Abstract
Current strategies for genetic therapy using Moloney retroviruses require ex vivo manipulation of hematopoietic cells to facilitate stable integration of the transgene. While many studies have evaluated the impact of ex vivo culture on normal murine and human stem/progenitor cells, the cellular consequences of ex vivo manipulation of stem cells with intrinsic defects in genome stability are incompletely understood. Here we show that ex vivo culture of Fancc-/- bone marrow cells results in a time-dependent increase in apoptosis of primitive Fancc-/- progenitor cells in conditions that promote the proliferation of wild-type stem/progenitor cells. Further, recipients reconstituted with the surviving Fancc-/- cells have a high incidence of cytogenetic abnormalities and myeloid malignancies that are associated with an acquired resistance to tumor necrosis factor α (TNF-α). Collectively, these data indicate that the intrinsic defects in the genomic stability of Fancc-/- stem/progenitor cells provide a selective pressure for cells that are resistant to apoptosis and have a propensity for the evolution to clonal hematopoiesis and malignancy. These studies could have implications for the design of genetic therapies for treatment of Fanconi anemia and potentially other genetic diseases with intrinsic defects in genome stability.
Introduction
Fanconi anemia (FA) is an autosomal recessive disorder characterized by multiple congenital abnormalities, bone marrow (BM) failure, and susceptibility to cancer.1 Ninety percent of all deaths result from complications of a progressive BM failure or a generally late malignant myeloid transformation, especially acute myeloid leukemia (AML).2,3 To date, 11 complementation groups (FANC A, B, C, D1, D2, E, F, G, I, J, L) have been identified and the cDNAs of 9 of these genes that collectively account for over 95% of all FA patients have been cloned.4-6 The identification of these genes raises the potential of using gene transfer technology to express the functional cDNA in autologous hematopoietic stem cells.
Recent unanticipated sequelae from gene transfer protocols underscore the need to assess as fully as possible the biologic consequences of all aspects of gene transfer protocols prior to clinical use.7-9 Fancc knockout (Fancc-/-) mice recapitulate many aspects of the human disease including hypersensitivity to DNA cross-linking agents10,11 and inhibitory cytokines,12-14 defects in germ cells and hematopoietic stem cells,10,15 and a predisposition to malignancies.16 This murine model provides a preclinical platform to examine in detail the disease pathogenesis and to study in vivo the biologic consequences of stem and progenitor cell function following the manipulation of bone marrow cells in experimental protocols. Previously, we demonstrated that introduction of recombinant Fancc into Fancc-/- stem cells via a Moloney retrovirus enhances stable long-term repopulating ability to near wild-type levels in primary and secondary recipient mice.17 However, some mice reconstituted with Fancc-/- cells that lacked the Fancc transgene acquired either BM hypoplasia or histologic features consistent with myelodysplasia (MDS). These observations prompted the current studies to explore the pathologic underpinnings of ex vivo culture of Fancc-/- stem cells. Here we show that ex vivo culture of Fancc-/- stem/progenitor cells that promote proliferation, required for transduction with Moloney retroviruses, is associated with a time-dependent increase in apoptosis of a primitive phenotypic population of Fancc-/- cells. Further, while irradiated recipients reconstituted with freshly isolated Fancc-/- cells did not acquire myeloid malignancies, recipients reconstituted with Fancc-/- bone marrow cells cultured for either 2 or 4 days had a high incidence of MDS, myeloproliferative disease (MPD), and AML, associated with a range of cytogenetic abnormalities. Finally, in contrast to progenitors cultured from the bone marrow of unaffected Fancc-/- recipients (which are hypersensitive to a range of inhibitory cytokines), progenitors from recipients with AML and MDS were resistant to tumor necrosis factor α (TNF-α)–induced apoptosis. Collectively, these data provide a unique model of stem cell exhaustion and clonal evolution in FA. These studies also have potential implications regarding the design of gene transfer protocols in Fanconi anemia for the treatment of autologous hematopoietic stem cells.
Materials and methods
Mice
Purification and culture of SCL cells
BM cells were harvested from littermates as described,13 incubated with lineage-specific antibodies conjugated with fluorescein isothiocyanate (FITC; CD3, B220, Mac1, Gr1, and Ter119) as well as antibodies to antigens that select for immature hematopoietic cell populations (Sca1 conjugated with phycoerythrin [PE] and c-kit with allophycocyanin [APC]; BD PharMingen, San Diego, CA), and sorted using a Becton Dickinson (San Jose, CA) FACSTAR sorter as previously described.19 Selected Sca1+c-kit+Lin- (SCL) cells were cultured in Iscoves Modified Dulbecco media (Gibco-BRL, Gaithersburg, MD) supplemented with 20% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT) at an initial cell density of 105 cells/mL in the presence of interleukin 6 (IL-6; 200 U/mL) and stem cell factor (SCF; 100 ng/mL; Peprotech Rocky Hill, NJ).13,19
TUNEL assay
Freshly isolated or cultured SCL cells were fixed in 1% paraformaldehyde19 and evaluated for apoptosis using a TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuridine triphosphate [dUTP] nick end labeling) assay (ApopTag Apoptosis Detection Kit; Intergen, Purchase, NY) as suggested by the manufacturer and analyzed using fluorescence cytometry.
Clonogenic assays
Freshly isolated or cultured SCL cells were cultured at a cell density of 2 × 103 cells/mL in triplicate on 35-mm plates (BD, Franklin Lakes, NJ). Cells and recombinant growth factors were added (0.66%-1%) to agar for growth of low proliferating potential (LPP) colony-forming cells (CFCs) and high proliferating potential (HPP)–CFCs and the solution was thoroughly mixed before plating as previously described.20 Growth factors used for culture include IL-1, macrophage colony-stimulating factor (M-CSF; R&D Research Laboratories, Minneapolis, MN), SCF, granulocyte M-CSF (GM-CSF), and IL-3 (Peprotech). To evaluate growth inhibitory effects of TNF-α on myeloid progenitor cells, CD45.2+ BM cells from recipients that were isolated by the deletion of CD45.1+ cells via fluorescence cytometry were plated in the absence or presence of TNF-α (0-10 ng/mL) as conducted previously.13,17 Freshly isolated BM cells from 2-month-old Fancc-/- and WT mice were used as controls for determining the sensitivity of the test cells to TNF-α.
Competitive repopulation assays and animal monitoring
BM low-density mononuclear cells (LDMNCs) from Fancc-/- and WT mice that had either been freshly isolated or cultured for 2 or 4 days in the presence of IL-6 and SCF were used as “test” cells. A test-to-competitor cell ratio of 6:1 was used for recipients of Fancc-/- cells while a 3:1 test-to-competitor cell ratio was used for mice that received transplants of WT test cells. Viability of transplanted cells was assessed by trypan blue exclusion. In some instances viability was also evaluated by TUNEL, which closely correlated with trypan blue exclusion. Cell mixtures with a total number of 3 × 106 trypan blue–negative low-density mononuclear cells were injected into the tail vein of 7 lethally irradiated C57Bl/6 recipients (1100 cGy split dose) per experimental group. CD45.2 test cell chimerism in peripheral blood was determined using fluorescence cytometry as described.18 Complete blood counts were performed monthly in an automated cell counter (Sysmex, Kobe, Japan). The accuracy of abnormal blood counts was verified by direct examination of blood smears. Spleen weight and BM cellularity were obtained at the time that the mice were killed. Hematopoietic tissues were collected for clonogenic assays and histologic and cytogenetic analysis.17 Secondary transplantation was conducted using 2 × 106 LDMNC BM cells from primary recipients. MPD, MDS, and AML were defined according to Bethesda proposals for classification of nonlymphoid neoplasms in mice.21
SKY analysis
Spectral karyotyping (SKY) analysis was performed on BM cells. The initiation of short-term (24-72 hours) cultures, metaphase cell preparation, and spectral karyotyping were performed as described elsewhere.22 A minimum of 10 metaphase cells were analyzed per sample.
Histology
Portions of the spleen and femur from selected mice were fixed in buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. Sections were analyzed using Zeiss Axioskop microscopy (Zeiss, Oberkochen, Germany) with Spot Advanced software (Spot, Amsterdam, The Netherlands).
Statistical analysis and graphs
Computations were carried out using the Graph Pad Prism software package (San Diego, CA). Student t test and Fisher exact analysis were used to compare the various groups. A P value of less than .05 was considered statistically significant.
Results
Ex vivo culture results in reduced numbers of primitive and mature Fancc-/- BM progenitors
To determine the consequences of ex vivo culture on primitive phenotypic and clonogenic Fancc-/- populations, Sca1+c-kit+Lin- (SCL) cells from Fancc-/- and WT BM were cultured in recombinant stem cell factor–, interleukin-6–, and fetal calf serum–enriched media.19 The number of Sca1+c-kit+ cells, as well as the number of primitive and mature myeloid progenitors, was determined 2 and 4 days after the initiation of culture.A2- to 4-fold increase in the number of Sca1+c-kit+ cells in cultures containing WT cells was observed, whereas cultures containing an equivalent phenotypic population of Fancc-/- cells failed to expand (Figure 1A). Furthermore, while ex vivo culture of WT SCL cells maintained the number of primitive clonogenic myeloid progenitor populations, called high proliferating potential colony-forming cells (HPP-CFCs), there was a decline in this primitive clonogenic population in cultures of Fancc-/- cells 2 days following initiation of cultures, and this population was nearly absent in cultures harvested 4 days following culture initiation (Figure 1B). Additionally, while a 6-fold expansion of low proliferating potential colony-forming cells (LPP-CFCs) was observed at the conclusion of liquid culture containing WT SCL cells, this expansion was significantly less (50% of WT) in cultures containing Fancc-/- cells (Figure 1B).
Effect of ex vivo culture on proliferation and apoptosis of Fancc-/- Sca1 +c-kit+Lin- (SCL) cells. (A) Expansion of SCL cells in liquid culture. SCL cells were incubated for 48 or 96 hours and the number of Sca1 +c-kit+ cells was determined using fluorescence cytometry. The mean fold increase of Sca1 +c-kit+ cells from 5 independent experiments is shown. Error bars represent the standard error of the mean (SEM). *P < .01, **P < .001 comparing Fancc-/- with WT. (B) Evaluation of primitive and mature clonogenic myeloid cells following ex vivo culture. SCL (106) cells were maintained in liquid culture for 0 to 4 days and then 2 × 103 cells were plated in semisolid media in triplicate cultures for growth of high proliferating potential colony-forming cells (HPP-CFCs) and low proliferating potential colony forming cells (LPP-CFCs). The mean cumulative colony numbers of HPP-CFCs and LPP-CFCs per culture from 5 independent experiments are shown. The error bars represent the SEM. *P < .05, **P < .01, ***P < .001 comparing Fancc-/- with WT. (C) Evaluation of apoptotic Sca1 +kit+ (SC) cells following ex vivo culture. (Left) A representative experiment demonstrating the gating method used for scoring apoptotic SC cells. SC cells were identified as described in “Purification and culture of SCL cells” and then evaluated for apoptosis by TUNEL. Bars indicate the percentage of apoptotic SC cells. The graph (right) shows the mean percentage of apoptotic SC cells from 4 independent experiments ± SEM. Error bars indicate SEM. *P < .01, **P < .001 comparing Fancc-/- with WT.
Effect of ex vivo culture on proliferation and apoptosis of Fancc-/- Sca1 +c-kit+Lin- (SCL) cells. (A) Expansion of SCL cells in liquid culture. SCL cells were incubated for 48 or 96 hours and the number of Sca1 +c-kit+ cells was determined using fluorescence cytometry. The mean fold increase of Sca1 +c-kit+ cells from 5 independent experiments is shown. Error bars represent the standard error of the mean (SEM). *P < .01, **P < .001 comparing Fancc-/- with WT. (B) Evaluation of primitive and mature clonogenic myeloid cells following ex vivo culture. SCL (106) cells were maintained in liquid culture for 0 to 4 days and then 2 × 103 cells were plated in semisolid media in triplicate cultures for growth of high proliferating potential colony-forming cells (HPP-CFCs) and low proliferating potential colony forming cells (LPP-CFCs). The mean cumulative colony numbers of HPP-CFCs and LPP-CFCs per culture from 5 independent experiments are shown. The error bars represent the SEM. *P < .05, **P < .01, ***P < .001 comparing Fancc-/- with WT. (C) Evaluation of apoptotic Sca1 +kit+ (SC) cells following ex vivo culture. (Left) A representative experiment demonstrating the gating method used for scoring apoptotic SC cells. SC cells were identified as described in “Purification and culture of SCL cells” and then evaluated for apoptosis by TUNEL. Bars indicate the percentage of apoptotic SC cells. The graph (right) shows the mean percentage of apoptotic SC cells from 4 independent experiments ± SEM. Error bars indicate SEM. *P < .01, **P < .001 comparing Fancc-/- with WT.
Fancc-/- cells have an increased propensity for apoptosis following exposure to a range of genotoxic and nongenotoxic stresses.11-13,23 To examine whether the reduced number of progenitors isolated from ex vivo cultures was a consequence of an increased propensity of Fancc-/- cells to undergo apoptosis, the proportion of Sca1+c-kit+ cells that were apoptotic as determined by TUNEL assays were scored. While the number of TUNEL-positive Sca1+c-kit+ cells was low at the initiation and the conclusion of cultures containing WT cells, there was a 4-fold increase in the number of TUNEL-positive Fancc-/- Sca1+c-kit+ cells by 48 hours and a 9-fold increase 4 days following the initiation of culture (Figure 1C). Collectively, Fancc-/- primitive hematopoietic cell populations are predisposed to undergo apoptosis when cultured ex vivo.
Reduced repopulating ability and accelerated development of clonal diseases in recipients of cultured Fancc-/- cells
To determine whether the long-term repopulating cell function of Fancc-/- cells was affected by the length of ex vivo culture, analogous to myeloid progenitors, competitive repopulation assays were established. Freshly isolated LDMNCs or LDMNCs cultured ex vivo for 2 or 4 days were transplanted with a common pool of competitor cells. Because of the previously published defect in freshly isolated Fancc-/- stem cells,17-19 a 6:1 test-to-competitor cell ratio was used in recipients reconstituted with viable Fancc-/- cells compared with a 3:1 test-to-competitor cell ratio in recipients reconstituted with WT cells. Mice reconstituted with WT cells displayed comparable levels of test cell chimerism 4 months following transplantation, regardless of whether cells were freshly isolated or cultured ex vivo (Figure 2A). In contrast, recipients reconstituted with Fancc-/- bone marrow cells showed a length of culture-dependent decline in long-term repopulating ability when evaluated 4 months following transplantation (Figure 2A).
Ex vivo culture of Fancc-/- hematopoietic stem cells alters graft stability in competitive repopulation assays. Following culture for 0, 2, or 4 days in SCF and IL-6, Fancc-/- and WT “test cells” that express the CD45.2 isoantigen were cotransplanted into irradiated syngeneic recipients with isogenic bone marrow cells that express the CD45.1 antigen. WT cells were transplanted at a 3:1 test-competitor cell ratio and Fancc-/- cells were transplanted at a 6:1 test-competitor cell ratio. (A) Evaluation of test cell chimerism as a function of time. Peripheral blood test cell chimerism was determined using fluorescence cytometry at the indicated time points. The length of cell culture and the genotype of test cells are indicated. Each symbol represents the test cell chimerism of an individual recipient. Symbols with numbers or letters indicate specimens where SKY analysis was determined at postmortem. Red symbols indicate recipients with abnormal karyotypes. Horizontal lines indicate the mean chimerism of the experimental group. (B) The relationship between cytogenetic abnormalities and the flux in test cell chimerism following transplantation of Fancc-/- cells is shown. Symbols indicate individual mice.
Ex vivo culture of Fancc-/- hematopoietic stem cells alters graft stability in competitive repopulation assays. Following culture for 0, 2, or 4 days in SCF and IL-6, Fancc-/- and WT “test cells” that express the CD45.2 isoantigen were cotransplanted into irradiated syngeneic recipients with isogenic bone marrow cells that express the CD45.1 antigen. WT cells were transplanted at a 3:1 test-competitor cell ratio and Fancc-/- cells were transplanted at a 6:1 test-competitor cell ratio. (A) Evaluation of test cell chimerism as a function of time. Peripheral blood test cell chimerism was determined using fluorescence cytometry at the indicated time points. The length of cell culture and the genotype of test cells are indicated. Each symbol represents the test cell chimerism of an individual recipient. Symbols with numbers or letters indicate specimens where SKY analysis was determined at postmortem. Red symbols indicate recipients with abnormal karyotypes. Horizontal lines indicate the mean chimerism of the experimental group. (B) The relationship between cytogenetic abnormalities and the flux in test cell chimerism following transplantation of Fancc-/- cells is shown. Symbols indicate individual mice.
Other investigators have established that chimerism in mice becomes stable 4 months following engraftment.24,25 The test cell chimerism of mice that received transplants of all groups of WT cells, as well as freshly isolated Fancc-/- cells, was stable up to 14 months following transplantation. However, the test cell chimerism of a subset of mice that received transplants of cultured Fancc-/- cells diverged radically as a function of time (Figure 2A) such that the test cell chimerism of these recipients progressively out-competed the WT competitor cells. This variability in chimerism was observed in recipients of cells that were cultured for 4 days prior to transplantation, as in previous studies,17 and in recipients of cells that had been cultured for 2 days only.
To determine whether the incremental increase in chimerism of recipients of cultured Fancc-/- bone marrow was associated with the development of cytogenetic abnormalities and/or myeloid malignancies, bone marrow and splenic cellularity and architecture, as well as spectral karyotyping of bone marrow cells, were determined at autopsy. In general, the bone marrow cellularity of mice reconstituted with previously cultured Fancc-/- bone marrow was lower than the cellularity of recipients reconstituted with freshly isolated Fancc-/- or WT cells (Table 1). SKY analysis was conducted on 20 recipients of freshly isolated or cultured Fancc-/- cells or WT cells (Table 1). These recipients are identified individually by numbers (Fancc-/- test cells) or letters (WT test cells; Figure 2A; Table 1) and recipients with cytogenetic abnormalities are indicated in red (Figure 2A). Seven other recipients of cultured Fancc-/- cells died unexpectedly of apparent bone marrow failure as evidenced by bone marrow hypoplasia and a very small pale spleen (data not shown). As expected, recipients reconstituted with WT cells had no cytogenetic abnormalities regardless of the length of ex vivo culture. Similarly, only 1 of 7 recipients reconstituted with freshly isolated Fancc-/- cells had a cytogenetic abnormality. In contrast, 5 of 7 recipients of ex vivo–cultured Fancc-/- cells acquired a range of cytogenetic abnormalities (Table 1) including deletions, reciprocal translocations, and unbalanced translocations of autosomes as well as loss of sex chromosomes. To determine the relationship between the unstable chimerism found in recipients reconstituted with Fancc-/- cells and the presence of cytogenetic abnormalities in bone marrow cells from those recipients, the change in chimerism of individual mice was evaluated as a function of time (Figure 2B). In contrast to the stable chimerism observed in recipients reconstituted with cells of either genotype with normal karyotypes, 4 of 6 mice with cytogenetic abnormalities had a progressive increase (> 25%) in test cell chimerism (P < .05; Figure 2B).
Cytogenetic and hematologic characteristics of mice that received transplants of ex vivo-cultured BM cells
Donor cell genotype and mouse no. . | Culture time, d . | Karyotype* . | BM cellularity, × 106 LDMNCs . | Spleen weight, mg . | TNF-α sensitivity . | BM and spleen histology . | BM and spleen histology from secondary BMT . |
|---|---|---|---|---|---|---|---|
| +/+ | |||||||
| a-f | 0,2,4 | Normal | 16.8-22.8 | 91-150 | R | Normal | Normal |
| -/- | |||||||
| 1-6 | 0 | Normal | 18.4-22.4 | 97-160 | S | Normal | Normal |
| 7 | 0 | 40,X, - X or - Y, +6(30%)/39,XX,t(8;9)(C3;F4), - 14(10%) | 16.3 | 286 | S | Reactive proliferation† | Normal |
| 8 | 2 | Normal | 18.5 | 167 | S | Hypoplasia | Hypoplasia |
| 9 | 2 | 40,XX,t(4;17)(D3;E4)(10%)/40,XX,del(6)(DF3)(10%)/38,X, - X or - Y,t(4;9)(E1;F1), - 9(10%)/40,XY,der(15)t(15;16)(E;D)(10%) | 17.0 | 224 | R | Myeloproliferative disease | MDS |
| 10 | 2 | 40,XY,t(8;17)(D1;A2)(20%) | 12.7 | 358 | R | Myeloproliferative disease | Acute myeloid leukemia |
| 11 | 4 | Normal | 9.0 | 83 | S | Hypoplasia | Hypoplasia |
| 12 | 4 | 39,X, - X or - Y(30%) | 6.1 | 62 | R | Hypoplasia | MDS |
| 13 | 4 | 39,XY, - 13der(19)t(13;19)(B;D2)(10%) | 11.4 | 120 | S | Hypoplasia | NA‡ |
| 14 | 4 | 40,XY, - 4, +mar(10%) | 7.0 | 99 | R | Hypoplasia | Hypoplasia |
Donor cell genotype and mouse no. . | Culture time, d . | Karyotype* . | BM cellularity, × 106 LDMNCs . | Spleen weight, mg . | TNF-α sensitivity . | BM and spleen histology . | BM and spleen histology from secondary BMT . |
|---|---|---|---|---|---|---|---|
| +/+ | |||||||
| a-f | 0,2,4 | Normal | 16.8-22.8 | 91-150 | R | Normal | Normal |
| -/- | |||||||
| 1-6 | 0 | Normal | 18.4-22.4 | 97-160 | S | Normal | Normal |
| 7 | 0 | 40,X, - X or - Y, +6(30%)/39,XX,t(8;9)(C3;F4), - 14(10%) | 16.3 | 286 | S | Reactive proliferation† | Normal |
| 8 | 2 | Normal | 18.5 | 167 | S | Hypoplasia | Hypoplasia |
| 9 | 2 | 40,XX,t(4;17)(D3;E4)(10%)/40,XX,del(6)(DF3)(10%)/38,X, - X or - Y,t(4;9)(E1;F1), - 9(10%)/40,XY,der(15)t(15;16)(E;D)(10%) | 17.0 | 224 | R | Myeloproliferative disease | MDS |
| 10 | 2 | 40,XY,t(8;17)(D1;A2)(20%) | 12.7 | 358 | R | Myeloproliferative disease | Acute myeloid leukemia |
| 11 | 4 | Normal | 9.0 | 83 | S | Hypoplasia | Hypoplasia |
| 12 | 4 | 39,X, - X or - Y(30%) | 6.1 | 62 | R | Hypoplasia | MDS |
| 13 | 4 | 39,XY, - 13der(19)t(13;19)(B;D2)(10%) | 11.4 | 120 | S | Hypoplasia | NA‡ |
| 14 | 4 | 40,XY, - 4, +mar(10%) | 7.0 | 99 | R | Hypoplasia | Hypoplasia |
R indicates Fancc-/- myeloid progenitors that had < 10% reduction in maximal clonogenic numbers when cultured in the presence of 1 ng/mL of TNF-α, S,Fancc-/- myeloid progenitors that had ≤ 50% reduction in maximal clonogenic numbers when cultured in the presence of 1 ng/mL of TNF-α, and NA, not available.
Ten metaphase cells were analyzed from each sample; only cells with abnormal karyotypes are listed
This mouse had an eye infection at the time that it was killed
Recipient died unexpectedly after transplantation
We and others have previously established that Fancc-/- cells are hypersensitive to inhibitory cytokines, particularly TNF-α and interferon-γ (IFN-γ).12,13,26 Clonogenic growth of Fancc-/- progenitors is reduced by approximately 50% in the presence of 1 ng/mL of TNF-α compared with a 10% or less reduction in colonies cultured from WT mice.13 Further, progenitors from FA patients with MDS and AML,27 as well as Fancc-/- mice with histologic features of MDS from our previous work,17 are resistant to inhibitory cytokines including TNF-α. To determine whether there is an association between the development of cytogenetic abnormalities and the resistance of Fancc-/- cells to inhibitory cytokines, cultures for the growth of myeloid progenitors in a range of concentrations of TNF-α (0-10 ng/mL) were established as previously described.13,17 Myeloid progenitors cultured from 8 of 8 recipients reconstituted with Fancc-/- cells that had a normal karyotype were hypersensitive to TNF-α based on a reduction in clonogenic frequency in response to TNF-α as previously described (Table 1).13,17 In contrast, progenitors cultured from 4 of 6 recipients with cytogenetic abnormalities had less than a 10% reduction in clonogenic numbers and were resistant to TNF-α (P < .05; Table 1).
Ex vivo–cultured Fancc-/- stem cells have an increased risk of developing hematologic malignancies associated with cytogenetic abnormalities in secondary recipients
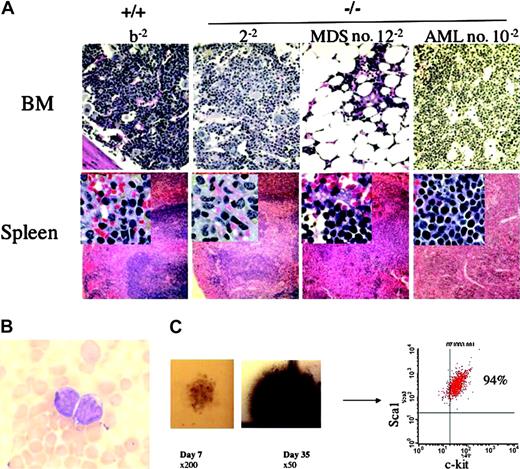
To pursue the phenotypic evolution of the ex vivo–cultured stem cells, bone marrow cells from 20 primary recipients were transplanted into secondary recipients and harvested 4 months following transplantation (Table 1). All secondary recipients reconstituted with WT or freshly isolated Fancc-/- cells retained normal marrow and splenic architecture. However, secondary recipients reconstituted with ex vivo–cultured cells developed MDS (2/7), AML (1/7), or BM hypoplasia (4/7; Table 1).All 3 mice with MDS orAML had cytogenetic abnormalities and 1 of 3 evaluable mice with bone marrow hypoplasia had cytogenetic abnormalities. Histologic sections of the bone marrow and spleen, as well as detailed cellularity of the hematopoietic organs from representative secondary recipients, are shown in Figure 3A. The recipient who acquired AML (no. 10-2) had low peripheral white blood cell counts that contained approximately 30% blast cells (Figure 3A-B, Table 2). Similarly, the BM and the red pulp of the spleen were infiltrated with immature cells, in which more than 50% cellularity of the bone marrow was Sca1+c-kit+ cells (Table 2). Attempts to culture myeloid progenitors from this animal yielded only very low numbers of small, dysplastic-appearing myeloid progenitors at day 7 of culture (Figure 3C). However, in contrast to granulocyte-macrophage progenitors whose colonies contain mature granulocytes and macrophages that begin to undergo apoptosis 7 to 10 days following the initiation of culture, colonies from the recipient with AML continued to grow in culture for 35 days (when the experiment was ended). Further, clonogenic cells retained a primitive phenotype (Figure 3C), indicating the inability of myeloid progenitors from this mouse to undergo appropriate differentiation.
Histologic and hematologic abnormalities in secondary recipients of cultured Fancc-/-cells. (A) Cellular and histologic evaluation of the hematopoietic organs. The test cell genotype and individual animal numbers are indicated. The top panel shows the bone marrow (BM) histology (original magnification × 200) subsequent to staining with hematoxylin and eosin. Specimens reconstituted with WT cells (b-2) or freshly isolated Fancc-/- cells (2-2) have normal histology, whereas the bone marrow from 2 recipients of Fancc-/- cells that had been cultured ex vivo prior to transplantation had significant hypoplasia (no. 12-2) or had a hyperplastic marrow with a homogeneous population of immature blasts (no. 10-2). The bottom panel shows the splenic histology (original magnification × 100) with insets of the red pulp of spleen (original magnification × 400). The b-2 and 2-2 recipients have normal splenic architecture, whereas there is a disruption of the normal red and white pulp with myeloid cell invasion in no. 12-2 and no. 10-2, respectively. (B) Representative blast cells from the peripheral blood of mouse no. 10-2 (original magnification × 1000). (C) The clonogenic growth of a representative small, dysplastic myeloid colony from mouse no. 10-2 on day 7 of culture is shown in the photomicrograph on the left (original magnification × 200). A photograph of the same colony is shown on day 35 of culture (middle; original magnification × 50). (Right) Cells isolated from that colony revealed a homogeneous population of immature phenotypic cells shown using fluorescence cytometry.
Histologic and hematologic abnormalities in secondary recipients of cultured Fancc-/-cells. (A) Cellular and histologic evaluation of the hematopoietic organs. The test cell genotype and individual animal numbers are indicated. The top panel shows the bone marrow (BM) histology (original magnification × 200) subsequent to staining with hematoxylin and eosin. Specimens reconstituted with WT cells (b-2) or freshly isolated Fancc-/- cells (2-2) have normal histology, whereas the bone marrow from 2 recipients of Fancc-/- cells that had been cultured ex vivo prior to transplantation had significant hypoplasia (no. 12-2) or had a hyperplastic marrow with a homogeneous population of immature blasts (no. 10-2). The bottom panel shows the splenic histology (original magnification × 100) with insets of the red pulp of spleen (original magnification × 400). The b-2 and 2-2 recipients have normal splenic architecture, whereas there is a disruption of the normal red and white pulp with myeloid cell invasion in no. 12-2 and no. 10-2, respectively. (B) Representative blast cells from the peripheral blood of mouse no. 10-2 (original magnification × 1000). (C) The clonogenic growth of a representative small, dysplastic myeloid colony from mouse no. 10-2 on day 7 of culture is shown in the photomicrograph on the left (original magnification × 200). A photograph of the same colony is shown on day 35 of culture (middle; original magnification × 50). (Right) Cells isolated from that colony revealed a homogeneous population of immature phenotypic cells shown using fluorescence cytometry.
Hematologic characteristics of secondary recipient mice
. | Mouse no. . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | b-2 . | 2-2 . | MDS no. 12-2 . | AML no. 10-2 . | |||
| WBC, × 103/μL (% blast cells) | 15.5 (0) | 12.5 (0) | 4.5 (2.0) | 5.5 (34) | |||
| BM cellularity, × 106 | 19.5 | 12.2 | 9.2 | 60.0 | |||
| Spleen weight, mg | 110 | 103 | 80 | 323 | |||
| Sca I+ c-kit+ cells, % | |||||||
| BM | 1.5 | 1.0 | 11.0 | 70.0 | |||
| Spleen | 1.0 | 0.5 | 33.0 | 53.0 | |||
| Peripheral blood | 0.8 | 0.4 | 2.0 | 31.0 | |||
| CFU-GM/2 × 104 LDMNC, mean ± SEM | 138 ± 21 | 107 ± 14 | 67 ± 9.0 | 8.0 ± 1.2 | |||
. | Mouse no. . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | b-2 . | 2-2 . | MDS no. 12-2 . | AML no. 10-2 . | |||
| WBC, × 103/μL (% blast cells) | 15.5 (0) | 12.5 (0) | 4.5 (2.0) | 5.5 (34) | |||
| BM cellularity, × 106 | 19.5 | 12.2 | 9.2 | 60.0 | |||
| Spleen weight, mg | 110 | 103 | 80 | 323 | |||
| Sca I+ c-kit+ cells, % | |||||||
| BM | 1.5 | 1.0 | 11.0 | 70.0 | |||
| Spleen | 1.0 | 0.5 | 33.0 | 53.0 | |||
| Peripheral blood | 0.8 | 0.4 | 2.0 | 31.0 | |||
| CFU-GM/2 × 104 LDMNC, mean ± SEM | 138 ± 21 | 107 ± 14 | 67 ± 9.0 | 8.0 ± 1.2 | |||
Discussion
Genome integrity is of vital importance in the maintenance of cell viability and the prevention of neoplastic transformation.28 This basic principle is underscored in the hematologic manifestations of Fanconi anemia, a genomic instability syndrome characterized by the development of a progressive bone marrow failure in childhood and the late development of myeloid malignancies. Although the details of the function of FA proteins in the maintenance of genome stability remain to be resolved, the close physical and functional interaction of FA proteins with proteins involved in DNA repair, cell cycle control, and apoptosis implicate the importance of the FA proteins in maintenance of genome integrity to endogenous as well as exogenous sources of DNA damage.23,29-31
FA cells are known to have an increased propensity to undergo apoptosis in response to both DNA-damaging agents and inhibitory cytokines.11-13,23,26 Data here additionally show an increased loss of phenotypically primitive precursors via apoptosis and functional losses of repopulating stem cell activity when bone marrow cells are cultured ex vivo for 48 or 96 hours in the presence of growth factors (SCF and IL-6) that support both survival as well as cell division in wild-type cells. In other studies where Fancc-/- SCL cells were cultured with single growth factors (SCF or IL-6) that promote survival but not proliferation of WT cells, a 5-fold loss of primitive progenitors was observed in Fancc-/- SCL cells compared with WT SCL cells (F.-C.Y. and D.W.C., unpublished results, September 2004).
Approximately 50% of mice reconstituted with ex vivo–cultured Fancc-/- bone marrow cells displayed progressively unstable chimerism in competitive repopulation assays. To determine whether this observation was associated with changes in cytogenetic abnormalities, spectral karyotyping was conducted. The data demonstrated that recipients whose test cell chimerism progressively increased over 12 to 14 months had cytogenetic abnormalities. A number of clonal and nonclonal deletions, reciprocal translocations, and unbalanced rearrangements of autosomes were observed. Although FA patients have been reported to have an increased incidence of abnormalities, particularly aberrations involving 1q, 3q25-q29, or monosomy 7,32-34 the cytogenetic abnormalities observed in these experimental mice did not involve the syntenic regions to those abnormalities found in FA patients. Additionally, 3 of 6 mice with cytogenetic abnormalities had a loss of sex chromosomes, a finding that is frequently observed in mice with myeloid malignancies.22
We were next interested in understanding the cellular mechanisms that predispose Fancc-/- stem cells to develop myelodysplasia and acute myeloid leukemia. We and others have previously shown that FA progenitors are hypersensitive to inhibitory cytokines that normally function as physiologic signals to inhibit cell division and induce apoptosis in hematopoietic and nonhematopoietic cells.12,13 Given that resistance to apoptosis is often a requirement for transformation and the genesis of myeloid malignancies,35-37 together with observations that myeloid progenitors cultured from FA patients with MDS/AML are resistant to inhibitory cytokines,27 we were interested in evaluating the responsiveness of the myeloid progenitors from the respective recipients to TNF-α. We found that all mice with hematologic malignancies and 4 of 6 mice with cytogenetic abnormalities were resistant to TNF-α, consistent with data in FA patients.27 Since these same mice had a progressive increase in Fancc-/- test cell chimerism as a function of time, the data suggest that the resistance to apoptosis in response to TNF-α and other physiologic cues provide an important selection pressure as initiating events leading to the predisposition of FA patients to develop clonal hematopoietic diseases. This type of selection pressure has recently been recognized as being central to the development of solid tumors in other disease models as well.38,39
While we have seen a range of cytogenetic abnormalities and myeloid malignancies in these murine studies, a gene transfer study involving 4 FANCC patients40 did not observe the development of myeloid malignancies or bone marrow failure in any of the patients. It is possible that there are potential species-specific differences between mice and humans that may account for these outcomes. However, there are also differences in the design of the current study and the previous clinical study that would be anticipated to influence the engraftment of exogenous cells as well as the ability to examine their fates. First, the murine recipients received myeloablative ionizing radiation, which requires the engraftment of exogenous repopulating cells in order for the recipient to survive. In contrast, in the previous phase 1 trial, 3 of 4 patients did not receive myelopreparation and a fourth patient received sublethal ionizing radiation treatment for a nonhematopoietic malignancy. Second, the contribution of exogenous Fancc-/- cells to the chimerism of each recipient was readily assessed by evaluating CD45.1/CD45.2 isoantigens on peripheral blood cells specimens. Fancc-/- cells contributed to at least 30% of the total chimerism in all recipients. In contrast, while a low level of retroviral-marked cells was observed for a few weeks to a few months after transplantation (< 1%-5%) in the human phase 1 trial, methodologies do not exist that allow accurate assessment of the engraftment of untransduced exogenous cells. Given the known reductions in engraftment of normal cells following ex vivo culture,41-43 it is unclear as to the proportion of untransduced FANCC repopulating cells that engrafted in the absence of myelopreparation in the clinical protocol.
The current study does point out potential unanticipated consequences of prolonged ex vivo culture for the purpose of attempting to expand Fancc mutant stem/progenitor cells or transduce them with Moloney retroviruses. The study also points out that the development of this process in mice is relatively slow in nature and points toward the need to follow murine recipients for 10 to 14 months to detect this pathology. Given that no gene transfer strategy is totally efficient, an important consideration in future gene transfer protocols will be to develop conditions that limit apoptosis of stem/progenitor cells and maintain genome integrity. Several potential strategies could be tested. Since the myeloid malignancies were not observed in recipients reconstituted with freshly isolated Fancc-/- stem cells, gene delivery strategies that limit ex vivo manipulation may allow an alternative strategy for genetic correction of the hematopoietic system in FA mutant mice. Mazurier et al44 recently achieved efficient transduction of human severe combined immunodeficiency–nonobese diabetic (SCID-NOD) repopulating cells in an overnight protocol using lentiviruses. Leurs et al45 also demonstrated efficient transduction of SCID-NOD repopulating stem cells using foamy viruses or lentiviruses in an abbreviated protocol. Alternatively, since some FA proteins, including FANCC, appear to have sequences that are redox sensitive,46-48 transducing cells in conditions using low concentrations of oxygen may be useful.
Finally, whether ex vivo manipulation of primary hematopoietic cells with other causes of genomic instability also have an increased propensity to undergo apoptosis and acquire cytogenetic abnormalities is unknown. Future studies evaluating whether comparable observations are found in other FA complementation groups or other diseases with genomic instability such as Werner or Bloom syndrome would be an important consideration of preclinical therapeutic studies for correction of hematopoietic stem cells.
Prepublished online as Blood First Edition Paper, January 11, 2005; DOI 10.1182/blood-2004-06-2483.
Supported by US Public Health Services Grants P01 HL53586, P30DK49218, R01 HL63219, K08 HLDK 040071-01, T32 HL-07910, and U01 CA84221 and the Riley Children's Foundation.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Dr Manuel Buchwald (Hospital for Sick Children, University of Toronto) for providing us with the Fancc-/- mice. We thank Drs Mervin Yoder and Mary Dinauer (Indiana University) for numerous helpful discussions and for reading the manuscript. We also thank Lee Ann Baldrige for excellent technical expertise in histologic analyses and Janice Walls for preparing the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal