reticulocyte incorporation of radiolabeled amino acids into α- and β-globin chains is balanced (data not shown). Taken together, these findings argue against a thalassemia, unstable hemoglobin, redox defect, or sideroblastic anemia as a cause of the anemia.
Automated blood analyses substantiate the light microscopic features of the nm1054 anemia. Table 1 describes the complete blood counts and red blood cell indices of 129B6F2 mutants and controls at 4, 8, 12, and 24 weeks of age. The anemia is moderately severe, with the hemoglobin reduced by at least one third at all ages examined. Red blood cells are microcytic (MCV decreased), and hypochromic (MCHC decreased). As is common in microcytic anemias, there is an erythrocytosis. The absolute reticulocyte count is increased to a variable degree at all ages, indicating a proliferative anemia, likely with a component of decreased red blood cell survival. The anemia tends to improve after 4 weeks of age. Platelet counts are consistently elevated in the mutant, and there is a trend toward an increased white blood cell count at all ages that cannot be accounted for by circulating erythroid precursors, which are never present.
Hematologic parameters of ?/+ and nm/nm animals
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ?/+, n = 8 . | nm/nm, n = 8 . | P . | ?/+, n = 11 . | nm/nm, n = 10 . | P . | ?/+, n = 8 . | nm/nm, n = 8 . | P . | ?/+, n = 10 . | nm/nm, n = 10 . | P . | ||||||||
| WBCs, × 103/μL | 2.35 ± 1.40 | 2.49 ± 0.28 | .810 | 1.30 ± 0.55 | 4.09 ± 1.65 | < .001 | 4.27 ± 2.63 | 5.85 ± 1.97 | .197 | 3.32 ± 2.48 | 5.80 ± 4.18 | .127 | ||||||||
| RBCs, × 106/μL | 8.17 ± 0.83 | 10.49 ± 1.03 | < .001 | 9.60 ± 0.51 | 14.01 ± 0.90 | < .001 | 9.30 ± 1.17 | 15.55 ± 1.04 | < .001 | 9.54 ± 0.52 | 15.86 ± 0.76 | < .001 | ||||||||
| Hemoglobin, g/dL | 13.1 ± 1.4 | 6.9 ± 0.5 | < .001 | 15.3 ± 1.0 | 9.0 ± 0.8 | < .001 | 14.3 ± 1.9 | 9.1 ± 0.7 | < .001 | 13.9 ± 0.9 | 8.8 ± 0.7 | < .001 | ||||||||
| Hematocrit, % | 47.3 ± 3.8 | 33.3 ± 3.4 | < .001 | 50.1 ± 2.0 | 36.2 ± 2.7 | < .001 | 48.4 ± 5.3 | 37.0 ± 2.9 | < .001 | 47.3 ± 2.2 | 37.2 ± 2.2 | < .001 | ||||||||
| MCV, fL | 58.1 ± 2.7 | 30.4 ± 2.8 | < .001 | 52.3 ± 1.4 | 25.9 ± 1.3 | < .001 | 52.2 ± 2.6 | 23.9 ± 2.1 | < .001 | 49.7 ± 2.2 | 23.5 ± 1.2 | < .001 | ||||||||
| MCH, pg | 16.0 ± 0.7 | 6.6 ± 0.5 | < .001 | 16.0 ± 0.8 | 6.4 ± 0.4 | < .001 | 15.4 ± 0.9 | 5.9 ± 0.4 | < .001 | 14.6 ± 1.2 | 5.6 ± 0.3 | < .001 | ||||||||
| MCHC, g/dL | 27.7 ± 1.8 | 20.9 ± 1.5 | < .001 | 30.5 ± 1.3 | 24.9 ± 1.5 | < .001 | 29.5 ± 0.4 | 24.6 ± 1.5 | < .001 | 29.4 ± 1.8 | 23.8 ± 1.1 | < .001 | ||||||||
| RDW, % | 14.4 ± 1.0 | 31.0 ± 2.2 | < .001 | 13.0 ± 0.8 | 28.9 ± 1.1 | < .001 | 13.6 ± 0.7 | 30.1 ± 1.1 | < .001 | 13.5 ± 0.8 | 28.2 ± 2.6 | < .001 | ||||||||
| Platelets, × 106/μL | 1144 ± 290 | 3798 ± 958 | < .001 | 1262 ± 159 | 2550 ± 603 | < .001 | 1154 ± 245 | 2286 ± 693 | .002 | 1301 ± 129 | 2183 ± 464 | < .001 | ||||||||
| MPV, fL | 7.8 ± 0.3 | 10.3 ± 1.7 | .004 | 8.1 ± 0.4 | 8.2 ± 0.7 | .730 | 8.1 ± 0.4 | 8.0 ± 0.6 | .687 | 8.3 ± 0.2 | 8.2 ± 0.8 | .785 | ||||||||
| Reticulocytes, % | 6.1 ± 2.4 | 21.1 ± 4.4 | < .001 | 1.6 ± 0.4 | 7.9 ± 2.5 | < .001 | 2.2 ± 0.8 | 7.3 ± 3.2 | .002 | 2.4 ± 1.3 | 4.3 ± 1.0 | < .001 | ||||||||
| Abs retic, × 106/μL | 0.491 ± 0.168 | 2.216 ± 0.518 | < .001 | 0.156 ± 0.041 | 1.095 ± 0.297 | < .001 | 0.200 ± 0.055 | 1.126 ± 0.428 | < .001 | 0.222 ± 0.109 | 0.684 ± 0.146 | .002 | ||||||||
| CHr, pg | 15.1 ± 0.4 | 7.5 ± 0.2 | < .001 | 14.6 ± 0.5 | 6.9 ± 0.3 | < .001 | 14.5 ± 0.7 | 7.1 ± 0.3 | < .001 | 14.6 ± 0.7 | 7.4 ± 0.3 | < .001 | ||||||||
| ZnPP/H, μmol/mol | 125 ± 47 | 532 ± 227 | .001 | 74 ± 8 | 285 ± 66 | < .001 | 74 ± 12 | 268 ± 65 | < .001 | 75 ± 19 | 196 ± 39 | < .001 | ||||||||
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ?/+, n = 8 . | nm/nm, n = 8 . | P . | ?/+, n = 11 . | nm/nm, n = 10 . | P . | ?/+, n = 8 . | nm/nm, n = 8 . | P . | ?/+, n = 10 . | nm/nm, n = 10 . | P . | ||||||||
| WBCs, × 103/μL | 2.35 ± 1.40 | 2.49 ± 0.28 | .810 | 1.30 ± 0.55 | 4.09 ± 1.65 | < .001 | 4.27 ± 2.63 | 5.85 ± 1.97 | .197 | 3.32 ± 2.48 | 5.80 ± 4.18 | .127 | ||||||||
| RBCs, × 106/μL | 8.17 ± 0.83 | 10.49 ± 1.03 | < .001 | 9.60 ± 0.51 | 14.01 ± 0.90 | < .001 | 9.30 ± 1.17 | 15.55 ± 1.04 | < .001 | 9.54 ± 0.52 | 15.86 ± 0.76 | < .001 | ||||||||
| Hemoglobin, g/dL | 13.1 ± 1.4 | 6.9 ± 0.5 | < .001 | 15.3 ± 1.0 | 9.0 ± 0.8 | < .001 | 14.3 ± 1.9 | 9.1 ± 0.7 | < .001 | 13.9 ± 0.9 | 8.8 ± 0.7 | < .001 | ||||||||
| Hematocrit, % | 47.3 ± 3.8 | 33.3 ± 3.4 | < .001 | 50.1 ± 2.0 | 36.2 ± 2.7 | < .001 | 48.4 ± 5.3 | 37.0 ± 2.9 | < .001 | 47.3 ± 2.2 | 37.2 ± 2.2 | < .001 | ||||||||
| MCV, fL | 58.1 ± 2.7 | 30.4 ± 2.8 | < .001 | 52.3 ± 1.4 | 25.9 ± 1.3 | < .001 | 52.2 ± 2.6 | 23.9 ± 2.1 | < .001 | 49.7 ± 2.2 | 23.5 ± 1.2 | < .001 | ||||||||
| MCH, pg | 16.0 ± 0.7 | 6.6 ± 0.5 | < .001 | 16.0 ± 0.8 | 6.4 ± 0.4 | < .001 | 15.4 ± 0.9 | 5.9 ± 0.4 | < .001 | 14.6 ± 1.2 | 5.6 ± 0.3 | < .001 | ||||||||
| MCHC, g/dL | 27.7 ± 1.8 | 20.9 ± 1.5 | < .001 | 30.5 ± 1.3 | 24.9 ± 1.5 | < .001 | 29.5 ± 0.4 | 24.6 ± 1.5 | < .001 | 29.4 ± 1.8 | 23.8 ± 1.1 | < .001 | ||||||||
| RDW, % | 14.4 ± 1.0 | 31.0 ± 2.2 | < .001 | 13.0 ± 0.8 | 28.9 ± 1.1 | < .001 | 13.6 ± 0.7 | 30.1 ± 1.1 | < .001 | 13.5 ± 0.8 | 28.2 ± 2.6 | < .001 | ||||||||
| Platelets, × 106/μL | 1144 ± 290 | 3798 ± 958 | < .001 | 1262 ± 159 | 2550 ± 603 | < .001 | 1154 ± 245 | 2286 ± 693 | .002 | 1301 ± 129 | 2183 ± 464 | < .001 | ||||||||
| MPV, fL | 7.8 ± 0.3 | 10.3 ± 1.7 | .004 | 8.1 ± 0.4 | 8.2 ± 0.7 | .730 | 8.1 ± 0.4 | 8.0 ± 0.6 | .687 | 8.3 ± 0.2 | 8.2 ± 0.8 | .785 | ||||||||
| Reticulocytes, % | 6.1 ± 2.4 | 21.1 ± 4.4 | < .001 | 1.6 ± 0.4 | 7.9 ± 2.5 | < .001 | 2.2 ± 0.8 | 7.3 ± 3.2 | .002 | 2.4 ± 1.3 | 4.3 ± 1.0 | < .001 | ||||||||
| Abs retic, × 106/μL | 0.491 ± 0.168 | 2.216 ± 0.518 | < .001 | 0.156 ± 0.041 | 1.095 ± 0.297 | < .001 | 0.200 ± 0.055 | 1.126 ± 0.428 | < .001 | 0.222 ± 0.109 | 0.684 ± 0.146 | .002 | ||||||||
| CHr, pg | 15.1 ± 0.4 | 7.5 ± 0.2 | < .001 | 14.6 ± 0.5 | 6.9 ± 0.3 | < .001 | 14.5 ± 0.7 | 7.1 ± 0.3 | < .001 | 14.6 ± 0.7 | 7.4 ± 0.3 | < .001 | ||||||||
| ZnPP/H, μmol/mol | 125 ± 47 | 532 ± 227 | .001 | 74 ± 8 | 285 ± 66 | < .001 | 74 ± 12 | 268 ± 65 | < .001 | 75 ± 19 | 196 ± 39 | < .001 | ||||||||
Complete blood counts and red blood cell zinc ratios of protoporphyrin to heme (ZnPP/H) were determined in 129B6F2 ?/+ and nm/nm animals at 4, 8, 12, and 24 weeks of age. Values are presented as the mean ± 1 SD. Statistical significance values (P) are reported for the two-tailed Student t test. WBCs indicates white blood cells; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; Abs retic, absolute reticulocytes; CHr, mean cellular hemoglobin of the reticulocytes.
Conversion to SI units is as follows: WBCs, Abs retic, and platelets, × 106 to achieve units in × 109/L; RBCs, × 106 to achieve units expressed in × 1012/L; hemoglobin and hematocrit, × 10 to achieve units in g/L; serum iron and TIBC, × 0.179 to achieve units in μM.
Mice with proliferative anemias typically have enlarged spleens because of expansion of erythropoiesis, either as a result of peripheral destruction, ineffective erythropoiesis, or both. Homozygous nm/nm animals have significantly ratios of larger spleen to body weight (Table 2). Histologically, sheets of erythroid precursors occupy the expanded red pulp, effacing the normal nodular white pulp architecture. A similar erythroid hyperplasia is also present in the marrow (data not shown). A slight cardiomegaly is seen in nm/nm homozygotes, particularly at earlier ages, and is likely secondary to chronic anemia and hypoxia. Both the splenomegaly and the cardiomegaly tend to improve with age, almost certainly as a result of improvement of the anemia. The cause of the mild hepatomegaly present at all ages is uncertain, but it is not due to extramedullary hematopoiesis. The testicular hypoplasia is commensurate with the male infertility.
Body weights and ratios of organ to body weight in ?/+ and nm/nm animals
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | ?/+, n = 19 . | nm/nm, n = 8 . | P . | ?/+, n = 22 . | nm/nm, n = 10 . | P . | ?/+, n = 13 . | nm/nm, n = 8 . | P . | ?/+, n = 19 . | nm/nm, n = 10 . | P . | ||||||||
| Body weight, g | 17.8 ± 2.4 | 11.7 ± 1.2 | < .001 | 21.4 ± 3.4 | 19.1 ± 2.6 | .050 | 25.9 ± 3.7 | 22.4 ± 3.2 | .243 | 35.9 ± 6.1 | 27.2 ± 3.9 | .002 | ||||||||
| Organ-body weight ratio, mg/g | ||||||||||||||||||||
| Liver | 45.1 ± 3.6 | 54.2 ± 2.1 | < .001 | 43.8 ± 5.3 | 50.7 ± 3.1 | < .001 | 40.7 ± 5.1 | 53.1 ± 5.6 | .001 | 42.8 ± 3.1 | 47.8 ± 2.8 | .001 | ||||||||
| Spleen | 3.8 ± 0.8 | 15.4 ± 3.3 | < .001 | 3.4 ± 1.0 | 7.2 ± 2.2 | < .001 | 3.0 ± 1.2 | 7.7 ± 3.6 | .032 | 2.8 ± 1.2 | 5.0 ± 1.3 | < .001 | ||||||||
| Kidneys | 12.0 ± 1.2 | 13.1 ± 1.0 | .028 | 12.6 ± 1.2 | 12.2 ± 1.5 | .384 | 13.1 ± 1.3 | 13.0 ± 1.2 | .708 | 12.3 ± 2.0 | 12.3 ± 1.4 | .985 | ||||||||
| Testes | 6.9 ± 0.8 | 4.5 ± 0.3 | < .001 | 7.5 ± 0.9 | 5.6 ± 1.5 | .149 | 7.8 ± 0.7 | 6.1 ± 0.7 | .018 | 7.0 ± 1.1 | 6.3 ± 0.8 | .149 | ||||||||
| Thymus | 4.3 ± 1.3 | 4.1 ± 0.9 | .573 | 2.9 ± 1.5 | 3.0 ± 0.8 | .907 | 1.7 ± 0.8 | 3.6 ± 3.7 | .231 | 1.3 ± 0.4 | 1.2 ± 0.3 | .251 | ||||||||
| Heart | 4.6 ± 0.6 | 6.7 ± 0.3 | < .001 | 4.9 ± 0.4 | 6.2 ± 1.2 | .007 | 4.5 ± 1.3 | 5.7 ± 0.7 | .017 | 4.5 ± 0.6 | 5.5 ± 0.5 | < .001 | ||||||||
| Brain | 24.0 ± 3.3 | 32.0 ± 2.5 | < .001 | 21.5 ± 3.2 | 21.9 ± 2.9 | .709 | 17.9 ± 2.8 | 18.6 ± 2.6 | .968 | 13.6 ± 2.7 | 15.6 ± 2.3 | .055 | ||||||||
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | ?/+, n = 19 . | nm/nm, n = 8 . | P . | ?/+, n = 22 . | nm/nm, n = 10 . | P . | ?/+, n = 13 . | nm/nm, n = 8 . | P . | ?/+, n = 19 . | nm/nm, n = 10 . | P . | ||||||||
| Body weight, g | 17.8 ± 2.4 | 11.7 ± 1.2 | < .001 | 21.4 ± 3.4 | 19.1 ± 2.6 | .050 | 25.9 ± 3.7 | 22.4 ± 3.2 | .243 | 35.9 ± 6.1 | 27.2 ± 3.9 | .002 | ||||||||
| Organ-body weight ratio, mg/g | ||||||||||||||||||||
| Liver | 45.1 ± 3.6 | 54.2 ± 2.1 | < .001 | 43.8 ± 5.3 | 50.7 ± 3.1 | < .001 | 40.7 ± 5.1 | 53.1 ± 5.6 | .001 | 42.8 ± 3.1 | 47.8 ± 2.8 | .001 | ||||||||
| Spleen | 3.8 ± 0.8 | 15.4 ± 3.3 | < .001 | 3.4 ± 1.0 | 7.2 ± 2.2 | < .001 | 3.0 ± 1.2 | 7.7 ± 3.6 | .032 | 2.8 ± 1.2 | 5.0 ± 1.3 | < .001 | ||||||||
| Kidneys | 12.0 ± 1.2 | 13.1 ± 1.0 | .028 | 12.6 ± 1.2 | 12.2 ± 1.5 | .384 | 13.1 ± 1.3 | 13.0 ± 1.2 | .708 | 12.3 ± 2.0 | 12.3 ± 1.4 | .985 | ||||||||
| Testes | 6.9 ± 0.8 | 4.5 ± 0.3 | < .001 | 7.5 ± 0.9 | 5.6 ± 1.5 | .149 | 7.8 ± 0.7 | 6.1 ± 0.7 | .018 | 7.0 ± 1.1 | 6.3 ± 0.8 | .149 | ||||||||
| Thymus | 4.3 ± 1.3 | 4.1 ± 0.9 | .573 | 2.9 ± 1.5 | 3.0 ± 0.8 | .907 | 1.7 ± 0.8 | 3.6 ± 3.7 | .231 | 1.3 ± 0.4 | 1.2 ± 0.3 | .251 | ||||||||
| Heart | 4.6 ± 0.6 | 6.7 ± 0.3 | < .001 | 4.9 ± 0.4 | 6.2 ± 1.2 | .007 | 4.5 ± 1.3 | 5.7 ± 0.7 | .017 | 4.5 ± 0.6 | 5.5 ± 0.5 | < .001 | ||||||||
| Brain | 24.0 ± 3.3 | 32.0 ± 2.5 | < .001 | 21.5 ± 3.2 | 21.9 ± 2.9 | .709 | 17.9 ± 2.8 | 18.6 ± 2.6 | .968 | 13.6 ± 2.7 | 15.6 ± 2.3 | .055 | ||||||||
Body weights and ratios of organ to body weight for 129B6F2-?/+ and -nm/nm animals at 4, 8, 12, and 24 weeks of age are reported. Values are presented as the mean ± 1 SD. Statistical significance values (P) are reported for the two-tailed Student t test.
A useful screen for defects in iron availability for heme biosynthesis is the erythrocyte zinc protoporphyrin IX to heme (ZnPP/H) ratio.43 When iron is not available for incorporation into heme, ferrochelatase, the terminal enzyme in heme biosynthesis, can use Zn2+ in lieu of Fe2+ as a substrate, yielding ZnPP. The ZnPP/H ratio is characteristically increased in iron deficiency and may be increased slightly in anemias in which there is a relative deficiency of iron as a result of severe hemolysis, inefficient iron recycling, or ineffective erythropoiesis. Certain sideroblastic anemias, in which there is inefficient utilization of intramitochondrial iron, may also have increased ZnPP/H ratios.44 As is shown in Table 1, the ZnPP/H ratio is increased nearly 4-fold in 4-week-old nm/nm homozygotes compared with?/+ controls, suggesting that a defect in heme biosynthesis prior to and including the ferrochelatase enzyme is unlikely. For comparison, erythrocytes of 2 strains, MK/ReJ-Slc11a2mk/mk and C57BL/6J-hbd/hbd, known to have intraerythroid defects in Fe metabolism, have 3.2- and 8.1-fold increases in the ZnPP/H ratio compared with appropriate controls, respectively. Furthermore, mice with a recessive, severe partial loss of function mutation in ferrochelatase, BALB/cJ-Fechm1Pas/m1Pas,45 have a mild microcytic anemia and a decreased or normal ZnPP/H ratio (data not shown).
Systemic iron metabolism in nm1054
If the increase in the ZnPP/H ratio were due to a defect in iron metabolism, it could be due to systemic iron deficiency, secondary to an iron utilization defect within red blood cell precursors, or possibly a recycling defect in cells such as hepatocytes or the macrophages of the reticuloendothelial system. To assess these possibilities, we performed quantitative liver and spleen iron analyses and determined serum iron parameters in 129B6F2 mutant and control animals at 4, 8, 12, and 24 weeks of age. Table 3 describes the results. The elevated liver iron at each of the ages examined indicates that nm animals are not systemically iron deficient. Histologic examination of iron-stained tissue sections demonstrates that the iron is predominantly in hepatocytes, rather than Kupffer cells of the reticuloendothelial system. In addition, bone marrow reticuloendothelial iron is present in mutant animals (data not shown). The normalization of liver iron over time suggests that the relative iron overload is secondary to the anemia that improves with age. Spleen iron is decreased in the mutant on a per milligram of tissue basis; however, corrected for the weight of the spleen, there is no difference in the total splenic iron in mutants and wild-type animals at later ages. Although at 4 weeks of age serum iron, TIBC and transferrin saturations are significantly elevated in the mutant, these parameters eventually also normalize. This is also likely a reflection of the dynamic changes in the relative degree of anemia. The presence of a normal or elevated TIBC excludes a quantitative defect in transferrin as a cause of the anemia.
Serum iron parameters and tissue iron stores in ?/+ and nm/nm animals
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ?/+, n = 19 . | nm/nm, n = 8 . | P . | ?/+, n = 14 . | nm/nm, n = 10 . | P . | ?/+, n = 13 . | nm/nm, n = 8 . | P . | ?/+, n = 19 . | nm/nm, n = 10 . | P . | ||||||||
| Serum iron, μg/dL | 166 ± 33 | 246 ± 30 | < .001 | 216 ± 62 | 199 ± 30 | .371 | 148 ± 41 | 121 ± 20 | .136 | 155 ± 55 | 228 ± 54 | .003 | ||||||||
| TIBC, μg/dL | 531 ± 73 | 646 ± 52 | < .001 | 353 ± 48 | 344 ± 59 | .713 | 255 ± 65 | 233 ± 72 | .551 | 311 ± 44 | 339 ± 53 | .178 | ||||||||
| Transferrin saturation, % | 31 ± 5 | 38 ± 3 | .001 | 61 ± 13 | 59 ± 11 | .704 | 56 ± 13 | 55 ± 11 | .600 | 49 ± 15 | 67 ± 10 | .001 | ||||||||
| Liver iron, μg/g | 84 ± 21 | 333 ± 107 | < .001 | 89 ± 16 | 201 ± 51 | < .001 | 95 ± 34 | 136 ± 46 | .273 | 116 ± 53 | 120 ± 60 | .829 | ||||||||
| Spleen iron, μg/g | 90 ± 20 | 50 ± 13 | < .001 | 345 ± 134 | 206 ± 59 | .003 | 618 ± 365 | 261 ± 129 | .014 | 1116 ± 559 | 441 ± 370 | .001 | ||||||||
| Total spleen iron, μg | 5.9 ± 1.7 | 8.7 ± 2.5 | < .001 | 24.3 ± 13.3 | 27.7 ± 12.0 | .522 | 47.9 ± 31.9 | 38.9 ± 13.6 | .330 | 104 ± 68 | 64 ± 73 | .159 | ||||||||
. | 4 weeks . | . | . | 8 weeks . | . | . | 12 weeks . | . | . | 24 weeks . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | ?/+, n = 19 . | nm/nm, n = 8 . | P . | ?/+, n = 14 . | nm/nm, n = 10 . | P . | ?/+, n = 13 . | nm/nm, n = 8 . | P . | ?/+, n = 19 . | nm/nm, n = 10 . | P . | ||||||||
| Serum iron, μg/dL | 166 ± 33 | 246 ± 30 | < .001 | 216 ± 62 | 199 ± 30 | .371 | 148 ± 41 | 121 ± 20 | .136 | 155 ± 55 | 228 ± 54 | .003 | ||||||||
| TIBC, μg/dL | 531 ± 73 | 646 ± 52 | < .001 | 353 ± 48 | 344 ± 59 | .713 | 255 ± 65 | 233 ± 72 | .551 | 311 ± 44 | 339 ± 53 | .178 | ||||||||
| Transferrin saturation, % | 31 ± 5 | 38 ± 3 | .001 | 61 ± 13 | 59 ± 11 | .704 | 56 ± 13 | 55 ± 11 | .600 | 49 ± 15 | 67 ± 10 | .001 | ||||||||
| Liver iron, μg/g | 84 ± 21 | 333 ± 107 | < .001 | 89 ± 16 | 201 ± 51 | < .001 | 95 ± 34 | 136 ± 46 | .273 | 116 ± 53 | 120 ± 60 | .829 | ||||||||
| Spleen iron, μg/g | 90 ± 20 | 50 ± 13 | < .001 | 345 ± 134 | 206 ± 59 | .003 | 618 ± 365 | 261 ± 129 | .014 | 1116 ± 559 | 441 ± 370 | .001 | ||||||||
| Total spleen iron, μg | 5.9 ± 1.7 | 8.7 ± 2.5 | < .001 | 24.3 ± 13.3 | 27.7 ± 12.0 | .522 | 47.9 ± 31.9 | 38.9 ± 13.6 | .330 | 104 ± 68 | 64 ± 73 | .159 | ||||||||
Serum iron parameters and tissue nonheme iron stores for 129B6F2-?/+ and -nm/nm animals at 4, 8, 12, and 24 weeks of age are reported. Values are presented as the mean ± 1 SD. Statistical significance values (P) are reported for the two-tailed Student t test.
TIBC indicates total iron binding concentration; total spleen iron = spleen weight × spleen iron.
Conversion factors for SI units are as described for Table 1.
nm1054 anemia is transplantable
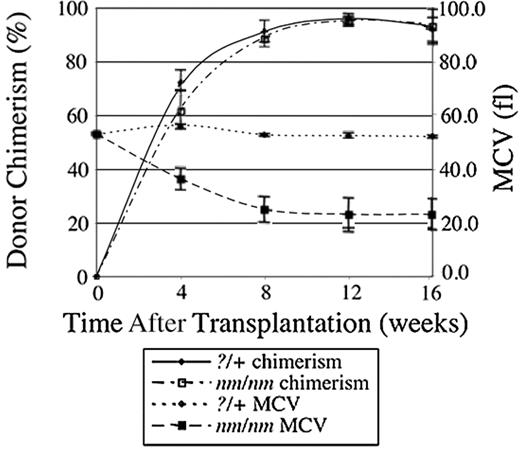
To determine whether a component of the nm1054 anemia was due to an intrinsic, transplantable defect in hematopoietic cells, we transplanted fetal liver cells from E16.5?/+ and nm/nm donors into congenic, histocompatible +/+ recipients. Recipients and donors differed at the glucose-6-phosphate isomerase 1 (Gpi1) locus, allowing the extent of RBC chimerism to be assessed with an enzymatic assay. As shown in Figure 2, by 12 to 16 weeks, a comparable, stable, high-level chimerism had been established in both groups. At this time, the morphology of red cells in mutant recipient chimeras was indistinguishable from nonchimeric mutants of like age on a 129B6F2 background (data not shown). Similarly, the degree of anemia, the red blood cell indices, the elevation of the ZnPP/H ratio, the thrombocytosis, and the spleen–body weight ratio were also comparable in the mutant chimeras and older 129B6F2 mutants (Table 4). Furthermore, the livers of chimeric animals that had received mutant fetal liver cells had demonstrably more iron than their control counterparts (Table 5). In these animals, the iron was preferentially deposited in hepatocytes (data not shown). Although we have not performed the reciprocal transplantation, wild-type fetal liver cells into mutant hosts, because of the inviability of the adult mutants on the C57BL/6J background, we conclude that the great majority of the anemia phenotype can be accounted for by an intrinsic hematopoietic defect that secondarily results in slight iron loading.
Hematologic parameters of ?/+ and nm/nm fetal liver transplant chimeras
Parameter . | ?/+, n = 6 . | nm/nm, n = 5 . | P . |
|---|---|---|---|
| WBCs, × 103/μL | 6.38 ± 2.45 | 6.79 ± 1.32 | .741 |
| RBCs, × 106/μL | 9.52 ± 0.3 | 16.84 ± 0.83 | < .001 |
| Hemoglobin, g/dL | 13.7 ± 0.5 | 8.7 ± 0.6 | < .001 |
| Hematocrit, % | 49.7 ± 1.5 | 37.6 ± 2.1 | < .001 |
| MCV, fL | 52.1 ± 0.4 | 22.3 ± 0.4 | < .001 |
| MCH, pg | 14.3 ± 0.2 | 5.2 ± 0.1 | < .001 |
| MCHC, g/dL | 27.5 ± 0.4 | 23.2 ± 0.8 | < .001 |
| RDW, % | 13.5 ± 0.2 | 29.8 ± 0.4 | < .001 |
| PLT, × 106/μL | 1394 ± 72 | 2634 ± 307 | < .001 |
| MPV, fL | 7.9 ± 0.2 | 8.0 ± 0.2 | .173 |
| Reticulocytes, % | 2.0 ± 0.1 | 7.0 ± 0.4 | < .001 |
| Abs retic, × 106/μL | 0.193 ± 0.015 | 1.181 ± 0.111 | < .001 |
| CHr, pg | 15.0 ± 0.2 | 6.9 ± 0.1 | < .001 |
| ZnPP/H, μmol/mol | 77 ± 13 | 383 ± 46 | < .001 |
Parameter . | ?/+, n = 6 . | nm/nm, n = 5 . | P . |
|---|---|---|---|
| WBCs, × 103/μL | 6.38 ± 2.45 | 6.79 ± 1.32 | .741 |
| RBCs, × 106/μL | 9.52 ± 0.3 | 16.84 ± 0.83 | < .001 |
| Hemoglobin, g/dL | 13.7 ± 0.5 | 8.7 ± 0.6 | < .001 |
| Hematocrit, % | 49.7 ± 1.5 | 37.6 ± 2.1 | < .001 |
| MCV, fL | 52.1 ± 0.4 | 22.3 ± 0.4 | < .001 |
| MCH, pg | 14.3 ± 0.2 | 5.2 ± 0.1 | < .001 |
| MCHC, g/dL | 27.5 ± 0.4 | 23.2 ± 0.8 | < .001 |
| RDW, % | 13.5 ± 0.2 | 29.8 ± 0.4 | < .001 |
| PLT, × 106/μL | 1394 ± 72 | 2634 ± 307 | < .001 |
| MPV, fL | 7.9 ± 0.2 | 8.0 ± 0.2 | .173 |
| Reticulocytes, % | 2.0 ± 0.1 | 7.0 ± 0.4 | < .001 |
| Abs retic, × 106/μL | 0.193 ± 0.015 | 1.181 ± 0.111 | < .001 |
| CHr, pg | 15.0 ± 0.2 | 6.9 ± 0.1 | < .001 |
| ZnPP/H, μmol/mol | 77 ± 13 | 383 ± 46 | < .001 |
Complete blood counts and red blood cell ratios of zinc protoporphyrin to heme (ZnPP/H) were determined in fetal liver transplant chimeras at 16 weeks after transplantation. Values are presented as the mean ± 1 SD. Statistical significance values (P) are reported for the two-tailed Student t test.
Iron parameters of ?/+ and nm/nm fetal liver transplant recipients at 16 weeks after transplantation
Parameter . | ?/+, n = 6 . | nm/nm, n = 5 . | P . |
|---|---|---|---|
| Serum iron, μg/dL | 76 ± 24 | 83 ± 43 | .778 |
| TIBC, μg/dL | 289 ± 28 | 290 ± 65 | .986 |
| Transferrin saturation, % | 26 ± 8 | 27 ± 8 | .807 |
| Liver iron, μg/g | 95 ± 8 | 202 ± 34 | .002 |
| Spleen iron, μg/g | 288 ± 63 | 380 ± 107 | .140 |
| Total spleen iron, μg | 21 ± 5 | 52 ± 6 | < .001 |
Parameter . | ?/+, n = 6 . | nm/nm, n = 5 . | P . |
|---|---|---|---|
| Serum iron, μg/dL | 76 ± 24 | 83 ± 43 | .778 |
| TIBC, μg/dL | 289 ± 28 | 290 ± 65 | .986 |
| Transferrin saturation, % | 26 ± 8 | 27 ± 8 | .807 |
| Liver iron, μg/g | 95 ± 8 | 202 ± 34 | .002 |
| Spleen iron, μg/g | 288 ± 63 | 380 ± 107 | .140 |
| Total spleen iron, μg | 21 ± 5 | 52 ± 6 | < .001 |
Serum iron parameters and tissue nonheme iron stores for fetal liver cell transplant chimeras are reported at 16 weeks after transplantation. Values are presented as the mean ± 1 SD. Statistical significance values (P) are reported for the two-tailed Student t test.
TIBC indicates total iron binding concentration. Total spleen iron = spleen weight × spleen iron.
Conversion factors for SI units are as described for Table 1.
nm1054 reticulocytes are defective in transferrin-mediated iron uptake
Iron uptake studies in reticulocytes from?/+ and nm/nm mice were conducted to investigate further the physiologic basis of the anemia. Although the increased ZnPP/H ratio and transplantation data suggested an intrinsic hematopoietic defect in iron metabolism, it was not clear whether the defect was specifically due to an inability of red blood cell precursors per se to assimilate iron for the purpose of heme biosynthesis. As has been done with other murine mutants with suspected red cell iron uptake defects, we assessed the ability of nm/nm reticulocytes to take up and use transferrin-bound iron.
Kinetics of bone marrow transplantation with nm/nm and ?/+ donors. Irradiated C57BL/6J-Igha Thy1a Gpi1a +/+ animals that had received C57BL/6J-Ighb Thy1b Gpi1b ?/+ (n = 6) or nm/nm (n = 5) fetal liver cells were serially monitored for RBC chimerism by Gpi1 isozyme analysis and blood parameters. The decrease in MCV closely follows engraftment by nm/nm cells. Error bars indicate plus or minus 1 standard deviation (SD).
Kinetics of bone marrow transplantation with nm/nm and ?/+ donors. Irradiated C57BL/6J-Igha Thy1a Gpi1a +/+ animals that had received C57BL/6J-Ighb Thy1b Gpi1b ?/+ (n = 6) or nm/nm (n = 5) fetal liver cells were serially monitored for RBC chimerism by Gpi1 isozyme analysis and blood parameters. The decrease in MCV closely follows engraftment by nm/nm cells. Error bars indicate plus or minus 1 standard deviation (SD).
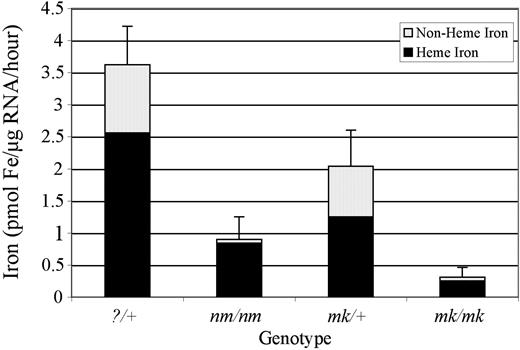
To do so, mutant and wild-type reticulocytes were incubated with 55Fe2-Tf, and the total intracellular iron and heme iron fractions were measured. Total iron uptake in nm/nm reticulocytes was decreased approximately 4-fold compared with?/+ control cells (0.90 ± 0.35 versus 3.62 ± 0.60 pmol Fe/μg RNA/h) (Figure 3). In addition to a decrease in total iron uptake, nm/nm reticulocytes also incorporated much less iron into heme compared with?/+ cells (0.84 ± 0.25 versus 2.56 ± 0.46 pmol Fe/μg RNA/h). However, the proportion of iron in the cell incorporated into heme was slightly higher in nm/nm reticulocytes (94% ± 8% versus 71% ± 9%). Qualitatively similar results were obtained using this assay with MK/ReJ-Slc11a2mk/mk animals, known to have a defect in transferrin-mediated iron uptake, due to a mutation in the endosomal iron transporter divalent metal transporter-1 (Dmt1; Slc11a2). The findings demonstrate that nm/nm animals have a defect in iron assimilation into the red cell precursor and suggest that they do not have a defect in incorporation of intraerythroid iron into heme.
To determine whether the defect in nm/nm reticulocytes was specifically due to a defect in receptor-mediated endocytosis of transferrin-bound iron, similar to Slc11a2mk/mk and hbd mice and Slc11a2b/b rats, we determined the efficiency with which iron internalized by endocytosis is irreversibly transferred to the cell. To do so, reticulocyte-rich RBCs were briefly loaded with 55Fe2-Tf, treated with Pronase (Sigma-Aldrich, Saint Louis, MO) to remove surface-bound iron, washed, transferred to normal mouse plasma, and incubated at 37°C, and the radioactivity remaining in the cell pellet was compared with that of cells incubated at 4°C. This procedure determines how much, if any, of the non–surface-bound, cell-associated iron, including iron in endosomes, can be released from the cell. In nm/nm cells, there was a significant loss of iron; on average of 31% ± 4% of the iron was lost compared with an average of 3% ± 3% loss in?/+ controls. Similar results were obtained when cells were incubated with plasma from nm/nm animals (data not shown), further confirming that the functional defect is not in a plasma component, such as transferrin, important for RBC iron assimilation. Overall, these results are consistent with an endosomal iron-processing defect in which there is inefficient transfer of endosomal iron to the cell.
Transferrin-bound iron uptake by nm/nm reticulocytes. Reticulocyte iron uptake and heme synthesis was measured in 129S6/SvEvTac-?/+ (n = 10) and -nm/nm (n = 5) as well as MK/ReJ-Slc11a2mk/+ (n = 4) and -Slc11a2mk/mk (n = 9) animals following incubation with 55Fe2-transferrin. Error bars are of total iron uptake ± 1 SD. Incubations were carried out for 30 minutes, when the assay was linear, but normalized to and presented as uptake per microgram of RNA per hour.
Transferrin-bound iron uptake by nm/nm reticulocytes. Reticulocyte iron uptake and heme synthesis was measured in 129S6/SvEvTac-?/+ (n = 10) and -nm/nm (n = 5) as well as MK/ReJ-Slc11a2mk/+ (n = 4) and -Slc11a2mk/mk (n = 9) animals following incubation with 55Fe2-transferrin. Error bars are of total iron uptake ± 1 SD. Incubations were carried out for 30 minutes, when the assay was linear, but normalized to and presented as uptake per microgram of RNA per hour.
nm1054 maps to chromosome 1
Because the anemic phenotype bears some resemblance to other microcytic anemias, we sought to determine whether it was allelic with other iron metabolism mutants with intrinsic erythroid defects, and, if not, whether it mapped to a novel locus. Breeding studies demonstrated that nm1054 was not an allele of microcytic anemia (Slc11a2mk),24 or hemoglobin deficit (hbd)32 (data not shown). Using pools of affected and unaffected [CBA × CAST]F2 intercross progeny segregating the nm1054 allele, we mapped the locus to chromosome 1. Genotypic analysis of individual animals in the affected pool with these and additional markers on chromosome 1 demonstrated that nm1054 mapped between D1Mit258 and D1Mit288: D1Mit258—2.2 ± 2.2 cM—nm1054—9.2 ± 4.7 cM— D1Mit288 (Figure 4). This map position excludes other genes known to, and that, based on current knowledge, could conceivably result in hypochromic, microcytic anemias, including the transferrin receptor, transferrin, duodenal cytochrome B (DcytB or Cyrbd1), the globin loci, and heme biosynthesis genes, which map to other chromosomes. Ferroportin 1 (Slc40a1) and natural resistance membrane protein 1 (Nramp1) both map to mouse chromosome 1; however, they are excluded as candidate genes by the recombination data. The candidate region is 41.6 megabases (Mb) and includes more than 200 genes encoding proteins that could conceivably result in an iron deficiency anemia phenotype. Among these are multiple proteins belonging to families involved in vesicle transport, including Ras-associated binding (RAB) proteins and kinesins, as well as several oxidoreductases, a vacuolar H+–adenosine triphosphatase (ATPase), and multiple cation transporters.
Discussion
In the absence of evidence for a thalassemia, hemoglobinopathy, or sideroblastic anemia, the presence of a transplantable, hypochromic, microcytic anemia with increased ZnPP/H and adequate tissue iron stores in nm1054 animals strongly suggests a primary defect in the delivery of iron to the red blood cell for the purpose of heme biosynthesis. Erythroid cells are uniquely dependent on the transferrin cycle to deliver iron sufficient for hemoglobin synthesis. The transplantability, normal TIBC, and lack of allelism with the Trfhpx hypotransferrinemia mouse mutant exclude a quantitative or qualitative defect in transferrin as the underlying cause of the anemia. Furthermore, the lack of allelism with a targeted mutation in the transferrin receptor-1 (Trfr1-/-), argues against a mutation in that gene as well. A unique abnormality in iron recycling from senescent red blood cells is also unlikely, because iron does not selectively accumulate in reticuloendothelial cells, and an iron uptake defect can be detected in reticulocytes in vitro. This deficit in reticulocyte transferrin-dependent iron uptake indicates that nm1054 is almost certainly due to a mutation in a gene either directly or indirectly necessary for efficient assimilation of iron from the transferrin cycle. The nm1054 functional defect is qualitatively similar to the erythroid defects in the microcytic anemia (mk) and hemoglobin deficit (hbd) murine mutants, the former of which is due to a mutation in the endosomal and apical intestinal iron transporter Dmt1 (Slc11a2). Iron uptake, normalized to reticulocyte RNA content, is reduced, and a substantial fraction of the iron initially internalized by endocytosis is released from the cell. Such functional abnormality could be the result of a defect in a component of the receptor-mediated endocytosis apparatus, an alternative, partially redundant endosomal iron transporter, a failure to acidify endosomes, or a failure to reduce iron from Fe3+ bound to transferrin to Fe2+ required for transport by Dmt1, among other possibilities. Ongoing mapping studies will eventually lead to the identification of the nm1054 gene and the function of its protein product and will further illuminate the pathogenesis of the anemia.
nm1054 maps to chromosome 1. Ideogram depicting the nm1054 region on mouse chromosome 1. The acromere is represented at the top. Simple sequence length polymorphism (SSLP) markers and recombination distances in centimorgans between adjacent markers are listed in the right- and the left-hand columns, respectively. The map is based on analysis of 23 affected [CBA × CAST]F2 intercross animals (46 meioses).
nm1054 maps to chromosome 1. Ideogram depicting the nm1054 region on mouse chromosome 1. The acromere is represented at the top. Simple sequence length polymorphism (SSLP) markers and recombination distances in centimorgans between adjacent markers are listed in the right- and the left-hand columns, respectively. The map is based on analysis of 23 affected [CBA × CAST]F2 intercross animals (46 meioses).
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2005-01-0379.
Supported by the National Institutes of Health (grants HL 074247 and DK 062474) (M.D.F.), the Pew Biomedical Scholars Program (M.D.F.), the Wilkes Fund of the Children's Hospital Boston, Department of Pathology (M.D.F.), and the National Institutes of Health (grants DK 49525 and DK 27726) (J.E.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Eva Eicher for recognizing and propagating the nm1054 mutation, and Nancy Hamblen who maintained the mice and organized the original characterization of the strain in the Barker laboratory. Howard Mulhern and James Edwards of the Children's Hospital, Department of Pathology Electron Microscopy Facility and the Histology Core Facility, respectively, provided expert technical assistance, and Drs Terence Law and Carlo Brugnara of the Clinical Core Laboratory at Children's Hospital offered invaluable technical advice on clinical laboratory tests. We thank Dr An-Ping Han for determining globin synthesis ratios and Dr Emanuela Gussoni for facilitating fetal liver transplantations. Members of the Andrews and Neufeld laboratories at Children's Hospital are acknowledged for ongoing critical evaluation of the project.

![Figure 4. nm1054 maps to chromosome 1. Ideogram depicting the nm1054 region on mouse chromosome 1. The acromere is represented at the top. Simple sequence length polymorphism (SSLP) markers and recombination distances in centimorgans between adjacent markers are listed in the right- and the left-hand columns, respectively. The map is based on analysis of 23 affected [CBA × CAST]F2 intercross animals (46 meioses).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-01-0379/6/m_zh80220587040004.jpeg?Expires=1771336945&Signature=WQg37--FMZhb8yybiXJaag4bjGW1cmqw85DtP94wDagXai5-BDdf79BhXnNkLFFSf1c4hJqyFUrybS8tdtHN-k0Pr7izJDMTeXASakCKWv5s1eB01QMZ~L08mbgX0m9YXZy2uE-G036kbioFVNpJZT4DzqxmawDa0xw3LpKES9yxDRc3nfVLoxsknVw9aaA54GIgJ-RqCguCu-4gUHsbYloglxLyRoy61fdafYEkFEjjo7ov7W~t3DVUL01XMxkDFt2AL3Mv8EzA9XvRv9wJ7VaZyaAINB-mchczJriGI0i1rCnhrLyB5iu78r8t8bxkDoEZ~840qHW7xDA5fKoDGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal