Activating mutations of Flt3 are found in approximately one third of patients with acute myeloid leukemia (AML) and are an attractive drug target. Two classes of Flt3 mutations occur: internal tandem duplications (ITDs) in the juxtamembrane and point mutations in the tyrosine kinase domain (TKD). We and others have shown that Flt3-ITD induced aberrant signaling including strong activation of signal transducer and activator of transcription 5 (STAT5) and repression of CCAAT/estradiol-binding protein α (c/EBPα) and Pu.1. Here, we compared the signaling properties of Flt3-ITD versus Flt3-TKD in myeloid progenitor cells. We demonstrate that Flt3-TKD mutations induced autonomous growth of 32D cells in suspension cultures. However, in contrast to Flt3-ITD and similar to wild-type Flt3 (Flt3-WT), Flt3-TKD cannot support colony formation in semisolid media. Also, in contrast to Flt3-ITD, neither Flt3-WT nor Flt3-TKD induced activation or induction of STAT5 target genes. Flt3-TKD also failed to repress c/EBPα and Pu.1. No significant differences were observed in receptor autophosphorylation and the phosphorylation of Erk-1 and -2, Akt, and Shc. Importantly, TKD but not ITD mutations were a log power more sensitive toward the tyrosine kinase inhibitor protein kinase C 412 (PKC412) than Flt3-WT. In conclusion, Flt3-ITD and Flt3-TKD mutations display differences in their signaling properties that could have important implications for their transforming capacity and for the design of mutation-specific therapeutic approaches.
Introduction
The receptor tyrosine kinase (RTK) Flt3 is expressed on early hematopoietic progenitors, B-cell precursor cells, and macrophage precursors.1-3 It has important functions in early hematopoietic progenitor proliferation and survival as well as in macrophage and dendritic cell differentiation.1,4 In acute myeloid leukemia (AML), Flt3 is uniformly highly expressed, and it has been shown to be mutated in about one third of patients with AML.1,5 Two classes of mutations have been described. The first class of mutations consists of internal tandem duplications (ITDs) in exons 14 and 15 of the receptor, leading to the insertion of several amino acids in the juxtamembrane domain.6,7 In previous work, we and others have shown that Flt3-ITD induces factor-independent proliferation and radioresistance of growth factor-dependent myeloid cell lines.6,8 Furthermore, the ITD mutations enhance their leukemogenicity in vivo.6 Flt3-ITD mutations also induce a myeloproliferative disease if retrovirally transduced into donor bone marrow in transplantation experiments.9 If donor bone marrow is used that is transgenic for promyelocytic leukemia–retinoic acid receptor α (PML-RARα), Flt3-ITDs synergize with PML-RARα, and most recipient animals develop an acute promyelocytic leukemia (APL) that is normally only observed after a long latency period with a low penetrance.10,11
The other class of Flt3 mutations consists of substitutions, small deletions, or insertions in a highly conserved structure of RTKs, the activation loop of the tyrosine kinase domain (TKD). Activating mutations in this loop are found in approximately 7% of patients with AML.12-14
The most common TKD mutation of Flt3 (Flt3-TKD) involves either a substitution of the highly conserved aspartic acid 835 (D835) to other residues, most frequently to tyrosine, valine, or histidine. Also, the deletion of the equally conserved I836 has been described.12,14,15 TKD mutations have been shown to be activating, and they confer factor-independent growth to interleukin 3 (IL-3)–dependent cell lines.12,15,16
Large patient series have been characterized with respect to the clinical differences between cases with the expression of Flt3-ITD, wild-type Flt3 (Flt3-WT), and Flt3-TKD. It is now very well documented that Flt3-ITDs are associated with a poor prognosis.5,7,14,17-20 In contrast, the Flt3-TKD mutations have so far not been shown to be of similar prognostic relevance, despite several studies on large patient series.12-14,19 Also, differences in the clinical presentation of AML cases with Flt3-ITD and Flt3-TKD mutations have been described, that is, a higher white blood cell count in patients harboring Flt3-ITD mutations.12,14,21-23 In a detailed clinical study by Thiede et al,14 some chromosomal translocations, for example, -7/7q, +11/+13/+22/+22, inv(16), and t(16;16) were almost absent in patients with ITD mutations, but these translocations were present at a frequency close to that in the overall population in samples that harbored Flt3-TKD mutations.
Here, we directly compared the signaling properties of the 2 mutation classes and the wild-type receptor, their transforming capacity, and their response with the tyrosine kinase inhibitor protein kinase C 412 (PKC412). Flt3-TKD mutations resembled the signal transduction properties of Flt3-WT more closely than the Flt3-ITD mutations. Furthermore, Flt3-TKD mutations conferred higher drug sensitivity to Flt3 toward PKC412, not only when compared to Flt3-ITD, but also when compared to Flt3-WT.
Patients, materials, and methods
The use of human material for scientific purposes was approved by the human ethics committee of each participating institution.
Reagents
Recombinant human Flt3 ligand (FL) and recombinant murine IL-3 were purchased from PeproTech (Rocky Hill, NJ). Polyclonal rabbit anti-Shc antibody was purchased from Transduction Laboratories (Lexington, KY). The rabbit polyclonal anti–phospho-Shc, anti–phospho-Flt3 (Tyr591), and anti–phospho–Erk-1/2 antibodies were obtained from Cell Signaling Technology (Beverly, MA). The antibodies for anti–signal transducer and activator of transcription 5a/b (anti-STAT5a/b), anti-CCAAT/enhancer-binding protein α (C/EBPα), anti-PU.1, anti-Flt3, anti-Pim-2, and anti–cytokine-inducible SH2 protein (CIS) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies for anti–Erk-1/2 and anti–phospho-STAT5a/b were purchased from Upstate Biotechnology (Lake Placid, NY). The mouse monoclonal antiactin antibody was purchased from Sigma (Taufkirchen, Germany). Phycoerythrin (PE)–labeled monoclonal mouse anti–human Flt3 antibodies as well as appropriate isotype controls were obtained from PharMingen (San Diego, CA). The Flt3-specific inhibitor PKC412 was a kind gift from Novartis Pharmaceuticals (Basel, Switzerland) and SU11248 from Sugen (San Francisco, CA).
Cell lines
The IL-3–dependent murine myeloid cell line 32Dcl3 (kindly provided by Dr Felicitas Rosenthal, Freiburg, Germany) was cultured in RPMI 1640 supplemented with 10% Walter and Eliza Hall Institute (WEHI)–conditioned medium as a source of IL-3, 10% fetal calf serum (FCS), penicillin/streptomycin, and l-glutamine. Cells were cultured in a humidified incubator at 37°C with 5% CO2.
Flow cytometry
Cells were washed once with PBS and stained for 20 minutes with the indicated antibodies. Subsequently, the cells were washed twice in PBS/0.1% NaN3/0.1% bovine serum albumin (BSA) and analyzed with a FACSCalibur cytometer (Becton Dickinson, Heidelberg, Germany) using CellQuest software (BD Biosciences, Palo Alto, CA).
3H-thymidine incorporation
A total of 2 × 104 32D cells were IL-3 and serum starved (0.5% FCS) for 12 hours in 200 μL medium in a 96-well plate. Subsequently, cells were placed in medium with 10% FCS, supplemented with the indicated concentrations of IL-3 or FL. The SU11248 or PKC412, wherever used, was added 1 hour prior to addition of growth factors. After an 8-hour incubation period at 37°C, 1 μCi (0.037 MBq) 3H-thymidine was added to each well, and cells were incubated for additional 12 hours. Cells were harvested onto glass fiber filters, and β emission of bound DNA was analyzed in a scintillation counter. Each data point represents the mean and the SD of triplicates from one of at least 3 independent experiments.
Analysis of cell growth
The 32D cells expressing Flt3 constructs were washed twice with PBS and resuspended in RPMI 1640 with 10% FCS alone or supplemented with indicated growth factors. The viable cells, determined by trypan blue exclusion, were counted every day until day 6. Cells were seeded at a concentration of 2 × 105 cells/mL and cell numbers were adjusted every day.
Site-directed mutagenesis
Site-directed mutagenesis was performed using the QuickChange site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's instructions. The ITD 1 (YEYDLK) and ITD W51 (REYEYDL) mutations were introduced in Flt3-WT cDNA. The mutation to D835Y or D835H or ΔI836 was introduced into the previously described Flt3-WT or Flt3-ITD (NEYFYVDFREYE) cDNA.24 All mutations were confirmed by DNA sequencing.
Immunoprecipitation and Western blotting
32D cells transfected with Flt3 constructs were washed twice with PBS and starved overnight in medium containing 0.5% serum and were then stimulated for 5 minutes at 37°C with 40 ng/mL FL. Subsequently, cells were washed once with ice-cold PBS and lysed with buffer containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 10% glycerol, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethylene glycol tetraacetic acid), 50 μM ZnCl2, 25 mM NaF, proteinase inhibitors (Complete; Boehringer Mannheim, Mannheim, Germany), 1 μM pepstatin, and 1 mM sodium orthovanadate. After incubation on ice for 10 minutes, cell lysates were clarified at 20 000g for 10 minutes at 4°C. For immunoprecipitation, the cell lysates were incubated either with control rabbit polyclonal serum or with an antibody to Flt3 (Sc-480; Santa Cruz Biotechnology) overnight, then Protein A/G-Plus-Sepharose (Santa Cruz Biotechnology) was added for an additional 2 hours. The immunoprecipitates were washed 4 times with lysis buffer, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon P membranes (Millipore, Bedford, MA), and probed either with 4G10 (a phosphotyrosine-specific antibody) or with anti–phospho-Flt3 (an antibody that recognizes Flt3 phosphorylated on Y591) antibodies overnight at 4°C. Immunodetection was performed using ECL-Plus (Amersham Pharmacia, Uppsala, Sweden).
Patient samples
Bone marrow samples processing and lysate preparation was done as described earlier.25 The cells were stored at -80° in FCS plus 10% dimethyl sulfoxide (DMSO). For lysate preparation cells were thawed and whole cell lysates were prepared in lysis buffer (see “Immunoprecipitation and Western blotting”). Written informed consent was obtained from all patients.
Kinase assays
The kinase assays were performed as described previously.26 Briefly, 32D cells expressing Flt3 constructs were starved from IL-3 and grown in presence of 100 nM PKC412 for 6 hours. The cells were lysed in cell lysis buffer and Flt3 was immunoprecipitated as described (see “Immunoprecipitation and Western blotting”). Immunoprecipitates were washed 3 times with lysis buffer and once with kinase buffer containing 50 mM HEPES, pH 7.4, 5 mM MnCl2, and 0.1 mM sodium orthovanadate. The immunoprecipitates were resedimented and resuspended in 20 μL kinase buffer containing 0.5 mM cold adenosine triphosphate (ATP) and 10 μCi (0.37 MBq) [γ-32P] ATP. The kinase reaction was allowed to proceed at 30°C for 20 minutes. The samples were boiled with SDS-PAGE sample buffer and analyzed by SDS-PAGE and autoradiography.
Generation of the Flt3-expressing 32D cell lines
Construction of human Flt3-WT, Flt3-ITD, and Flt3-TKD mutant expressing 32D cell lines was essentially performed as described previously.6 Several bulk cultures were generated for Flt3-D835Y mutant, 2 bulk cultures D835Y-1 and D835Y-2 were used as indicated; the D835Y-1 was used unless specified. All cell lines were generated and maintained in medium supplemented with WEHI-conditioned medium as source of IL-3 until being used for the experiments.
Surface expression and autophosphorylation of Flt3 receptors. 32D cells were transfected with constructs encoding either Flt3-WT or Flt3-D835Y or Flt3-ITD. (A) Flow cytometric analyses of Flt3 surface expression on 32D cells. The shaded curves show staining with anti-Flt3 antibody; the open curves, the isotype control. (B) Flt3 was immunoprecipitated from 32D cells expressing the indicated Flt3 constructs. Immunoprecipitates were resolved on 4% to 15% SDS-PAGE and immunoblotted for 4G10, an antibody specific for phosphotyrosine. Equal loading was confirmed by reprobing with an antibody recognizing activation independent state of Flt3.
Surface expression and autophosphorylation of Flt3 receptors. 32D cells were transfected with constructs encoding either Flt3-WT or Flt3-D835Y or Flt3-ITD. (A) Flow cytometric analyses of Flt3 surface expression on 32D cells. The shaded curves show staining with anti-Flt3 antibody; the open curves, the isotype control. (B) Flt3 was immunoprecipitated from 32D cells expressing the indicated Flt3 constructs. Immunoprecipitates were resolved on 4% to 15% SDS-PAGE and immunoblotted for 4G10, an antibody specific for phosphotyrosine. Equal loading was confirmed by reprobing with an antibody recognizing activation independent state of Flt3.
Clonal growth in methylcellulose
To analyze clonal growth, 1 mL of a culture mixture containing Iscove modified Dulbecco medium (Life Technologies, Grand Island, NY), 1% methylcellulose, and 10% FCS was plated on a 35-mm culture dish in the presence of either with FL (40 ng/mL), IL-3 (2 ng/mL), or no cytokine. Stably transfected 32D cells expressing Flt3 constructs were seeded at a concentration of 1 × 103 cells/dish. The assays were plated as triplicates, and colonies were photographed and counted on day 6. The results shown are representative of one of at least 3 independent experiments per construct.
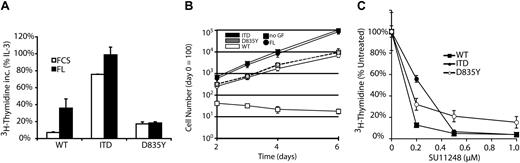
Factor-independent growth of 32D cells expressing either Flt3-D835Y or Flt3-ITD. (A) Ligand-independent 3H-thymidine incorporation by Flt3-ITD and Flt3-D835Y mutants. 32D cells expressing different Flt3 constructs were starved for 12 hours in medium supplemented with 0.5% serum. Subsequently, cells were exposed to FL or IL-3 or left unstimulated. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. (B) Long-term growth of Flt3-expressing cells. 32D cells containing the indicated Flt3 constructs were seeded at 2 × 105/mL in suspension cultures. Cells were grown in the presence of 10% FCS alone or in combination with FL and were counted until day 6 by trypan blue exclusion method. GF indicates growth factor. (C) Ligand-independent proliferation of cells expressing Flt3 mutants is dependent on Flt3 kinase activity. 3H-thymidine incorporation was analyzed in the presence of the tyrosine kinase inhibitor SU11248 in the presence of FCS only (Flt3-ITD, Flt3-D835Y) or FCS and FL (Flt3-WT). Data represent mean values ± standard deviation (SD) of triplicates.
Factor-independent growth of 32D cells expressing either Flt3-D835Y or Flt3-ITD. (A) Ligand-independent 3H-thymidine incorporation by Flt3-ITD and Flt3-D835Y mutants. 32D cells expressing different Flt3 constructs were starved for 12 hours in medium supplemented with 0.5% serum. Subsequently, cells were exposed to FL or IL-3 or left unstimulated. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. (B) Long-term growth of Flt3-expressing cells. 32D cells containing the indicated Flt3 constructs were seeded at 2 × 105/mL in suspension cultures. Cells were grown in the presence of 10% FCS alone or in combination with FL and were counted until day 6 by trypan blue exclusion method. GF indicates growth factor. (C) Ligand-independent proliferation of cells expressing Flt3 mutants is dependent on Flt3 kinase activity. 3H-thymidine incorporation was analyzed in the presence of the tyrosine kinase inhibitor SU11248 in the presence of FCS only (Flt3-ITD, Flt3-D835Y) or FCS and FL (Flt3-WT). Data represent mean values ± standard deviation (SD) of triplicates.
Radiation-induced apoptosis
The assay was performed as described before.6 Briefly, 32D cells expressing the indicated Flt3 constructs were starved from IL-3 for 3 hours and subsequently irradiated with 5 Gy γ irradiation, in the presence or absence of the indicated growth factors. At the indicated time points, cells were stained with annexin V and propidium iodide as described before. Cells staining negative for annexin V and propidium iodide were counted as “viable.” Statistical analyses were performed using the Mann-Whitney U algorithm.
Results
Surface expression and phosphorylation analysis of Flt3-WT, Flt3-D835Y, and Flt3-ITD mutants
The substitution D835Y is the most frequently found Flt3-TKD mutation in AML. Therefore, we first compared the transforming properties of Flt3-ITD and Flt3-WT versus Flt3-D835Y that we stably expressed in 32D cells. Pools of 32D cells expressing either Flt3-WT, Flt3-ITD, or Flt3-D835Y were generated as described previously.6,27 Flow cytometric analyses revealed equal surface expression of all receptor isoforms (Figure 1A). Western blot analyses of Flt3 immunoprecipitates for phosphotyrosine revealed strong ligand-independent autophosphorylation of Flt3-ITD and Flt3-D835Y after a starvation period of the cells from IL-3 and serum for 12 hours. In the absence of ligand, Flt3-WT was phosphorylated only at basal levels. Incubation of the cells with FL for 10 minutes before lysis resulted in strong phosphorylation of Flt3-WT without a significant change in the phosphorylation of the 2 mutant Flt3 isoforms (Figure 1B). Therefore, Flt3-ITD and Flt3-D835Y display comparable levels of constitutive Flt3 autophosphorylation.
Differential transforming potentials of Flt3-D835Y and Flt3-ITD mutant receptors
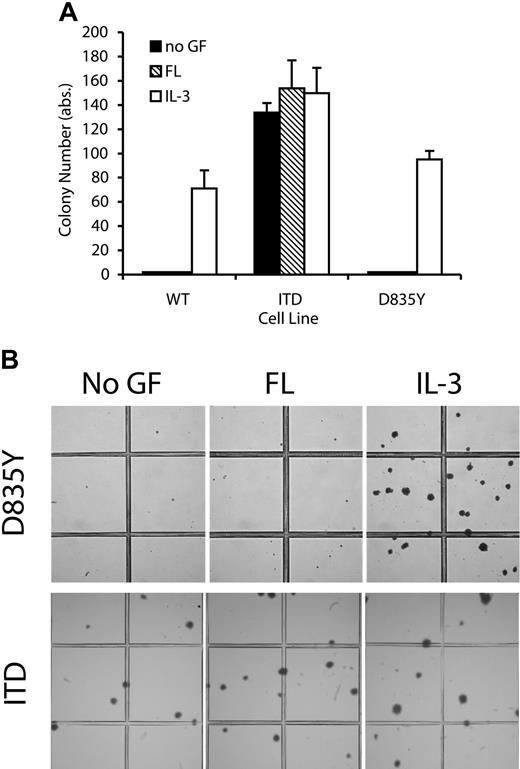
It was reported that both Flt3-ITD and Flt3-TKD mutations confer factor-independent growth to IL-3–dependent hematopoietic cells in vitro.12,16 Here, we directly compared the growth properties of 32D cells expressing either Flt3-WT, Flt3-ITD, or Flt3-D835Y. Transfection of 32D cells either with Flt3-ITD or Flt3-D835Y led to factor-independent growth in suspension cultures, whereas 32D-Flt3-WT cells remained factor-dependent and proliferated only in the presence of FL (Figure 2A-B). In comparison to Flt3-ITD, Flt3-D835Y induced significantly less pronounced autonomous growth of the cells, both in short-term (Figure 2A) as well as in long-term growth assays (Figure 2B). The factor-independent growth of cells that expressed mutant Flt3 was dependent on constitutive activation of the receptor because incubation with SU11248, a specific inhibitor for Flt3,28 led to strong dose-dependent growth inhibition (Figure 2C), which could be rescued by the addition of IL-3 (data not shown). To further investigate the transforming potential of the 2 mutant receptors, we examined their ability to confer clonogenic growth to 32D cells, as a marker of full transformation of hematopoietic cells in vitro. Cells expressing Flt3-ITD receptors readily formed colonies in semisolid media, regardless of the presence or absence of growth factors (Figure 3), as we have previously reported.6 To our surprise, 32D-Flt3-D835Y cells were unable to form colonies in semisolid media, even in presence of FL. This is reminiscent of the effects of Flt3-WT, which also was unable to support colony growth in presence of FL (Figure 3A).6 However, we repeatedly observed that Flt3-D835Y cells formed very few small cell clusters of no more than 10 cells both in the absence and presence of FL that died off at around day 4 (Figure 3B). To exclude the possibility of a general growth problem of these cells, we analyzed their colony formation ability in the presence of IL-3, where they displayed growth properties very similar to Flt3-ITD cells (Figure 3). To rule out clonal effects, we repeated the transfection procedure several times, also with different vectors, always used bulk cultures for the experiments, and always kept the cell lines in IL-3 to avoid selection effects. Similar results were obtained in all these experiments. Taken together, these results demonstrate differential transforming capacities of 2 Flt3 receptor mutations in vitro.
Flt3-D835Y does not induce clonogenic growth of 32D cells in semisolid media. The 32Dcl3 cells stably expressing the indicated Flt3 constructs were plated in triplicate at a concentration of 1000 cells/dish. Colonies were analyzed on day 6. (A) Colony numbers. Each bar represents the mean ± SD of a representative triplicate experiment. (B) Low-power photograph of representative areas of the plates demonstrates the lack of colony growth in 32D/Flt3-D835Y in the absence of IL-3. Photographs of the colony assays were taken with an Olympus C5050 digital camera attached to an Olympus CKX1 inverted microscope equipped with an Olympus Plan 4 ×/0.1 objective lens (Olympus, Hamburg, Germany).
Flt3-D835Y does not induce clonogenic growth of 32D cells in semisolid media. The 32Dcl3 cells stably expressing the indicated Flt3 constructs were plated in triplicate at a concentration of 1000 cells/dish. Colonies were analyzed on day 6. (A) Colony numbers. Each bar represents the mean ± SD of a representative triplicate experiment. (B) Low-power photograph of representative areas of the plates demonstrates the lack of colony growth in 32D/Flt3-D835Y in the absence of IL-3. Photographs of the colony assays were taken with an Olympus C5050 digital camera attached to an Olympus CKX1 inverted microscope equipped with an Olympus Plan 4 ×/0.1 objective lens (Olympus, Hamburg, Germany).
Both Flt3-ITD and Flt3-D835Y mutations induce resistance to radiation-induced apoptosis of 32D cells
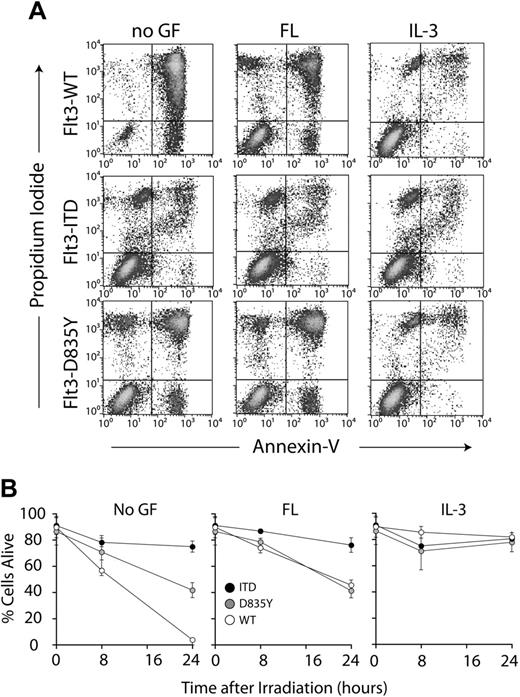
Next, we analyzed whether Flt3-D835Y provides resistance against radiation-induced apoptosis in 32D cells. For this purpose, 32D cells expressing the different Flt3 isoforms were starved from cytokines in the presence of 0.5% serum for 3 hours prior to γ irradiation and were analyzed for up to 24 hours thereafter for the induction of apoptosis, in the presence or absence of growth factors. As shown in Figure 4, 32D-Flt3-WT cells rapidly underwent apoptosis in the absence of exogenously added growth factors. Exposure to 40 ng/mL FL could rescue about 50% of these cells. In line with our previous reports,6 and in contrast to ligand-stimulated Flt3-WT, Flt3-ITD could almost completely rescue the cells from apoptosis, even in the absence of growth factors. In the absence of FL, Flt3-D835Y only partially protected 32D cells from apoptotic death following irradiation. This effect was not further enhanced by the addition of exogenous FL. These results again corroborated distinct biologic effects of Flt3-ITD and Flt3-D835Y.
Expression of Flt3-D835Y and Flt3-ITD induces radiation-induced apoptosis resistance in myeloid cells. Cells were washed and cytokine starved in 0.5% FCS for 3 hours and exposed to 5 Gy γ irradiation. Thereafter, cells were incubated for up to 24 hours in the presence or absence of the indicated cytokines, stained with annexin V and propidium iodide, and analyzed by flow cytometry. (A) Dot plots of the flow cytometric analyses up to 24 hours after irradiation. (B) Time course of cell viability after irradiation. Cells that were negative for annexin V and propidium iodide were counted as viable cells. Viability was calculated as a percentage of these cells over the total cell population. The data shown represent the average of 3 independent experiments ± SD. The observed differences of WT versus ITD versus D835Y (left) and ITD versus WT and D835Y (middle) were statistically significant (P < .01). No significant induction of apoptosis was observed under the chosen experimental conditions in the absence of irradiation (data not shown).
Expression of Flt3-D835Y and Flt3-ITD induces radiation-induced apoptosis resistance in myeloid cells. Cells were washed and cytokine starved in 0.5% FCS for 3 hours and exposed to 5 Gy γ irradiation. Thereafter, cells were incubated for up to 24 hours in the presence or absence of the indicated cytokines, stained with annexin V and propidium iodide, and analyzed by flow cytometry. (A) Dot plots of the flow cytometric analyses up to 24 hours after irradiation. (B) Time course of cell viability after irradiation. Cells that were negative for annexin V and propidium iodide were counted as viable cells. Viability was calculated as a percentage of these cells over the total cell population. The data shown represent the average of 3 independent experiments ± SD. The observed differences of WT versus ITD versus D835Y (left) and ITD versus WT and D835Y (middle) were statistically significant (P < .01). No significant induction of apoptosis was observed under the chosen experimental conditions in the absence of irradiation (data not shown).
Constitutive activation of downstream signaling pathways
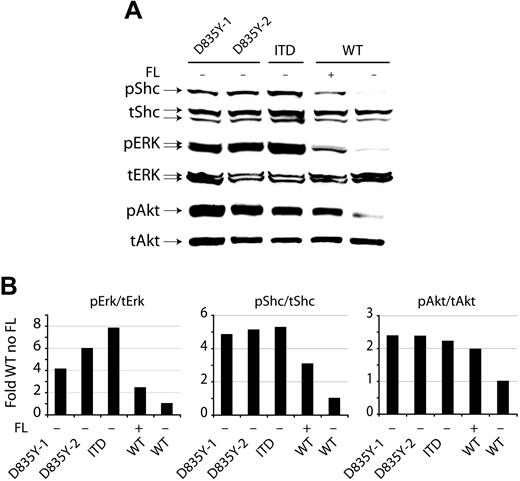
The different transforming potentials of the 2 mutant receptors prompted us to further investigate the underlying mechanisms. Therefore, we compared the various signaling pathways activated by the Flt3 isoforms. For direct comparison, 32D cells expressing Flt3-WT, Flt3-ITD, or Flt3-D835Y were starved from IL-3 and serum for 12 hours, and cell lysates were prepared and subjected to immunoblotting using activation-specific antibodies against signal transduction intermediates. As shown in Figure 5A-B, we observed constitutive activation of Erk-1 and Erk-2, Akt, and of Shc by both Flt3-ITD and Flt3-D835Y. Flt3-D835Y–expressing cells induced a somewhat weaker phosphorylation of Erk-1 and Erk-2, but constitutive phosphorylation of Shc and Akt was comparable with the Flt3-ITD–expressing cells. Flt3-WT activated all these signaling intermediates in a ligand-dependent manner. Collectively, these results demonstrate that both the ITD and D835Y mutations induce constitutive activation of Flt3 signaling pathways.
Constitutive activation of MAP kinase, Akt, and Shc by Flt3-ITD and Flt3-D835Y. (A) Constitutive activation of mitogen-activated protein (MAP) kinase Akt and Shc by Flt3 mutants. The 32D cells stably expressing the indicated Flt3 constructs were starved overnight in medium containing 0.5% FCS with or without the addition of FL as indicated. Total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained with the indicated activation-specific antibodies. phospho-Erk recognizes Erk-1 (p44), and Erk-2 (p42), phosphorylated on Tyr204; phospho-Akt is specific for Akt phosphorylated on S473 and phospho-Shc recognizes Shc phosphorylated on Y239/240. Subsequently, the blots were stripped and stained with the indicated antibodies recognizing above proteins in their nonactivated state to demonstrate equal loading. (B) The activation of Shc, Erk, and Akt was quantitated by densitometry from Western blots (from panel A).
Constitutive activation of MAP kinase, Akt, and Shc by Flt3-ITD and Flt3-D835Y. (A) Constitutive activation of mitogen-activated protein (MAP) kinase Akt and Shc by Flt3 mutants. The 32D cells stably expressing the indicated Flt3 constructs were starved overnight in medium containing 0.5% FCS with or without the addition of FL as indicated. Total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained with the indicated activation-specific antibodies. phospho-Erk recognizes Erk-1 (p44), and Erk-2 (p42), phosphorylated on Tyr204; phospho-Akt is specific for Akt phosphorylated on S473 and phospho-Shc recognizes Shc phosphorylated on Y239/240. Subsequently, the blots were stripped and stained with the indicated antibodies recognizing above proteins in their nonactivated state to demonstrate equal loading. (B) The activation of Shc, Erk, and Akt was quantitated by densitometry from Western blots (from panel A).
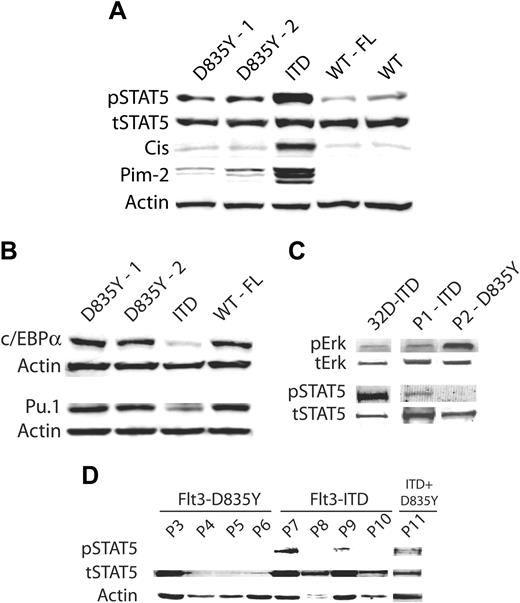
Differential activation of signaling pathways by the Flt3 mutation classes. (A) Differential STAT5 activation. The 32D cells stably expressing the indicated Flt3 constructs were starved overnight in medium containing 0.5% FCS with or without the addition of FL as indicated. Total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained using an activation state specific phospho-STAT5 (Y694/699) antibody. Subsequently, the membrane was stripped and reprobed using an anti-CIS antibody, as indicated. Equal loading was shown by reprobing the membrane with an antibody recognizing total STAT5. For analysis of the Pim-2 expression levels, the proteins were resolved on a 15% gel and stained with a Pim-2 antibody. Equal loading was confirmed by reprobing the blot with an antibody specific for β-actin. (B) The transcription factors Pu.1 and C/EBP-α are specifically repressed by Flt3-ITD mutations. Cells were grown for 36 hours in the absence of IL-3 and with (Flt3-WT) or without (Flt3-D835Y and Flt3-ITD) FL in medium supplemented with 10% FCS. The lysates were resolved by SDS-PAGE and immunoblotted with antibodies specific for c/EBP-α or Pu.1, as indicated. Membranes were stripped off and reprobed for β-actin to ensure equal loading. (C) Differential activation of STAT5, but not of Erk, by ITD versus D835Y in primary AML samples. Cell lysates were prepared from frozen bone marrow and total cell lysates were analyzed for activation of MAP kinase or STAT5 using activation state-specific antibodies, as described. (D) Preferential activation of STAT5 by Flt3-ITD in AML samples. The activation of STAT5 was analyzed as described in panel C. P indicates patient.
Differential activation of signaling pathways by the Flt3 mutation classes. (A) Differential STAT5 activation. The 32D cells stably expressing the indicated Flt3 constructs were starved overnight in medium containing 0.5% FCS with or without the addition of FL as indicated. Total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained using an activation state specific phospho-STAT5 (Y694/699) antibody. Subsequently, the membrane was stripped and reprobed using an anti-CIS antibody, as indicated. Equal loading was shown by reprobing the membrane with an antibody recognizing total STAT5. For analysis of the Pim-2 expression levels, the proteins were resolved on a 15% gel and stained with a Pim-2 antibody. Equal loading was confirmed by reprobing the blot with an antibody specific for β-actin. (B) The transcription factors Pu.1 and C/EBP-α are specifically repressed by Flt3-ITD mutations. Cells were grown for 36 hours in the absence of IL-3 and with (Flt3-WT) or without (Flt3-D835Y and Flt3-ITD) FL in medium supplemented with 10% FCS. The lysates were resolved by SDS-PAGE and immunoblotted with antibodies specific for c/EBP-α or Pu.1, as indicated. Membranes were stripped off and reprobed for β-actin to ensure equal loading. (C) Differential activation of STAT5, but not of Erk, by ITD versus D835Y in primary AML samples. Cell lysates were prepared from frozen bone marrow and total cell lysates were analyzed for activation of MAP kinase or STAT5 using activation state-specific antibodies, as described. (D) Preferential activation of STAT5 by Flt3-ITD in AML samples. The activation of STAT5 was analyzed as described in panel C. P indicates patient.
D835Y marginally activates STAT5 and weakly induces STAT5 downstream targets
STAT proteins are constitutively activated in many types of cancers and are thought to play a crucial role in disease development and progression.29 STAT5 is found to be constitutively activated in the majority of samples from patients with AML and is strongly activated by Flt3-ITD.6,30 Here, we compared the STAT5 activation potential of 2 different activating mutations of Flt3. The expression of Flt3-ITD, but not of ligand-activated Flt3-WT, led to constitutive and strong activation of STAT5 in 32D cells (Figure 6A). In contrast, STAT5 was only marginally activated by Flt3-D835Y in the absence (Figure 6A) or presence of FL (data not shown). The STAT5 target gene CIS, a member of the suppressor of cytokine signaling (SOCS) protein family, was highly induced by Flt3-ITD, whereas expression of Flt3-D835Y was followed by weak induction of CIS. Activation of Flt3-WT by FL failed to induce this protein (Figure 6A). The serine-threonine kinase Pim-2 is a functionally relevant downstream target of STAT5.24 Here, we observed only a weak induction of Pim-2 by Flt3-D835Y compared to the effects of Flt3-ITD (Figure 6A). Taken together, Flt3-D835Y induced a very weak but measurable activation of STAT5, as analyzed by STAT phosphorylation and induction of STAT5 target genes.
Next, we analyzed the activation of STAT5 in primary AML samples. Interestingly, STAT5 protein expression was lost in 2 of 5 analyzed samples expressing Flt3-D835Y and it was quite weak in another sample. None of the analyzed samples displayed STAT5 phosphorylation. On the other hand, we could identify phosphorylated STAT5 proteins in 4 of 6 samples expressing Flt3-ITD (Figure 6C-D).
ITD mutations, but not TKD mutations, suppress myeloid transcription factors
Recently, we have shown that Flt3-ITD mutations regulate the expression and function of c/EBPα and Pu.1.24 We now compared their regulation by Flt3-ITD, Flt3-D835Y, and Flt3-WT. Both proteins play a crucial role in the regulation of myeloid differentiation.31-33 Their inactivation is thought to be crucial for the differentiation block found in AML.32,34
Cells were washed off from IL-3 and cultured in 10% FCS for 36 hours, with or without FL. The protein expression of both transcription factors was analyzed on total cell lysates by Western blot analyses. The presence of Flt3-ITD led to suppression of both c/EBPα and Pu.1 (Figure 6B). However, this effect was not observed in cells expressing Flt3-D835Y or Flt3-WT in the presence of FL.
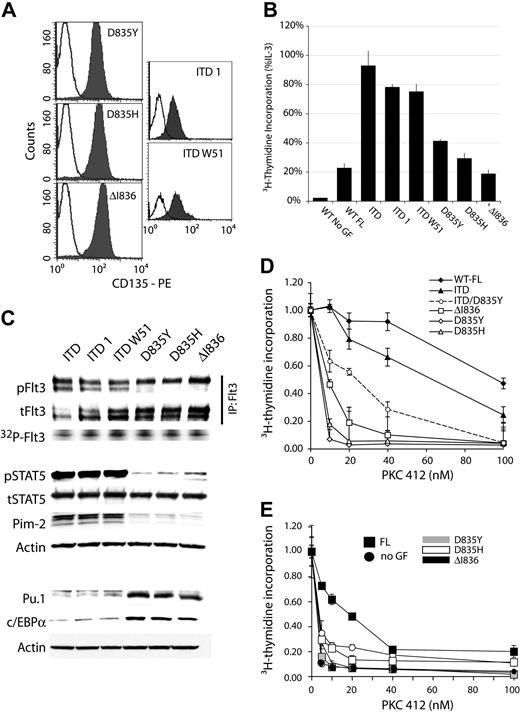
Similar signal transduction properties and sensitivity toward PKC412 of Flt3-TKD mutations. (A) Surface expression of Flt3-TKD and ITD mutations. The 32D cells expressing the indicated constructs were analyzed by flow cytometry using an anti–human Flt3 (CD135) antibody. The shaded curves show staining with anti-Flt3 antibody; open curves, the isotype control. (B) Comparison of the DNA synthesis of the different Flt3 mutant cell lines by 3H-thymidine incorporation. 32D cells expressing the indicated Flt3 constructs were starved for 12 hours in medium supplemented with 0.5% serum. Subsequently, cells were exposed to FL or IL-3 or left unstimulated. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. (C) Signaling properties of Flt3 mutants. The indicated cell lines were analyzed for Flt3 autophosphorylation using activation state-specific phospho-Flt3 (Y591) antibody. Flt3 kinase assays were performed as described in “Patients, materials, and methods.” The activation of the STAT5 pathway and expression of myeloid transcription factors was analyzed as described in “Patients, materials, and methods.” (D) The 32D cells containing the indicated Flt3 constructs were analyzed for their proliferation by 3H-thymidine incorporation as described in panel B under increasing concentrations of PKC412. Each data point represents the mean of 3H-thymidine incorporation of 3 samples ± SD. The numbers are given as fraction of DNA synthesis in the absence of PKC412. (E) Effect of FL on sensitivity of Flt3-TKD mutants toward PKC412 was analyzed in presence or absence of FL under increasing concentration of the inhibitor. Data represent mean values ± SD of triplicates.
Similar signal transduction properties and sensitivity toward PKC412 of Flt3-TKD mutations. (A) Surface expression of Flt3-TKD and ITD mutations. The 32D cells expressing the indicated constructs were analyzed by flow cytometry using an anti–human Flt3 (CD135) antibody. The shaded curves show staining with anti-Flt3 antibody; open curves, the isotype control. (B) Comparison of the DNA synthesis of the different Flt3 mutant cell lines by 3H-thymidine incorporation. 32D cells expressing the indicated Flt3 constructs were starved for 12 hours in medium supplemented with 0.5% serum. Subsequently, cells were exposed to FL or IL-3 or left unstimulated. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. (C) Signaling properties of Flt3 mutants. The indicated cell lines were analyzed for Flt3 autophosphorylation using activation state-specific phospho-Flt3 (Y591) antibody. Flt3 kinase assays were performed as described in “Patients, materials, and methods.” The activation of the STAT5 pathway and expression of myeloid transcription factors was analyzed as described in “Patients, materials, and methods.” (D) The 32D cells containing the indicated Flt3 constructs were analyzed for their proliferation by 3H-thymidine incorporation as described in panel B under increasing concentrations of PKC412. Each data point represents the mean of 3H-thymidine incorporation of 3 samples ± SD. The numbers are given as fraction of DNA synthesis in the absence of PKC412. (E) Effect of FL on sensitivity of Flt3-TKD mutants toward PKC412 was analyzed in presence or absence of FL under increasing concentration of the inhibitor. Data represent mean values ± SD of triplicates.
The effects of D835Y are recapitulated by other Flt3-TKD mutations
Although D835Y is the most frequent AML-associated mutation found in the TKD of Flt3, several other activating mutations were reported in this region including other substitutions of D835 and small deletion or insertion mutations.12-15 To analyze the signal transduction properties of ITD and TKD mutations and gain insight into the effects of Flt3-TKD mutations as a class, we introduced 2 additional mutations into Flt3, D835H and ΔI836, and expressed both constructs in 32D cells. Similarly, to generalize our findings of ITD, we generated 2 additional previously characterized Flt3-ITD mutants,6,9 and stably expressed them in 32D cells. All receptor isoforms were expressed at the cell surface (Figure 7A) and showed evidence of constitutive autophosphorylation (Figure 7C). All Flt3 mutants conferred factor-independent proliferation to 32D cells as analyzed by 3H-thymidine incorporation assays (Figure 7B) and long-term growth of the culture (data not shown). However, similar to Flt3-D835Y, the other TKD mutations induced a less pronounced proliferative response in suspension cultures and also failed to support 32D clonogenic growth (data not shown). When we analyzed the signaling properties of D835H and ΔI836, we also found a striking resemblance of the signaling properties of D835Y that was in contrast to Flt3-ITD (Figure 7C), including the lack of significant effects of the TKD mutations on STAT5 activation or repression of myeloid transcription factors c/EBPα and Pu.1 (Figure 7C). Taken together, these experiments established the finding that the TKD mutations indeed induce similar signaling alterations and have similar biologic consequences, which is different from ITD mutations, so that they can be considered as a functionally different mutation class.
Flt3-TKD confers high sensitivity to PKC412
Finally, we analyzed the relative sensitivity of Flt3-TKD mutations in comparison to Flt3-WT and Flt3-ITD toward the tyrosine kinase inhibitor PKC412.35 We incubated 32D cells containing either Flt3-WT, one of the Flt3-TKD mutations, or Flt3-ITD with increasing concentrations of PKC412 and analyzed cellular proliferation. Interestingly, 32D/Flt3-D835Y cells were much more sensitive toward PKC412 than 32D/Flt3-ITD or 32D/Flt3-WT cells (Figure 7D). All cell lines were not significantly affected by PKC412 up to 100 nM in the presence of IL-3 (data not shown). The higher sensitivity of Flt3-TKD mutants toward PKC412 in comparison to Flt3-ITD was not merely due to a “weaker” signal emanating from the Flt3-D835Y mutant. Ligand activation of Flt3-WT induced similar biologic and biochemical responses as the Flt3-TKD mutants. However, this receptor was more than a log power more resistant to PKC412. Also, we constructed a doublemutant receptor containing both an ITD-mutation and the D835Y substitution and included this receptor in our analyses. As shown in Figure 7D, the double-mutant receptor was more sensitive to Flt3 kinase inhibition than Flt3-ITD. Taken together, the TKD mutations confer high sensitivity toward PKC412 to Flt3, an effect that is dominant over the increased transforming potential of the Flt3-ITD mutations. These data clearly show that TKD mutations render Flt3 receptor more sensitive to PKC412. Recently, coexpression of Flt3 and its ligand on primary AML samples and leukemia cell lines was reported to cause autocrine signaling in these cells.36 Here, we analyzed the protective effect of FL on PKC412 sensitivity of Flt3-TKD mutants. Although the presence of FL had hardly any effect on PKC412 sensitivity of cells expressing Flt3-D835Y and D835H, surprisingly, the presence of FL could provide a protective role on Flt3-I836del–expressing cells (Figure 7E). Because the inhibitory mechanisms of PKC412 are not known at present, it is not clear from our data how FL partially rescued Flt3-I836del cells. But it is tempting to speculate that binding of FL to some Flt3 mutant receptors may lead to a slight change in the conformation/orientation of receptor dimers, which are less favorable to inhibitor binding. Taken together, our data suggest that the presence of Flt3-TKD mutations increases PKC412 sensitivity of all receptors tested in this study and elsewhere.16,35,37 However, the presence of FL had differential protective effects depending on the type of TKD mutation.
Discussion
Mutations in the Flt3 receptor represent the most common known genetic alteration in AML blasts. These mutations cluster in 2 different regions of Flt3, the juxtamembrane region and the TKD. Here, we report that Flt3-TKD mutations constitute a mutation class with similar signaling properties and transforming abilities. However, its functional properties differ from Flt3-ITD mutations and resemble more closely the properties of ligand-activated wild-type receptors. Importantly, we show that Flt3-TKD mutations are more sensitive to the tyrosine kinase inhibitor PKC12 and that this sensitivity is dominantly transferred to the receptor by a mutation in the TKD.
When we analyzed the transforming potential of the Flt3-ITD versus the Flt3-TKD mutations, we found in suspension cultures small but consistent differences in their effects on proliferation and survival. Interestingly, the extent to which the Flt3-TKD mutations could support these functions always matched the function of ligand-activated wild-type receptors. Our data confirm previous findings that overexpression of Flt3-TKD could induce factor-independent proliferation of hematopoietic cells.12,15,16,38 However, in these studies, the differences in proliferation between cells expressing Flt3-ITD and Flt3-TKDs were either not directly compared or they seemed too subtle to raise the attention of the authors. Here, we show for the first time that Flt3-TKD mutations, like Flt3-WT, fail to support clonogenic growth in 32D cells. This finding was highly reproducible in several vector systems with both human and mouse Flt3 constructs (data not shown). Taken together, our comparative analyses of Flt3-WT, Flt3-ITD, and Flt3-TKD receptors for the first time revealed differences in the biologic effects of these mutations in that the Flt3-ITD gains a function over ligand-activated Flt3-WT that is not gained by Flt3-TKD mutations.
We found comparable Akt and Shc activation between Flt3-ITD and Flt3-TKD mutations, whereas the activation of Erk kinases was slightly lower. These findings support the hypothesis that both types of mutations are equally activating and argue against the suggestion that the observed differences in the biologic effects of the Flt3 mutation classes are due to a dose effect of qualitatively similar signaling events. In contrast, we found strong differences between Flt3-ITD versus Flt3-WT and Flt3-TKD in the activation of STAT5.
Preferential activation of the STAT5 pathway by Flt3-ITD but not by ligand-activated Flt3-WT has been described by several groups including our own.6,8,16,24,39,40 The mechanistic basis of this signaling difference has been addressed by a recent paper that described the involvement of Janus kinase (JAK) kinases in Flt3-ITD but not in Flt3-WT signaling.40 However, when we analyzed the ability of Flt3-ITD to activate STAT5 in cells deficient for JAK kinases, we still observed differential STAT5 activation by Flt3-ITD (C.C. and H.S., unpublished data, August 2003). Our observation of a very weak activation of STAT-dependent signal transduction by Flt3-TKD is in contrast to some previous reports where Flt3-D835Y activated STAT5 similar to Flt3-ITD. These differences could be attributed to the fact that in these reports the extent of STAT5 activation was not directly analyzed in comparison to Flt3-ITD or that a lymphoid or fibroblast cellular background was used for the experiments.16,38,41 Our findings are further supported by the fact that Flt3-TKD induced 2 proteins only weakly that have been characterized as STAT5 target genes (Pim-2 and CIS). In contrast, Flt3-ITD strongly regulated their expression, providing another piece of evidence that differential induction of STAT5 signaling is an important difference between Flt3-ITD and Flt3-TKD mutants. Given the crucial role that has been attributed to STAT5 signal transduction in AML leukemogenicity,29,30,42 this may explain some of the observed differences of the transforming potential of the 2 Flt3 mutation classes. Also, in primary AML samples we found activation of the Erk kinase pathways by both ITD and D835Y mutant receptors, whereas STAT5 was preferentially activated by FLt3-ITD. The fact that we could not observe phosphorylated STAT5 in all samples positive for Flt3-ITD is in accord with previously published data, may reflect methodologic variation due to clinical sample processing, and may not reflect the true situation in vivo. However, the lack of STAT5 activation by Flt3-D835Y is surprisingly consistent with our data in 32D cells as well as with concurrently published data by Grundler et al43 that show similar differences in the ability of Flt3-TKD and Flt3-ITD mutations to activate STAT5 in primary mouse bone marrow and Ba/F3 cells.
Many reports suggest that the myeloid transcription factors c/EBPα and Pu.1 play an important role in AML pathophysiology. The fusion product of t(8;21), AML1-ETO, inhibits c/EBPα and Pu.1 expression and function, and inactivating mutations of c/EBPα and Pu.1 occur in AML blasts.34,44-49 Very recently, it has been reported that mice expressing hypomorphic Pu.1 alleles leading to low levels of Pu.1 expression have a high propensity to develop AML.50 We and others have previously reported that Flt3-ITD mutations suppressed the expression of both transcription factors.24,51 Thus repression of Pu.1 and c/EBPα is a significant event in AML transformation, and Flt3-ITD may contribute to this event. Here, our data show that Flt3-TKD mutants do not efficiently repress both transcription factors in comparison to Flt3-ITD.
The presented results raise interesting questions about the role of Flt3-TKD mutations in the molecular pathogenesis of AML. Our and other groups' data clearly suggest that Flt3-TKD mutations provide proliferative advantages to leukemic clones, possibly allowing AML progenitors independence from exogenous FL. However, they very weakly, if at all, activate Flt3-ITD–specific signaling pathways that are thought to be important for its transforming potential. If Flt3-ITD and Flt3-TKD mutations were functionally redundant, both mutations should not occur in the same patient. However, the proportion of patients simultaneously expressing both mutations is considerable. In one large series of patients, the frequency of Flt3-TKD mutations in cases positive for Flt3-ITD was roughly the same as in the total population (6%-7%), and vice versa, the frequency of Flt3-ITD in the Flt3-TKD+ population was at about 20%, similar to the overall population.14 This strongly argues for the hypothesis arising from our data that Flt3-ITD and Flt3-TKD have differential signaling properties and thus play differential roles in AML transformation.
Our results suggest that expression of Flt3-TKD does not offer a selection advantage for hematopoietic progenitors that surpasses ligand activation of Flt3-WT, a presumably frequent event in Flt3+ leukemic progenitors in the bone marrow with widespread expression of FL. Thus, the expression of Flt3-TKD can be expected to be rather unstable in the course of the disease. The results from several small published patient series are available that indeed report the loss of Flt3-TKD mutations in relapse in up to 60% of the cases. As expected, loss of Flt3-ITD that provides additional selection advantages over Flt3-WT has been shown at much lower frequencies.18,52,53
The data also suggest specific response patterns of Flt3-TKD+ cases to the tyrosine kinase inhibitor PKC412. It has been reported previously that Flt3-ITD mutants are less sensitive toward PKC412 than Flt3-TKD mutations.16,35 However, we report for the first time that the high sensitivity of Flt3-TKD mutations toward PKC412 is also in contrast to Flt3-WT and that it is transferred to Flt3 by mutations in the TKD domain in a dominant fashion. The high sensitivity of Flt3-TKD mutations predicts that patients with TKD mutations should rapidly respond to even low doses of PKC412. However, Flt3-TKD- clones residing in FL+ niches should be a rapid source for relapse, unless therapeutic concentrations of PKC412 are reached at which Flt3-WT is efficiently inhibited. Our data also hint that the sensitivity of PKC412 in primary AML could be influenced by several factors including the type of Flt3 mutation and the presence of FL in the bone marrow.
The role of Flt3-WT in AML is an open question. Recent papers certainly argue for a role of high levels of Flt3-WT in AML, possibly through autocrine stimulation of the receptor.36,54 However, clinical results comparing the efficacy of tyrosine kinase inhibitors on Flt3-WT, Flt3-TKD, and Flt3-ITD cases are needed to definitely answer this issue. Our data suggest that the situation is decisively different for Flt3-ITD+ cases. Here, we might be able to not only inhibit physiologic signaling pathways with enhanced activity, but also aberrant signals that in this combination are relevant for leukemic transformation.
In conclusion, we report significant differences in the transforming ability and signal transduction properties of 2 Flt3 mutation classes. Our results suggest different roles of the 2 mutation classes in AML pathogenesis and may help to explain the clinical differences observed between patients containing the 2 Flt3 mutation classes. This has implications for the treatment of AML with agents targeting Flt3 signal transduction.
Prepublished online as Blood First Edition Paper, March 15, 2005; DOI 10.1182/blood-2004-07-2942.
Supported by the Deutsche Forschungsgemeinschaft (SE 600 3-1) and the Innovative Medizinische Forschung (IMF) at the University of Münster.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Justus Duyster, MD, Technical University of Munich, Munich, Germany, for providing us with cDNA constructs encoding murine Flt3 D835Y and Flt3-ITD. We are highly grateful to our laboratory members for their technical advice and fruitful discussions. PKC412 was kind gift from Norvatis Pharmaceuticals (Basel, Switzerland), and SU11248 was provided by Sugen Inc.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal