Cell adhesion and migration are both regulated by the rapid synthesis of lipids in the plasma membrane by phosphatidylinositol 3-kinase (PI3K).
Phosphatidylinositol 3-kinases (PI3Ks) are a diverse family of enzymes that phosphorylate the D-3 position of the inositol ring of phosphatidylinositol to produce phosphatidylinositol 3-phosphate (PI3-P), phosphatidylinositol 3,4-bisphosphates (PI3,4-P2), and phosphatidylinositol 3,4,5-triphosphates (PI3,4,5-P3 or PIP3). The 4 human isoforms of PI3K that generate PIP3 are classified by their catalytic subunit: p110α, p110β, p110γ, and p110δ. The p110α, p110β, and p110δ isoforms (PI3Kα, PI3Kβ, and PI3Kδ) associate with the regulatory subunit p85, and are tightly linked to signaling by growth factor, immunoglobulin, and cytokine receptors. The p110γ isoform (PI3Kγ) is stimulated by G-protein βγ heterodimers (Gβγ) and associates with p101, a regulatory subunit that has no similarity to p85. Although it was originally described in myeloid cells, PI3Kγ has been found in other blood cells as well as the endothelium.1,2
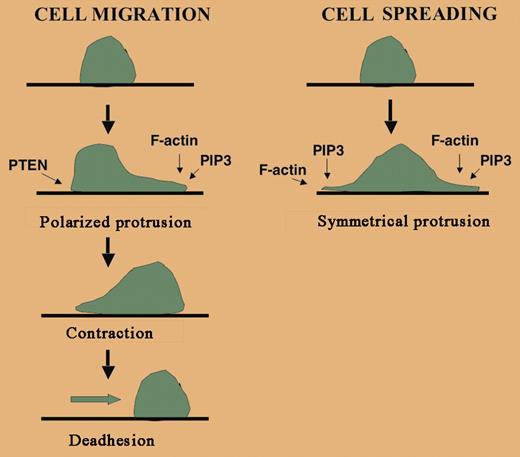
Cells undergoing chemotaxis develop a polarity with a lamellipodia in the front of the cell that is rich in F-actin and a uropod in the rear of the cell that also contains actin-binding proteins such as moesin, intercellular adhesion molecule 3 (ICAM-3), and myosin. Chemotaxis occurs by the alignment of this polarity along a gradient of chemoattractant. Studies in Dictyostelium and murine hematopoietic cells have shown that PI3K is critical for chemotaxis, but the particular isoform required appears to depend on the cell type. For example, neutrophil chemotaxis requires PI3Kγ and PI3Kδ3,4 but monocyte chemotaxis requires PI3Kβ and PI3Kγ.5,6 In these examples, a chemoattractant stimulates the transient accumulation of PIP3, and therefore PIP3-binding proteins, at the plasma membrane along the leading edge of the cell. Phosphatase and tensin homolog (PTEN), a phospholipid phosphatase localized at the rear of the cell, ensures the relative dearth of PIP3 and its binding partners at the trailing edge (see figure).FIG1
Although both spreading and migration involve actin polymerization and protrusion, cell locomotion is complex and requires cell polarity, contraction, and deadhesion, none of which are needed for spreading. PI3K at the leading edge of a cell is important for generating PIP3. Localized PIP3 binds specific actin regulatory proteins and leads to F-actin assembly. PTEN is an inositol phosphatase localized at the trailing edge of the migrating cell that dephosphorylates PIP3 into PIP2.
Although both spreading and migration involve actin polymerization and protrusion, cell locomotion is complex and requires cell polarity, contraction, and deadhesion, none of which are needed for spreading. PI3K at the leading edge of a cell is important for generating PIP3. Localized PIP3 binds specific actin regulatory proteins and leads to F-actin assembly. PTEN is an inositol phosphatase localized at the trailing edge of the migrating cell that dephosphorylates PIP3 into PIP2.
Like cell migration, adhesion also involves lamellipodia formation and PI3K-induced actin polymerization. But cell migration is more complex because it requires cell polarity, contraction, and de-adhesion, none of which are needed for simple adhesion. For example, nonlocalized activation of Rac leads a nonpolarized increase in F-actin and adhesion that retards cell migration.
In this issue of Blood, 2 articles address the relationship between specific PI3K isoforms and the migration and adhesion of hematopoietic cells. First, Munugalavadla and colleagues rigorously characterized the defects in monocytes lacking the dominant isoform of p85 (and hence the majority of PI3Kα, PI3Kβ, and PI3Kδ activity). Using several independent assays of directional cell migration, they found marked defects in both cell adhesion and migration. Second, Puri and colleagues analyzed the role of PI3K in neutrophil migration along inflamed blood vessel walls. Given the known role of PI3K in cell adhesion, they found the expected result that neutrophil adhesion to the endothelium was markedly impaired in PI3Kγ and PI3Kδ knockout mice. Remarkably, they found that wild-type neutrophils were also impaired in their ability to adhere to PI3Kγ knockout endothelial cells. This demonstrates the importance of a PI3Kγ-dependent signaling cascade within endothelial cells, that is, cells required for their adhesion to neutrophils. Puri and colleagues speculate that endothelial cell PI3Kγ is required for an endothelial cell adhesion receptor, E-selectin, to tether neutrophils. Although at this point, other possible explanations still exist.
In addition to chemotaxis and adhesion, PI3Ks are involved in diverse cellular events, including the prevention of apoptosis, regulation of glucose metabolism, and cell proliferation. Lessons from pharmacologic inhibitors, knockout mice, and single-cell microscopy studies have advanced our knowledge of these processes considerably over the past few years. The challenge will be to inhibit specific PI3K isoforms in discreet regions of cells to target migration without affecting other PI3K-dependent processes. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal