Abstract
The devastating systemic effects of bacterial superantigens may be explained by powerful proinflammatory synergy with lipopolysaccharide (LPS). However, the mechanism underlying this phenomenon remains unclear and has never been investigated in humans. Specifically, there is no known link between superantigen-induced immune effects and the pattern recognition of LPS at toll-like receptor 4 (TLR4). Here we show that bacterial superantigens induce rapid transcription and increased membrane expression of TLR4 in primary human monocytes by ligation of major histocompatibility complex (MHC) class II. We also demonstrate that superantigens are solely responsible for monocyte TLR4 up-regulation induced by products from Gram-positive bacteria. In parallel with enhanced TLR4 expression, priming of purified monocytes or mixed peripheral blood mononuclear cells with superantigens significantly enhanced the induction of proinflammatory cytokines by known TLR4 ligands. Staphylococcal enterotoxin A constructs containing targeted mutations were used to demonstrate a requirement for MHC class II ligation in both TLR4 up-regulation and enhanced responses to endotoxin. In contrast to results from animal models, superantigen-endotoxin interaction was not dependent on T-cell receptor ligation by superantigen or interferon gamma production. Pattern recognition of bacterial superantigens by MHC class II receptors may exacerbate the proinflammatory response of monocytes to Gram-negative infection or endotoxin by up-regulation of TLR4.
Introduction
The most potent microbial products implicated in the pathogenesis of septic shock are Gram-positive derived superantigenic exotoxins and Gram-negative bacterial endotoxin.1 Both types of toxin induce comparable proinflammatory cytokines from human mononuclear cells in vitro, cause lethal shock in vivo, and have been identified in the bloodstream of critically ill patients.2-8 Despite these striking similarities, there appears to be a quite distinct immunology underlying the shock states induced by these 2 bacterial products. Bacterial superantigens ligate both T-cell receptor (TCR) and major histocompatibility complex (MHC) class II without antigen processing.9 This leads to the activation of up to 20% of all peripheral circulating T cells, determined by TCR V beta repertoire and MHC haplotype, provoking massive cytokine production.3,10,11 In contrast, endotoxin is detected by a complex macromolecular complex dominated by CD14, toll-like receptor 4 (TLR4) and MD2, a key accessory molecule for TLR4.12,13 The endotoxin pattern-recognition complex is present on both epithelial and endothelial surfaces in addition to leukocytes such as monocytes/macrophages, mast cells, and granulocytes.14 Ligation of TLR4 leads to activation of transcriptional control elements such as nuclear factor κB (NF-κB), via a well-documented signal transduction cascade, and the induction of cytokines.1,14,15
Despite different proinflammatory pathways, there is a wide array of literature suggesting a major interaction between Gram-positive superantigenic exotoxins and Gram-negative endotoxin in animal shock models, where superantigens are reported to enhance the lethality of endotoxin by between 100- and 50 000-fold.16-19 The mechanism underlying this powerful in vivo effect has not been fully delineated. Although some workers have suggested a predominant role for T cells and the lymphokine interferon γ (IFN-γ), other cells and mediators may be involved.18,20-26 Surprisingly, biologically relevant concentrations of superantigens have never been shown to produce a comparable effect in human cells. Moreover, to our knowledge, the mechanism underlying superantigen-endotoxin synergy has never been investigated in any human system.
Here, we examine the effect of biologically relevant concentrations of staphylococcal and streptococcal superantigens on TLR4 expression in primary human monocytes. In addition, we describe the effect of superantigen exposure on subsequent responsiveness of human monocytes to TLR4 ligands. We also use staphylococcal enterotoxin (SE) A constructs with targeted mutations in key binding sites to investigate the relative dependence of superantigen-endotoxin interaction on MHC class II and TCR ligation.
Materials and methods
TLR agonists, heat-killed Staphylococcus aureus
Gel-filtered Pseudomonas aeruginosa lipopolysaccharide (LPS) (serotype 10) and Escherichia coli 0111.B4 LPS (purified on an ion-exchange column to protein/DNA content < 1%) were obtained from Sigma (Poole, United Kingdom). E coli F515 hexa-bis lipid A was kindly donated by U. Zahringer (Borstel, Germany)27 and endotoxin-free S aureus lipoteichoic acid (LTA) was kindly donated by T. Hartung (Konstanz, Germany).28 Heat-killed S aureus (HKSA) was generated from a single colony of S aureus H380, a clinical bacteremia isolate, which was cultured overnight in RPMI 1640 tissue-culture medium supplemented with 10% fetal calf serum (FCS; Labtech, Ringmer, United Kingdom)/2 mM l-glutamine (Gibco, Paisley, United Kingdom). After centrifugation, bacterial pellets were washed, resuspended in saline, and quantified on blood agar prior to heat killing at 70°C for 1 hour.
Purified superantigens and mutant constructs
Native toxic shock syndrome toxin-1 (TSST-1), SEA (staphylococcal endotoxin A), and SEB (staphylococcal endotoxin B) were obtained from Sigma (Poole, United Kingdom); streptococcal pyrogenic exotoxin A (SPEA) was from Toxin Technology (Sarasota, FL); and recombinant streptococcal mitogenic exotoxin Z–1 (rSMEZ-1) and rSMEZ-2 were kindly donated by T. Proft (Auckland, New Zealand). Wild-type (WT) and site-directed mutant constructs of rSEA were generated as described previously.29 SEA F47S.L48A.Y92A was defective in MHC class II α-chain binding; SEA-H225A in β-chain binding; SEA-H225A.F47S in both α and β-chain binding; and SEA-N25A.Y94A in TCR binding. LPS content of superantigens was below 0.1 pg/ng of toxin by Limulus assay.
Bacterial supernatants
Overnight cultures of isogenic S pyogenes strains, grown in glutamine-supplemented RPMI 1640, were centrifuged and supernatants were filter-sterilized. Aliquots were plated onto blood agar to ascertain equivalent growth. Strains used were parent strain H293, a clinical isolate (smez+), H377 (smez-mutant), and H432 (complemented with smez as previously described).30,31 Supernatants were incubated with monocytes at a dilution of 1:10 and were endotoxin-free by Limulus assay.
Reagents
Neutralizing anti–IFN-γ (R&D Systems, Abingdon, United Kingdom) was used in the range of 0.02 μg/mL to 2 μg/mL with appropriate isotype-matched antibody controls. Genestein, cycloheximide, and phytohemagglutinin (PHA) were obtained from Sigma. Genestein (0.037 μM-37 μM) and cycloheximide (0.36 nM-360 nM) were dissolved in dimethyl sulfoxide (DMSO) and applied in parallel with appropriate vehicle-only controls.
Monocyte, nonmonocyte, T-cell, and PBMC cultures
Peripheral blood mononuclear cells (PBMCs) were extracted from the whole blood of healthy volunteers (Hammersmith Hospital ethics reference: 2002/6418) by differential gradient centrifugation over endotoxin-free Ficoll-Paque Plus (Amersham Biosciences, Amersham, United Kingdom). Monocytes and positively selected `nonmonocytes' were extracted from PBMCs using Monocyte Isolation Kit II (Miltenyi Biotech, Bisley, United Kingdom), according to the manufacturer's instructions. Briefly, PBMCs were incubated for 10 minutes at 4°C with biotin-conjugated anti-CD3, CD7, CD19, CD45RA, CD56, and immunoglobulin E (IgE) in phosphate-buffered saline (PBS) supplemented with 10% autologous human serum and FcR blocking reagent (Miltenyi Biotec). Magnetic microbeads conjugated to anti–biotin antibody were then added and coincubated for an additional 15 minutes. Following a PBS serum wash, the sample was passed across an LS column within a magnetic field (Midi-Macs; Miltenyi Biotech). Cells were seeded in sterile flow cytometry tubes (Becton Dickinson, Oxford, United Kingdom) or 96-well plates at a concentration of 2 × 105/well or tube in RPMI 1640/FCS supplemented with 50 U/mL penicillin G and 50 μg/mL streptomycin sulfate. They were incubated at 37°C in 5% CO2 for 12 hours then washed and recovered with fresh medium. For monocyte preparation, nonadherent cells were aspirated prior to washing to further increase purity. Pure T cells were similarly prepared using a pan-T selection kit (Miltenyi Biotec) by selecting with hapten-conjugated CD11b, CD16, CD19, CD36, and CD56 antibodies prior to separation with hapten-conjugated microbeads. Cell populations were double-stained with phycoerythrin (PE)–CD3 and fluorescein isothiocyanate (FITC)–CD14 for flow cytometric analysis. Monocyte populations contained less than 4% CD3+ cells and T-cell populations contained less than 0.5% CD14+ cells.
Flow cytometry
Monocyte populations were exposed to tissue-culture medium (TCM), superantigens, lipid A, LPS, LTA, or heat-killed whole bacteria for various times at 37°C in 5% CO2. They were then washed and resuspended in 50 μL ice-cold FACS buffer (PBS/1% bovine serum albumin [BSA]) prior to dark incubation for 30 minutes on ice with PE anti-TLR4 and FITC anti-CD14 mouse, or IgG1-FITC, IgG2A-PE isotype-matched controls (ITMCs). Samples were washed and resuspended in 400 μL FACS buffer for flow cytometric analysis (FACSCalibur; Becton Dickinson) using appropriately single-stained samples to set compensations. The mean fluorescence intensity generated from resting or stimulated PE-TLR4–labeled cells in CD14-gated populations (n = 20 000) was measured and the difference between these groups (ΔMFI) was calculated for each experimental condition.
Quantitative RT-PCR
Pure monocyte cultures from 5 different donors were exposed to 1 ng/mL SEB, or tissue-culture medium alone for 3 hours, 7 hours, and 24 hours. In some cases, monocytes were also incubated with 1 ng/mL LPS 0111:B4. Cells were lysed with Trizol reagent (Invitrogen, Paisley, United Kingdom) and total RNA isolated according to the manufacturer's instructions. cDNA was prepared by priming with oligo-dT primer, and then applying ImProm-II reverse transcriptase (Promega, Southampton, United Kingdom). For reverse transcriptase–polymerase chain reaction (RT-PCR), TaqMan probe/primer sequences were determined using Primer Express software (Applied Biosystems, Warrington, United Kingdom) and synthesized by Biosource (Nivelles, Belgium): TLR4 (forward: 5′-CTGCAATGGATCAAGGACCAG′; reverse: 5′-TGC CCT GCT TAT CTG AAG GTG-3′; probe: 5′-FAM-CAGCTCTTGGTGGAAGTTGAACGAATG G-TAMRA-3′); MD2 (forward: 5′-TGAATCTTCCAAAGCGCAAA-3′; reverse 5′-TGTATTCACAGTCTCTCCCTTCAGA-3′; probe: 5′-FAM-TTATTTGCCGAGGATCTGATGACGATTACTCTT-TAMRA-3′); and β-actin (forward: 5′-TTGCCGACAGGATGCAGAA-3′; reverse: 5′-GCCGATCCACACGGAGTACT-3′; probe: 5′-FAM-TCAAGATCATTGCTCCTCCTGAGAGC-TAMRA-3′). Standard curves were constructed using dilutions of plasmids containing the PCR products cloned into pCR2.1 (Invitrogen). PCR was performed using qPCR mastermix (Eurogentec, Romsey, United Kingdom) and fluorescence measurement was conducted in the ABI Prism 7900HT sequence detection system (Applied Biosystems). Threshold cycle numbers were used to calculate target gene copy number in the samples by relating to the standard curves. Results were normalized against β-actin transcripts.
Cytokine quantification by ELISA
Monocytes or PBMCs were exposed to superantigen and/or LPS under varying conditions. Supernatants were stored at -80°C, prior to measurement of cytokines by enzyme-linked immunosorbent assay (ELISA; R&D Systems). Where PBMCs were primed with nonsuperantigen stimulants (LTA or heat-killed S aureus), the latter were used at a concentration that individually produced the same magnitude of cytokine production as solo superantigen stimulation.
T-cell proliferation
Two-day [3H] thymidine inclusion assay was used to assess the mitogenic function of wild-type SEA in comparison with mutant SEA constructs. Superantigens were added to PBMC cultures and pulsed with 1 μCi (0.037 MBq) 3H-thymidine/well (Amersham Biosciences) at 24 hours. Cells were harvested 24 hours later onto filter paper (Micro 96 harvester; Skatron Instruments, Lier, Norway) and tritium incorporation was measured using a scintillation counter (Wallac 1205 Betaplate, Turku, Finland). All samples were run in parallel with a PHA-only control.
Statistical analysis
Statistical analyses were provided by the Statistical Advisory Service (Imperial College, London, United Kingdom). All error bars shown represent standard deviations from the mean. Where statistical tests were applied to compare groups, significance was defined at the 5% level (P < .05).
For flow cytometry data, individual gated populations of monocytes (20 000 events) were used for each measurement. Change in mean fluorescence intensity from resting levels (ΔMFI) was determined for cells stimulated with toxin and for cells exposed only to TCM. The paired t test was used to compare ΔMFI values between different groups.
Analysis of RNA transcript changes in response to SEB over time was performed by repeated measures analysis of variance following Box-Cox transformation of RNA data; SEB-stimulated cells were compared with control cells stimulated with TCM alone.
To analyze the effect of superantigen-endotoxin combinations on induction of cytokines, mean cytokine production and standard deviations were calculated from triplicate repeats for each individual donor. All experiments were performed on at least 5 occasions. Observed cytokine release was compared with expected cytokine release (derived from the sum of cytokine production induced by single toxin stimulation). Where groups of 8 or more individuals were used, a 2-step nonparametric analysis was performed to demonstrate supra-additive effects. Median values for observed cytokine production were subtracted from expected values and the results compared with 0 using the Wilcoxon signed rank test (Figure 4A, 6A-E).
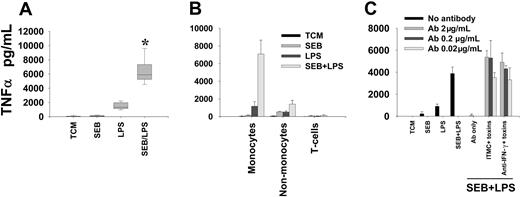
Stimulation of primary human monocytes with superantigens enhances production of TNF-α in response to subsequent LPS challenge. Monocytes were exposed to 1 ng/mL SEB for 3 hours with coincubation with 1 ng/mL E coli LPS 0111:B4 for an additional 4 hours. (A) Composite box-plot to illustrate magnitude of enhanced induction of TNF-α by SEB-LPS (n = 10). *Denotes significant difference from “expected” cytokine production, derived from the sum of TNF-α produced from individual toxin stimulations; P = .002. (B) Enhancement of LPS-induced TNF-α production by SEB pre-exposure is restricted to the monocyte subset. Mean and SD of data from triplicate wells are shown. Data are representative of 3 separate experiments. (C) Enhanced production of TNF-α is not inhibited by neutralizing concentrations of anti–IFN-γ. ITMC indicates isotype-matched control antibody. Mean and SD of data from triplicate wells are shown. Results are representative of 3 separate experiments.
Stimulation of primary human monocytes with superantigens enhances production of TNF-α in response to subsequent LPS challenge. Monocytes were exposed to 1 ng/mL SEB for 3 hours with coincubation with 1 ng/mL E coli LPS 0111:B4 for an additional 4 hours. (A) Composite box-plot to illustrate magnitude of enhanced induction of TNF-α by SEB-LPS (n = 10). *Denotes significant difference from “expected” cytokine production, derived from the sum of TNF-α produced from individual toxin stimulations; P = .002. (B) Enhancement of LPS-induced TNF-α production by SEB pre-exposure is restricted to the monocyte subset. Mean and SD of data from triplicate wells are shown. Data are representative of 3 separate experiments. (C) Enhanced production of TNF-α is not inhibited by neutralizing concentrations of anti–IFN-γ. ITMC indicates isotype-matched control antibody. Mean and SD of data from triplicate wells are shown. Results are representative of 3 separate experiments.
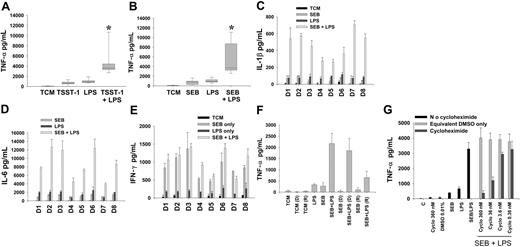
The enhancement of proinflammatory responses to LPS by superantigens in PBMCs is a reproducible and specific cellular phenomenon. (A-B) TNF-α production by PBMCs stimulated with superantigen-endotoxin in expanded donor population. Cells were pre-exposed to 1 ng/mL superantigen (A, TSST-1, n = 21; B, SEB, n = 15) for 3 hours, then coincubated with LPS (1 ng/mL) for an additional 4 hours. Median and 10th, 25th, 75th, and 90th centiles are shown. *Represents significant supra-additive superantigen-endotoxin interaction; P < .001 for both superantigens. (C) IL-1β and (D) IL-6 cytokine induction by SEB-LPS compared with that expected from individual toxins was also supra-additive (P = .008). Results are shown for each individual donor (D1-D8). (E) IFN-γ production in response to SEB-LPS was not supra-additive from that predicted by levels induced by individual toxins. (F) In comparison with control PBMCs (incubated either alone or sequentially with SEB and LPS), SEB-exposed donor PBMCs retained hyperresponsiveness to LPS even when supernatants were removed and replaced with fresh medium (bars marked “D”). Supernatants from PBMCs pre-exposed to SEB did not directly transfer priming (ie, immediate enhanced LPS responsiveness) to naive recipient PBMCs (bars marked “R”). (G) Cycloheximide (cyclo) inhibited enhanced production of TNF-α by superantigen-endotoxin. Error bars reflect standard deviation of triplicate wells.
The enhancement of proinflammatory responses to LPS by superantigens in PBMCs is a reproducible and specific cellular phenomenon. (A-B) TNF-α production by PBMCs stimulated with superantigen-endotoxin in expanded donor population. Cells were pre-exposed to 1 ng/mL superantigen (A, TSST-1, n = 21; B, SEB, n = 15) for 3 hours, then coincubated with LPS (1 ng/mL) for an additional 4 hours. Median and 10th, 25th, 75th, and 90th centiles are shown. *Represents significant supra-additive superantigen-endotoxin interaction; P < .001 for both superantigens. (C) IL-1β and (D) IL-6 cytokine induction by SEB-LPS compared with that expected from individual toxins was also supra-additive (P = .008). Results are shown for each individual donor (D1-D8). (E) IFN-γ production in response to SEB-LPS was not supra-additive from that predicted by levels induced by individual toxins. (F) In comparison with control PBMCs (incubated either alone or sequentially with SEB and LPS), SEB-exposed donor PBMCs retained hyperresponsiveness to LPS even when supernatants were removed and replaced with fresh medium (bars marked “D”). Supernatants from PBMCs pre-exposed to SEB did not directly transfer priming (ie, immediate enhanced LPS responsiveness) to naive recipient PBMCs (bars marked “R”). (G) Cycloheximide (cyclo) inhibited enhanced production of TNF-α by superantigen-endotoxin. Error bars reflect standard deviation of triplicate wells.
Results
Purified superantigens induced up-regulation of monocyte membrane TLR4 expression via ligation of MHC class II
Reproducible increases in TLR4 membrane expression were found following exposure of monocytes to purified streptococcal and staphylococcal superantigens, even in cultures where CD3+ cells were undetectable by flow cytometry (Figure 1). Superantigen-induced shifts in mean fluorescence intensity were not observed in isotype-matched control-stained cells or in experiments where monocytes were stained with a different isotype-matched antibody, anti–TLR-1-PE. The dose-response relationship between SEB and TLR4 expression is shown in Figure 1B. SEB consistently produced maximal TLR4 up-regulation at a concentration of 1 ng/mL. TLR4 up-regulation was reproducibly observed from 3 hours after SEB exposure and was maximal by 16 hours. All tested superantigens induced increased membrane expression of TLR4 at concentrations above 10 pg/mL, maximal in magnitude at superantigen concentrations between 1 ng/mL and 100 ng/mL. Importantly, up-regulation of TLR4 therefore occurred at toxin concentrations compatible with those thought to occur in vivo in the context of severe sepsis.6-8 The reproducibility of this effect for both staphylococcal and streptococcal superantigens was tested in multiple healthy subjects (Figure 1C). Up-regulation of TLR4 was specific to superantigens and was not seen with LTA (0.1 μg/mL-10 μg/mL), whole, heat-killed Gram-positive bacteria (S aureus 10-105 CFU [colony-forming units]/mL), or any TLR4 agonist (purified LPS from E coli and Pseudomonas spp, hexa-bis lipid A). Indeed, 100 ng/mL lipid A significantly depressed TLR4 membrane expression relative to the low levels observed on resting monocytes. The latter observation is consistent with published observations in murine macrophages.32
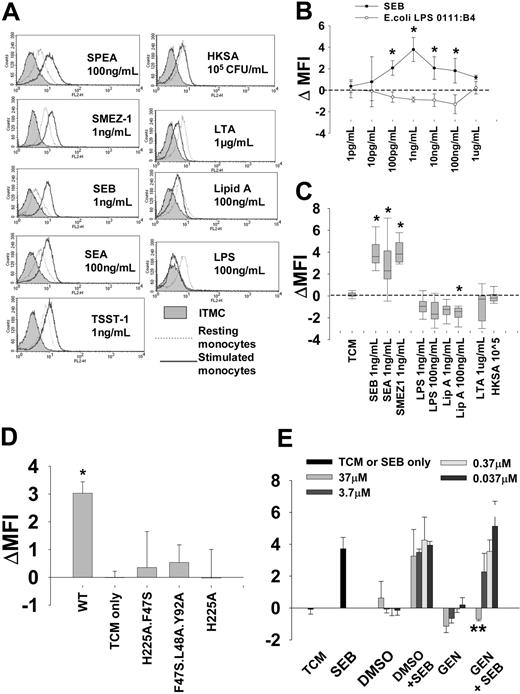
Surface TLR4 expression in monocytes is specifically up-regulated by superantigens via MHC class II ligation. (A) Membrane TLR4 expression following incubation of purified monocytes with medium alone (resting monocytes; dotted line) or with different microbial products (stimulated monocytes; solid line). Left panel, superantigens; right panel, other microbial products. Representative flow cytometry histograms are shown at the superantigen concentrations found to induce maximal TLR4 up-regulation. Cells stained with isotype-matched control (ITMC) antibody are shown in shaded region. Other microbial products, in particular LTA and E coli LPS 0111:B4, did not support TLR4 up-regulation at any concentration tested and in some cases down-regulated TLR4 expression. (B) Dose-dependence of SEB (⬡) and LPS (E coli 0111:B4; ○) effects on membrane TLR4 expression relative to unstimulated cells (ΔMFI): mean ± SD of 4 separate donor monocyte preparations. *Denotes difference from TCM-only controls. (C) Membrane expression of TLR4 in monocytes from expanded donor pool stimulated with 1 ng/mL SEB (n = 35), SEA (n = 16), SMEZ-1 (n = 9), LPS (n = 12), and lipid A (n = 9). Additional results are shown for cells stimulated with 10 ng/mL LPS, 10 ng/mL lipid A, 1 μg/mL LTA, and 105 cfu/mL HKSA (n = 9) Median and 10th, 25th, 75th, and 90th centiles are shown: concentrations shown are those used in subsequent cytokine induction experiments. (D) In contrast to WT SEA, MHC nonbinding mutant constructs fail to support TLR4 up-regulation. Mean and SD of 3 experiments. *Denotes significant difference from TCM-only controls. (E) Genestein (37 μM) inhibits SEB-induced TLR4 up-regulation. Mean and SD of 3 experiments. **Denotes significant difference from SEB-only TLR4 up-regulation.
Surface TLR4 expression in monocytes is specifically up-regulated by superantigens via MHC class II ligation. (A) Membrane TLR4 expression following incubation of purified monocytes with medium alone (resting monocytes; dotted line) or with different microbial products (stimulated monocytes; solid line). Left panel, superantigens; right panel, other microbial products. Representative flow cytometry histograms are shown at the superantigen concentrations found to induce maximal TLR4 up-regulation. Cells stained with isotype-matched control (ITMC) antibody are shown in shaded region. Other microbial products, in particular LTA and E coli LPS 0111:B4, did not support TLR4 up-regulation at any concentration tested and in some cases down-regulated TLR4 expression. (B) Dose-dependence of SEB (⬡) and LPS (E coli 0111:B4; ○) effects on membrane TLR4 expression relative to unstimulated cells (ΔMFI): mean ± SD of 4 separate donor monocyte preparations. *Denotes difference from TCM-only controls. (C) Membrane expression of TLR4 in monocytes from expanded donor pool stimulated with 1 ng/mL SEB (n = 35), SEA (n = 16), SMEZ-1 (n = 9), LPS (n = 12), and lipid A (n = 9). Additional results are shown for cells stimulated with 10 ng/mL LPS, 10 ng/mL lipid A, 1 μg/mL LTA, and 105 cfu/mL HKSA (n = 9) Median and 10th, 25th, 75th, and 90th centiles are shown: concentrations shown are those used in subsequent cytokine induction experiments. (D) In contrast to WT SEA, MHC nonbinding mutant constructs fail to support TLR4 up-regulation. Mean and SD of 3 experiments. *Denotes significant difference from TCM-only controls. (E) Genestein (37 μM) inhibits SEB-induced TLR4 up-regulation. Mean and SD of 3 experiments. **Denotes significant difference from SEB-only TLR4 up-regulation.
Importantly, up-regulation of TLR4 by superantigen was absolutely dependent on MHC class II ligation (Figure 1D). Up-regulation of TLR4 was not supported by mutant constructs of SEA designed to prevent binding to MHC class II, or by SEB in the presence of genestein (37 μM), an inhibitor of protein tyrosine kinase known to be important in superantigen signaling via class II33 (Figure 1E). This concentration of genestein did not induce cell death or changes in forward- or side-scatter characteristics in monocytes.
The up-regulation of TLR4 on monocytes induced by streptococcal supernatants is solely due to superantigen production
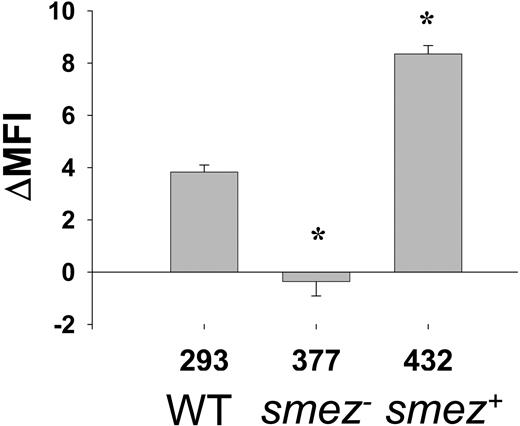
The biologic relevance of the above findings was underlined by the observation that dilute sterile supernatants from a clinical S pyogenes isolate (H293), which expresses the superantigen SMEZ, also enhanced monocyte membrane TLR4 expression (Figure 2). Moreover, disruption of the smez gene in strain H377 prevented up-regulation of TLR4 by derived bacterial supernatant. Interestingly, reconstitution of smez by plasmid complementation (strain H432) increased the capacity of supernatant to enhance TLR4 expression beyond that seen for the parent strain. This is likely to be due to the presence of 2 to 3 copies of smez in the complemented strain.31
Up-regulation of TLR4 in monocytes by S pyogenes supernatants is solely due to superantigens. Dilute (1/10) supernatant from a superantigen-producing strain of S pyogenes (H293) up-regulates TLR4 on human monocytes. Disruption of smez gene (strain H377) abrogates TLR4 up-regulation. Complementation with smez (strain H432) restores TLR4 up-regulation beyond that of wild-type (WT). *Denotes significant difference from H293 parent strain. Mean and SD of triplicate monocyte cultures; results are representative of separate experiments in 3 different individuals.
Up-regulation of TLR4 in monocytes by S pyogenes supernatants is solely due to superantigens. Dilute (1/10) supernatant from a superantigen-producing strain of S pyogenes (H293) up-regulates TLR4 on human monocytes. Disruption of smez gene (strain H377) abrogates TLR4 up-regulation. Complementation with smez (strain H432) restores TLR4 up-regulation beyond that of wild-type (WT). *Denotes significant difference from H293 parent strain. Mean and SD of triplicate monocyte cultures; results are representative of separate experiments in 3 different individuals.
TLR4 up-regulation was not observed using supernatant from nonsuperantigen producing streptococci (S viridans, S pneumoniae) and, as with purified TLR4 ligands, TLR4 expression was down-regulated by supernatant from Gram-negative bacteria (P.A.H., unpublished observations, February 2004).
Superantigens induced up-regulation of TLR4 and MD2 mRNA in human monocytes
In comparison to control cells, primary human monocytes stimulated with 1 ng/mL SEB demonstrated significant up-regulation of TLR4 transcription over the time course studied, which was maximal at 7 hours and diminished by 24 hours (Figure 3A). Interestingly, we found a parallel up-regulation of MD2 transcription by SEB (Figure 3B). Endotoxin had no apparent effect on TLR4 and MD2 gene transcription relative to that seen in resting monocytes.
TLR4 and MD2 mRNA transcripts are up-regulated in purified primary human monocytes by SEB. Quantities of TLR4 (A) and MD2 (B) mRNA were corrected relative to the constitutively produced β-actin gene product. Individual results are shown for SEB-stimulated (⬡) or TCM-only exposed (○) monocytes from 5 donors at 3 hours, 7 hours, and 24 hours. The difference in TLR4 and MD2 expression in SEB- and TCM-stimulated cells was significant (repeated measures analysis of variance, P < .05). The result was representative of 2 independent experiments.
TLR4 and MD2 mRNA transcripts are up-regulated in purified primary human monocytes by SEB. Quantities of TLR4 (A) and MD2 (B) mRNA were corrected relative to the constitutively produced β-actin gene product. Individual results are shown for SEB-stimulated (⬡) or TCM-only exposed (○) monocytes from 5 donors at 3 hours, 7 hours, and 24 hours. The difference in TLR4 and MD2 expression in SEB- and TCM-stimulated cells was significant (repeated measures analysis of variance, P < .05). The result was representative of 2 independent experiments.
Superantigens enhance LPS-induced human monocyte TNF-α production
The biologic consequence of TLR4 up-regulation at the level of both transcription and membrane expression was tested by the sequential stimulation of monocytes with SEB (1 ng/mL) followed by exposure to the TLR4 agonist E coli LPS 0111:B4 (1 ng/mL). Monocytes were exposed to SEB for 3 hours and then coincubated with LPS for an additional 4 hours (total superantigen exposure: 7 hours). The amount of tumor necrosis factor (TNF)–α produced from sequential stimulation of purified monocytes with superantigen-LPS greatly exceeded any additive effects (Figure 4A). Enhanced production of TNF-α was seen only from monocytes, and was not observed from positively selected nonmonocytes or negatively selected purified T cells, which were stimulated sequentially with SEB and LPS in parallel (Figure 4B). Neutralizing anti–IFN-γ (2.0 μg/mL) did not impair enhancement of TNF-α production from purified monocytes exposed sequentially to SEB and LPS (Figure 4C). This concentration of anti–IFN-γ is saturating (manufacturer's data) and blocked HLA class II up-regulation by exogenous IFN-γ (5 ng/mL). In contrast to murine shock models, the current data suggest that a proinflammatory interaction between superantigen and endotoxin can occur without the presence of T cells or IFN-γ.
Pre-exposure to superantigens specifically enhances TLR4 ligand–induced human PBMC TNF-α production
In order to further characterize the interaction of superantigen and LPS observed in primary monocytes in a more biologically relevant setting and assess the interaction in more detail, experiments were performed using primary human PBMCs. Preliminary titration experiments using superantigen-E coli LPS 0111:B4 stimulation were used to define the optimal toxin concentrations for maximal induction of TNF-α production by PBMCs stimulated sequentially by both toxin types (Figure 5A). SEB concentrations between 100 pg/mL and 1 ng/mL were optimal for enhancing subsequent responses to LPS; the effect was not observed at SEB concentrations below 10 pg/mL. To define the temporal relationship between the 2 toxin types that was necessary for interaction, the duration of pre-exposure to superantigen was varied (Figure 5B). To determine the rapidity of TNF-α release following LPS exposure by superantigen-primed PBMCs, the duration of exposure to LPS was varied (Figure 5C). Superantigen priming of PBMCs for a minimum of 3 hours followed by coincubation with LPS for an additional 4 hours consistently enhanced TNF-α production compared with either toxin alone. Further extension of superantigen priming interval or the period of exposure to LPS did not further increase TNF-α compared with that resulting from stimulation with the individual toxins. Reversal of toxin addition led to complete loss of enhanced cytokine induction (Figure 5D). Proinflammatory priming was supported by all other superantigens tested (TSST-1, SEA, SPEA, SMEZ-1, SMEZ-2) at similar optimal concentrations (not shown). HKSA and LTA were unable to prime PBMCs to respond to LPS to the extent shown by superantigens. While proinflammatory superantigen-LPS interactions were observed over a range of superantigen concentrations, any apparent interactions between LTA-LPS or HKSA-LPS were low in magnitude and restricted to single priming stimulant concentrations. (Figure 5E-F). With regard to specificity of the secondary stimulus, other TLR4 ligands tested were able to act as secondary stimuli (LPS from Pseudomonas spp and lipid A) and produced enhanced levels of TNF-α in superantigen-primed PBMCs (Figure 5G-H). LTA and superantigens could not act as secondary stimuli and did not induce enhanced levels of TNF-α from superantigen-primed monocytes (not shown). Hence, the priming effect of superantigens may have specificity for TLR4 ligands.
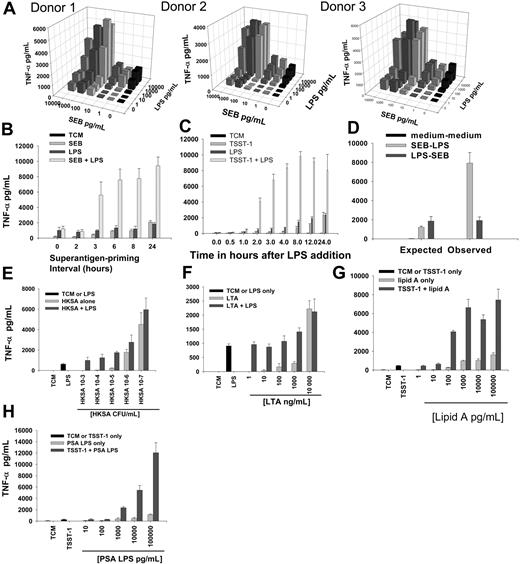
Sequential PBMC stimulation by superantigen-LPS enhances TNF production. (A) Three-dimensional dose-response bar chart showing mean TNF-α by SEB-LPS sequentially stimulated PBMCs from 3 different subjects. Means were derived from triplicate observations at each toxin concentration. Data were representative for those obtained for TSST-1 and SMEZ-1. (B) LPS-induced TNF-α release by PBMCs: effect of duration of SEB pre-exposure. Enhanced TNF-α production required a minimum period of 3 hours of pre-exposure to SEB. (C) LPS-induced TNF-α release by PBMCs following 3 hours of pre-exposure to TSST-1: effect of duration of LPS exposure. Enhanced TNF-α production was maximal 4 hours after LPS stimulation. (D) Reversal in order of exposure (LPS-SEB instead of SEB-LPS) did not produce enhanced observed levels of TNF-α when compared with expected levels. (E-F) HKSA and LTA were unable to prime PBMCs for enhancement of LPS-induced TNF-α production. (G) Enhanced TNF-α production was also seen when TSST-1–primed PBMCs were exposed to lipid A as a secondary stimulant or (H) LPS from Pseudomonas aeruginosa (PSA LPS; threshold concentration one log higher than for E coli LPS or lipid A). All results are mean and SD of triplicate stimulations. Results are representative of 5 separate experiments and were repeated with both TSST-1 and SEB.
Sequential PBMC stimulation by superantigen-LPS enhances TNF production. (A) Three-dimensional dose-response bar chart showing mean TNF-α by SEB-LPS sequentially stimulated PBMCs from 3 different subjects. Means were derived from triplicate observations at each toxin concentration. Data were representative for those obtained for TSST-1 and SMEZ-1. (B) LPS-induced TNF-α release by PBMCs: effect of duration of SEB pre-exposure. Enhanced TNF-α production required a minimum period of 3 hours of pre-exposure to SEB. (C) LPS-induced TNF-α release by PBMCs following 3 hours of pre-exposure to TSST-1: effect of duration of LPS exposure. Enhanced TNF-α production was maximal 4 hours after LPS stimulation. (D) Reversal in order of exposure (LPS-SEB instead of SEB-LPS) did not produce enhanced observed levels of TNF-α when compared with expected levels. (E-F) HKSA and LTA were unable to prime PBMCs for enhancement of LPS-induced TNF-α production. (G) Enhanced TNF-α production was also seen when TSST-1–primed PBMCs were exposed to lipid A as a secondary stimulant or (H) LPS from Pseudomonas aeruginosa (PSA LPS; threshold concentration one log higher than for E coli LPS or lipid A). All results are mean and SD of triplicate stimulations. Results are representative of 5 separate experiments and were repeated with both TSST-1 and SEB.
The enhancement of PBMC cytokine responses to TLR4 ligands by superantigens is reproducible and extends to IL-1β and IL-6, though not IFN-γ
Using a sequential stimulation approach (superantigen for 3 hours followed by coincubation with LPS for an additional 4 hours) the proinflammatory interaction between SEB or TSST-1 and LPS was then examined in an expanded number of PBMC donors, to ascertain variablility. Data are shown for superantigen and LPS concentrations of 1 ng/mL although similar distribution was also seen for both superantigens at 100 pg/mL (Figure 6A-B).
Interestingly, although significantly enhanced quantities of IL-6 and IL-1β were also produced by PBMCs exposed sequentially to superantigen-LPS, enhancement of IFN-γ production was not seen when the 2 types of toxin were added sequentially (Figure 6C-E). Removal of supernatant from superantigen-primed donor PBMCs and replacement with fresh medium did not impair subsequent enhancement of TNF-α responses to LPS, showing that continuing presence of superantigen or soluble factors, such as CD14, in medium were not essential for the enhancement of TNF-α production by superantigen. This was further confirmed by the failure of transferred primed supernatants to increase responsiveness of naive recipient PBMCs to LPS (Figure 6F). Taken together, these data suggested that the priming effect of superantigen was cell-associated rather than secreted. The inhibition of superantigen priming effects by cycloheximide confirmed a requirement for protein synthesis, and excluded effects due to the release of TNF-α from a preformed pool of cytokine (Figure 6G).
Superantigens enhance the early TNF-α response of human PBMCs to TLR4 ligands independent of TCR ligation or IFN-γ production
rSEA mutant constructs with impaired MHC class II receptor binding capacity were unable to support priming of PBMCs for LPS response, consistent with the data pertaining to TLR4 up-regulation. In contrast, however, SEA-N25A.Y94A, with impaired TCR binding (and mitogenic potential), was shown to retain full priming potential for subsequent LPS stimulation (Figure 7A-B). In addition, as in pure monocyte cultures, anti–IFN-γ failed to disrupt superantigen-endotoxin proinflammatory cooperation (Figure 7C).
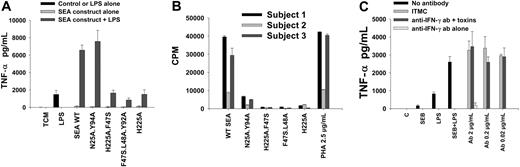
The enhancement of proinflammatory responses to LPS by superantigens in PBMCs is not dependent on superantigen ligation of TCR or IFN-γ production. (A) Enhancement of LPS-induced TNF-α production was supported by TCR nonbinding but not MHC class II nonbinding SEA constructs. Cells were exposed to SEA constructs (1 ng/mL) for 3 hours, then coincubated with LPS (1 ng/mL) for an additional 4 hours. Mean and SD of triplicate stimulations are shown and data are representative of 5 experiments. (B) T-cell mitogenic potential of SEA WT versus mutant constructs was confirmed in 3 donors. (C) Anti–IFN-γ did not inhibit enhanced production of TNF-α by PBMCs following superantigen-LPS exposure. ITMC indicates isotype-matched control antibody. Mean and SD of triplicate samples are shown; data are representative of 5 separate experiments.
The enhancement of proinflammatory responses to LPS by superantigens in PBMCs is not dependent on superantigen ligation of TCR or IFN-γ production. (A) Enhancement of LPS-induced TNF-α production was supported by TCR nonbinding but not MHC class II nonbinding SEA constructs. Cells were exposed to SEA constructs (1 ng/mL) for 3 hours, then coincubated with LPS (1 ng/mL) for an additional 4 hours. Mean and SD of triplicate stimulations are shown and data are representative of 5 experiments. (B) T-cell mitogenic potential of SEA WT versus mutant constructs was confirmed in 3 donors. (C) Anti–IFN-γ did not inhibit enhanced production of TNF-α by PBMCs following superantigen-LPS exposure. ITMC indicates isotype-matched control antibody. Mean and SD of triplicate samples are shown; data are representative of 5 separate experiments.
Discussion
Bacterial superantigenic exotoxins, derived predominantly from S pyogenes or S aureus, can provoke severe hemodynamic shock and multiorgan failure.1,3,11 The devastating pathophysiology underlying these conditions is thought to result from the ability of these microbial products to cross-link T-cell receptors and MHC class II without undergoing conventional antigen processing.9 This provokes a massive polyclonal T-cell expansion with the consequent release of harmful proinflammatory cytokines.3 However, this immunologic phenomenon may not provide the sole explanation for the potency of these toxins. First, superantigens can induce powerful immune effects in the host without a requirement for T-cell proliferation.22-26 Second, their capacity to induce proinflammatory injury can be greatly enhanced by Gram-negative bacterial endotoxin.16-19,34 The interaction of endotoxin with superantigens has been shown to be highly significant in several animal models. However, the mechanism for this powerful effect remains uncertain and has not been studied in a human system.
Using primary human monocytes we have demonstrated that superantigens can reproducibly enhance the membrane expression of TLR4, a pivotal component in mammalian recognition of endotoxin. We showed that this effect is optimal at biologically relevant concentrations of superantigen and that it is absolutely dependent on ligation of MHC class II. The up-regulation of TLR4 was specific to superantigens and did not occur with LTA, another immunologically active Gram-positive product or whole, heat-killed bacteria. We used genetic disruption or complementation of the superantigen gene smez to show that the ability of certain Gram-positive bacteria to induce increased expression of TLR4 was solely due to the presence of superantigens. Although increased expression of TLR4 has been reported in monocytes exposed to cytokines35 and in epithelial cells exposed to respiratory syncytial virus,36 this is the first demonstration of TLR4 up-regulation by any bacterial product.
In addition to increasing membrane expression of TLR4, superantigens were also observed to induce significant and rapid TLR4 transcription in monocytes (in excess of 50-fold over resting levels in some subjects). This finding is also novel. Although superantigens are known to induce important effects on transcription of cytokines such as TNF-alpha,33,37,38 they have not previously been reported to influence genes encoding toll-like receptors. Interestingly, we were also able to show a parallel increase in superantigen-induced MD2 transcription. MD2 is now known to be a critical accessory molecule for the function of TLR4 in endotoxin recognition.13
Critically, by describing superantigen-mediated amplification of monocyte responses to TLR4 ligands, we were also able to demonstrate a clear proinflammatory phenotype for cells that demonstrate superantigen-mediated up-regulation of TLR4. This is the first report to describe proinflammatory superantigen-endotoxin interactions in human monocytes using concentrations of superantigen found in sepsis. The presence of enhanced endotoxin responsiveness in monocytes primed with superantigen was unexpected, since animal studies have delineated a key role for T cells in this phenomenon.18,20,21 Superantigen-endotoxin proinflammatory interactions were therefore further investigated in human mixed PBMC cultures where SEA mutant constructs could be used to compare the relative contribution of superantigen ligation by MHC class II or TCR to the priming effect. In addition, the larger sample sizes available in PBMC experiments also enabled better definition and quantification of superantigen-endotoxin proinflammatory cooperation.
The sequential stimulation of PBMCs with superantigen-endotoxin again induced early- and high-magnitude induction of proinflammatory cytokines when compared with that produced by stimulation with individual toxins. This multiplicative effect was significant over a range of toxin concentrations. Interestingly, while greatly enhanced levels of TNF-α, IL-6, and IL-1β were found following superantigen-endotoxin addition, this was not true for IFN-γ. Although the optimal superantigen priming interval preceded maximal superantigen TLR4 membrane expression, it correlated well with the rapid rise in TLR4/MD2 transcription and the time point at which detectable up-regulation of TLR4 at the cell surface occurred. There may also be important functional enhancement of the TLR4 signaling pathway ahead of maximal TLR4 membrane expression as detectable by flow cytometry.
In addition to their failure to up-regulate TLR4, MHC class II nonbinding SEA constructs were also unable to enhance subsequent PBMC responses to endotoxin. It was therefore interesting to note that superantigens known to cross-link MHC class II (such as SEA)29,38 were not the most potent with respect to either TLR4 up-regulation or amplification of cytokine induction. Importantly, not only was proinflammatory priming invoked in full by the TCR nonbinding SEA construct, but it also could not be disrupted by saturating concentrations of anti–IFN-γ. Taken together, these data confirmed that superantigen-endotoxin proinflammatory cooperation in this system occurs independent of any interaction with T cells, and raises the possibility that monocytes may constitute a major early host responder cell in Gram-positive toxic shock.
We considered the possibility that trace amounts of endotoxin present in superantigen preparations might account for TLR4 up-regulation or for interactions with secondary LPS challenge. However, this possibility was discounted because exogenous endotoxin and mutant constructs of superantigens were unable to reproduce TLR4 up-regulation or priming of mononuclear cells. Furthermore, superantigen-containing bacterial supernatants were able to support TLR4 up-regulation despite being LPS-free.
The induction of significant TLR4 up-regulation and proinflammatory priming in monocytes by low concentrations of superantigen (100 pg/mL), raises the possibility that reported synergy between other Gram-positive microbial products and endotoxin could be mediated by contaminating superantigens. The suggestion that endotoxin may be important in the pathogenesis of Gram-positive toxic shock may also have important clinical relevance. This is because the systemic circulation of critically ill patients may be vulnerable to concurrent assault from both endotoxin and superantigens. Not only may both toxins be released during episodes of polymicrobial sepsis,7 but translocation of endotoxin may occur during Gram-positive sepsis where there is injury to the gastrointestinal tract or ventilated lung.5,39-41 Conversely, acquisition of nosocomial infection with superantigen-producing bacteria (such as S aureus) could adversely affect outcomes in patients with severe sepsis due to Gram-negative organisms, particularly if plasma levels of superantigen in excess of the apparent threshold (10 pg/mL-100 pg/mL) are reached. Intriguingly, the results from our study may offer an explanation for the observation that TLR4 is up-regulated in critically ill patients with Gram-positive sepsis (predominantly S aureus).42 Our observations may also explain apparent discrepancies between the presence of circulating superantigens and severity of illness.6,7 An absence of systemic endotoxin in some patients may blunt host injury due to superantigens.
In summary, we have demonstrated that by signaling through MHC class II, Gram-positive bacterial superantigenic exotoxins can up-regulate the transcription and membrane expression of TLR4 in human monocytes. These events are associated with a marked enhancement of proinflammatory cytokine production when superantigen-primed monocytes are stimulated with TLR4 ligands. Importantly, T cells were not necessary for the superantigen-endotoxin interactions observed in this system. Taken together, our observations suggest that general pattern recognition of superantigens from S aureus and S pyogenes by monocyte MHC class II can enhance endotoxin sensing and signal transduction and offer a novel and mechanistic explanation for the rapid and devastating effects that small quantities of bacterial superantigens provoke when released into the systemic circulation. This provides a further example of important cross-talk between Gram-positive and Gram-negative bacterial products and raises the possibility that endotoxin translocation in critically ill patients may be highly detrimental in the context of concurrent Gram-positive infection.
Prepublished online as Blood First Edition Paper, January 11, 2005; DOI 10.1182/blood-2004-07-2523.
Supported by an MRC Clinical Training Fellowship (P.A.H.), a grant from the Intensive Care Society (P.A.H.), and the Sheffield Teaching Hospitals Charitable Trust (A.C.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lee Faulkner (Gram Positive Molecular Pathogenesis Group, Imperial College, London) for advice and technical assistance. We also thank Dr Elena Kulinskaya and Dr Fabiana Gordon (Statistical Advisory Service, Imperial College, London) for advice regarding data analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal