Abstract
By real-time quantitative polymerase chain reaction (RQ-PCR), we evaluated BCL2/IgH+ cells in the bone marrow (BM) and peripheral blood (PB) from 86 patients with follicular lymphoma treated with the sequential administration of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab. At diagnosis, the amount of BCL2/IgH+ cells in the BM was low (1 BCL2/IgH+ cell in 1000-100 000 normal cells) in 43% of patients, intermediate (1 in 100-1000) in 34%, and high (> 1 in 100) in 23%. A 2 log decrease of BCL2/IgH+ cells was achieved after CHOP and an additional 2 log reduction following rituximab. By multivariate analysis, a low level of BCL2/IgH+ cells in the BM at diagnosis was the best predictor for the achievement of a complete clinical and molecular response. At 5 years, the event-free survival rates of patients with a low/intermediate or high tumor infiltration in the BM were 59% and 32%, respectively. The freedom from recurrence of patients who achieved a molecular response in the BM, no matter whether after CHOP alone or CHOP and rituximab, was 64% as compared to 32% for patients who did not (P < .006). RQ-PCR performed at presentation on BM samples predicts treatment response and long-term clinical outcome in patients with follicular lymphoma.
Introduction
With the possible exception of rare localized cases, follicular non-Hodgkin lymphomas (FL-NHLs) are usually incurable diseases.1 FL-NHLs exhibit a continuous tendency to relapse due to the incapacity of the available chemotherapeutic agents to eradicate the neoplastic clone. The use of qualitative polymerase chain reaction (PCR) allows a rapid detection of the chimeric BCL2IGH gene generated by the t(14;18) translocation, which identifies the neoplastic clone in the great majority (up to 80%) of these patients.2 This molecular marker has been proposed as an important tool in the diagnostic work-up at diagnosis and during the clinical follow-up.3 In a previous paper, we reported that the achievement of a PCR- status in the bone marrow (BM), no matter whether after CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy or after sequential CHOP and rituximab administration, is associated with a better freedom from recurrence (FFR).4 Although the clinical significance of circulating BCL2/IgH+ cells is still controversial,5,6 the persistence of these cells after conventional or high-dose chemotherapy programs correlates with a shorter clinical remission.7-9 During the last few years, different quantitative PCR assays have been developed.10-12 These methods provide a more reliable tool for an accurate evaluation of BCL2/IgH+ cells in the BM or peripheral blood (PB)13 and allow a better molecular monitoring of minimal residual disease after different therapeutic protocols. Moreover, some studies have provided evidence that real-time quantitative PCR (RQ-PCR) evaluation before and after autologous transplantation may predict the clinical course of these patients.14-16 Similar informative results have been gained by a quantitative molecular monitoring performed after donor leukocyte infusions in patients with a relapse following allogeneic hematopoietic stem cell transplantation.17,18 Based on these observations, the present study was designed to evaluate whether RQ-PCR in FL-NHL at diagnosis can be used to define the probability of achieving a clinical and molecular response after CHOP and rituximab given sequentially and to predict the long-term clinical outcome.
Patients, materials, and methods
A total of 128 patients with FL-NHL, PCR+ in the BM or PB for the major breakpoint region (MBR), or minor cluster region (mcr) BCL2IGH rearrangement were enrolled in a previously published study,4 designed to investigate whether the sequential administration of 4 weekly intravenous infusions of rituximab after 6 cycles of CHOP chemotherapy could induce a durable eradication of BCL2/IgH+ cells from BM and PB. Molecular evaluation was performed immediately before the beginning of CHOP chemotherapy. All patients were treated with 6 cycles of CHOP chemotherapy (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/m2). Three response categories were defined. Complete remission (CR) was defined as the complete disappearance of all clinically detectable disease (including BM infiltration) or reduction to less than 1 × 1 cm in size of all lymph nodes visible on computed tomography (CT) scans of the neck, chest, abdomen, and pelvis. Partial response was defined as more than 50% reduction in the sum of the products of bidimensional measurements for all measurable lesions. Progressive disease (PD) was considered as an increase in size of more than 25% of previously documented disease or the appearance of disease at any site.
At the end of chemotherapy, patients were eligible for rituximab treatment (375 mg/m2 weekly intravenous infusion for 4 weeks), if they were in partial or complete clinical remission and still PCR+ in the BM or PB (or both) as demonstrated on 2 molecular analyses performed 4 and 6 weeks after the sixth cycle of CHOP. Patients who achieved a PCR- status after CHOP were not eligible to receive rituximab. According to study protocol,4 patients were clinically and molecularly monitored at 12, 28, and 44 weeks after rituximab administration. Thereafter, patients were monitored at each institution according to local guidelines. Of the 128 patients who entered the previous study, 14 could not be studied because they had a mcr rearrangement of the BCL2IGH chimeric gene for which an accurate RQ-PCR analysis is not yet available. Seven patients were also excluded because of the presence of a MBR rearrangement larger than 500 bp, which similarly prevents a reproducible quantification of the chimeric gene.19 Of the remaining 107 patients with an MBR gene rearrangement, DNA collected at diagnosis was available for 86 patients (76 BM and 66 PB samples) who were clinically and molecularly evaluated. The clinical data of these patients are summarized in Table 1.
Clinical characteristics of the 86 patients analyzed by RQ-PCR
Characteristic . | Data . |
|---|---|
| Age, y | 51 |
| Median range, y | 28-66 |
| Older than 60 y, no. (%) | 13 (15) |
| Male, no. (%) | 55 (64) |
| Disease stage at diagnosis, no. (%) | |
| I | 1 (1) |
| II | 5 (6) |
| III | 24 (28) |
| IV | 56 (65) |
| BM involvement, no. (%) | 55 (64) |
| Extranodal sites other than BM, no. (%) | |
| 1 | 17 (20) |
| 2 or more | 3 (3) |
| B symptoms, no. (%) | 8 (9) |
| Bulky disease, no. (%)* | 15 (17) |
| Serum LDH level, no. (%) | |
| Normal | 73 (85) |
| Elevated | 10 (12) |
| International prognostic index, no. (%) | |
| 0-1 | 56 (65) |
| 2 or higher | 28 (33) |
| Unknown | 2 (2) |
| Response to CHOP chemotherapy, no. (%) | |
| Complete | 51 (59) |
| Partial | 28 (32) |
| No response | 7 (8) |
| Rituximab treatment, no. (%)† | |
| Yes | 49 (57) |
| No | 37 (43) |
Characteristic . | Data . |
|---|---|
| Age, y | 51 |
| Median range, y | 28-66 |
| Older than 60 y, no. (%) | 13 (15) |
| Male, no. (%) | 55 (64) |
| Disease stage at diagnosis, no. (%) | |
| I | 1 (1) |
| II | 5 (6) |
| III | 24 (28) |
| IV | 56 (65) |
| BM involvement, no. (%) | 55 (64) |
| Extranodal sites other than BM, no. (%) | |
| 1 | 17 (20) |
| 2 or more | 3 (3) |
| B symptoms, no. (%) | 8 (9) |
| Bulky disease, no. (%)* | 15 (17) |
| Serum LDH level, no. (%) | |
| Normal | 73 (85) |
| Elevated | 10 (12) |
| International prognostic index, no. (%) | |
| 0-1 | 56 (65) |
| 2 or higher | 28 (33) |
| Unknown | 2 (2) |
| Response to CHOP chemotherapy, no. (%) | |
| Complete | 51 (59) |
| Partial | 28 (32) |
| No response | 7 (8) |
| Rituximab treatment, no. (%)† | |
| Yes | 49 (57) |
| No | 37 (43) |
Bulky disease was defined as more than 10 cm
Patients not eligible for rituximab were those who had a molecular CR (n = 30) and those who had no response to CHOP (n = 7)
The study was conducted according to good clinical and laboratory practice rules, and the principles of the Declaration of Helsinki and the local Ethics Review Committees approved the protocol at each center.
Molecular analysis of BCL2/IgH+ cells by RQ-PCR
All samples were centralized and analyzed in one laboratory (Bergamo, Italy) and patients selected for this study were known to have rearrangements involving the MBR with amplicons of length between 120 and 300 bp. Cells bearing the t(14;18) translocation were quantified by real-time PCR using the ABI PRISM 7700 Detection System (PE Applied Biosystems, Foster City, CA). RQ-PCR was performed using oligos and a probe previously described by Ladetto et al.16 Each reaction was performed by amplifying triplicate samples containing 500 ng target DNA in a 25-μL volume using the following reagents: 12.5 μL TaqMan Universal Mastermix (PE Applied Biosystems), 400 nmol/L of primers, and 200 nmol/L of probe. Reaction of amplification was as follows: 2 minutes at 50°C, 10 minutes at 95°C, and then 50 cycles of 15 seconds at 95°C and 1 minute at 60°C. Standard curves were constructed using DNA extracted from the cell line Karpas 422.20 To ensure that all samples contained the same amount of DNA and were free of PCR inhibitors, for each patient, the albumin gene was amplified in duplicate.21 On the same assay plate, we added 4 no-template controls, 4 no-amplification controls (DNA extracted from mononuclear cells obtained from 8 healthy donors), and standard curves in duplicate for albumin gene and in triplicate for MBR t(14;18).
We tested the sensitivity and the reproducibility of our RQ-PCR assay by analyzing serial dilutions of DNA derived from Karpas 422 cells. A maximal sensitivity limit of 10-5 was achieved in all reactions. We analyzed 1.5 × 105 cells (1 cell = 6 pg DNA) so that our assay can detect a single lymphoma cell in a background of 83 000 normal cells. Positivity was defined if any of triplicate samples was positive by RQ-PCR analysis.
The specificity of our assay was documented by the absence of BCL2/IgH amplification in normal mononuclear cell-derived DNA, used in each experiment as negative control. Moreover, our quantitative data obtained by RQ-PCR are in keeping with the positive or negative results obtained by a qualitative nested PCR assay, for which a sensitivity level of 10-5 was repeatedly obtained using paired blind samples.4 To exclude PCR positivity due to contamination, BCL2/IgH RQ-PCR products were also run on agarose gel showing PCR products with an identical size to that found by a previous qualitative analysis.
Statistical analysis
Patients were analyzed for overall survival (OS) evaluated from the beginning of CHOP chemotherapy to the date of death or the date of last follow-up. Patients were also evaluated for event-free survival (EFS), calculated from the start of CHOP chemotherapy to the date of the first event (events being defined as treatment failure, relapse, evidence of disease progression, any additional treatment, or death for any cause) or last follow-up and for FFR measured from the time of response to CHOP chemotherapy (CR or PR) to the date of relapse, progression, or last follow-up. Curves were calculated according to the method of Kaplan-Meier.22 The log-rank test was used to analyze differences between curves. To identify prognostic variables of clinical and molecular remission, the following factors were examined by multivariate analysis using a multiple regression model: age, sex, serum lactic dehydrogenase (LDH) level, bulky disease, BM infiltration, and number of t(14;18)+ clones, detected by RQ-PCR in BM and PB at diagnosis. The Wilcoxon signed test was used to estimate the significance of reduction of t(14;18)+ cells after chemotherapy.
Results
Quantification of BCL2/IgH+ cells in previously untreated patients with FL-NHL
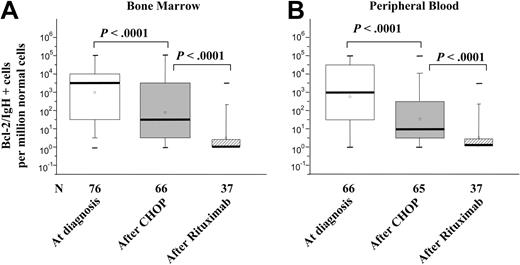
DNA samples were obtained at diagnosis from 76 BM and 66 PB samples from 86 previously untreated patients whose clinical data are summarized in Table 1. A median value of 1 BCL2/IgH+ cell in 103 normal cells (ranging from 1 in 10 up to 1 in 105 normal cells) was found at diagnosis within the BM. The amount of tumor cells detected in the PB was lower although within one order of magnitude (Figure 1A-B). Among the 56 patients for whom a BM and PB evaluation was available at diagnosis, the same quantity of disease was measured in the BM and PB of 25, a higher BM level was detected in 18 (median difference 1 log), and a higher PB level in 13 (median difference half a log). After 6 cycles of CHOP chemotherapy, a median 2 log reduction of tumor cells was documented both in the BM and PB (Figure 1A-B). At the end of CHOP, approximately 70% of patients still proved consistently PCR+ in the BM or PB and thus were selected to receive additional rituximab treatment (375 mg/m2 weekly for 4 weeks). In this subgroup of molecularly resistant patients, the amount of measurable disease at diagnosis was greater (almost 1 log) compared to that recorded in the unselected group of patients (data not shown). After rituximab treatment, an additional 2 log reduction of the neoplastic burden was documented and a molecular remission was eventually achieved in up to 70% of cases (Figure 1A-B).
Amount of t(14;18)+ cells at diagnosis, at the end of CHOP, and after rituximab. The amount of BCL2/IgH+ cells are expressed as the number of neoplastic cells per million normal cells. The black bold line indicates the median value observed. The box refers to the range defined by the 25th and 75th percentiles. The bars extend from the 5th to 95th percentile. The minus symbol indicates the extremes. Neoplastic cells were evaluated at diagnosis (□), at the end of CHOP chemotherapy (▦), and after rituximab (▨). Small squares inside boxes represent the mean value. Statistical analyses and P values were calculated by Wilcoxon test on paired samples.
Amount of t(14;18)+ cells at diagnosis, at the end of CHOP, and after rituximab. The amount of BCL2/IgH+ cells are expressed as the number of neoplastic cells per million normal cells. The black bold line indicates the median value observed. The box refers to the range defined by the 25th and 75th percentiles. The bars extend from the 5th to 95th percentile. The minus symbol indicates the extremes. Neoplastic cells were evaluated at diagnosis (□), at the end of CHOP chemotherapy (▦), and after rituximab (▨). Small squares inside boxes represent the mean value. Statistical analyses and P values were calculated by Wilcoxon test on paired samples.
Correlation between tumor burden at diagnosis and achievement of clinical and molecular CR
To evaluate the correlation between the amount of tumor cells at diagnosis and the probability of achieving a clinical CR, we divided our patients among those with a low (1 in 103-105 normal cells), intermediate (1 cell in 102-103 normal cells), or high (> 1 cell in 102 normal cells) BM tumor infiltration. As shown in Table 2, more than 70% of patients with a low amount of BCL2/IgH+ cells in the BM at diagnosis obtained a clinical CR after CHOP. On the other hand, among patients with a high number of BM BCL2/IgH+ cells only 26% achieved this result. In addition to the amount of BCL2/IgH+ cells detectable at diagnosis, other factors including sex, age, clinical stage, presence of bulky disease, LDH level, and histologically documented BM infiltration were tested as predictors of clinical and molecular response. By univariate and multivariate analyses, we found that the amount of BCL2/IgH+ cells in the BM along with the presence of bulky disease at diagnosis are significant predictors for the achievement of a clinical CR after CHOP (P < .03 and .006, respectively; data not shown). As expected, an association was observed between high levels of BCL2/IgH+ cells with a histologically documented BM infiltration (Pearson χ2 = 0.006), but not with bulky disease (Pearson χ2 = 0.7). Among patients with a histologically positive BM (n = 55), RQ-PCR analysis was performed on BM samples obtained from 52 patients. The presence of BCL2/IgH+ cells was invariably documented in all these cases even though the amount of tumor cells was heterogeneous, being high in 35%, intermediate in 35%, and low in the remaining 30%. A positive molecular result was also detected in all the 24 histologically negative patients who showed high, intermediate, or low amount of BCL2/IgH+ cells in 4%, 33%, and 63%, respectively.
Correlation between BCL2/IgH+ cells in the BM at diagnosis with clinical response after CHOP
. | Amount of BCL2/IgH+ cells in the BM . | . | . | . | ||
|---|---|---|---|---|---|---|
| Clinical response . | High (%) . | Intermediate (%) . | Low (%) . | P, χ2 . | ||
| Complete | 5 (26) | 16 (61) | 22 (71) | .007 | ||
| Partial | 10 (53) | 8 (31) | 8 (26) | .1 | ||
| None | 4 (21) | 2 (8) | 1 (3) | .1 | ||
. | Amount of BCL2/IgH+ cells in the BM . | . | . | . | ||
|---|---|---|---|---|---|---|
| Clinical response . | High (%) . | Intermediate (%) . | Low (%) . | P, χ2 . | ||
| Complete | 5 (26) | 16 (61) | 22 (71) | .007 | ||
| Partial | 10 (53) | 8 (31) | 8 (26) | .1 | ||
| None | 4 (21) | 2 (8) | 1 (3) | .1 | ||
For high, n = 19; intermediate, n = 26; low, n = 31.
By univariate analysis, the presence of bulky disease, clinical stage of III or higher, the histologic demonstration of lymphoma infiltration of the BM, and the amount of BCL2/IgH+ cells quantified in the BM by RQ-PCR were all predictors of a lower probability of achieving a clinical and molecular CR. However, by multivariate analysis, only the presence of bulky disease and a high amount of BCL2/IgH+ cells in the BM remained significant predictors of poor response (Table 3). Comparable results were obtained when multivariate analysis was performed using the amount of BCL2/IgH+ cells detected in PB at diagnosis (data not shown). Univariate and multivariate analysis were also performed using the same molecular and clinical parameters in patients who, after CHOP, were treated with rituximab because of the persistent lymphomatous infiltration at the end of chemotherapy. A low probability of achieving a molecular and clinical remission even after rituximab was confirmed in patients who, at diagnosis, had the highest level of BCL2/IgH+ cells in BM (> 1 in 102 normal cells). All the other clinical and biologic factors, with the exception of sex, were not predictors of CR achievement (Table 4).
Predictive factors of clinical and molecular CR after CHOP
. | Univariate . | . | Multivariate by logistic analysis . | . | ||
|---|---|---|---|---|---|---|
| Factors . | No. CR/no. total (%) . | P, χ2 . | OR (95% CI) . | P . | ||
| Sex | .46 | 0.80 (0.16-4.17) | .79 | |||
| Male | 14/48 (29) | |||||
| Female | 6/28 (21) | |||||
| Age | .77 | 2.85 (0.23-35.25) | .41 | |||
| 60 y or younger | 17/66 (26) | |||||
| Older than 60 y | 3/10 (30) | |||||
| LDH* | .67 | 1.35 (0.11-16.47) | ||||
| Yes | 3/9 (33) | |||||
| No | 17/64 (27) | |||||
| Bulky disease† | .018 | NA | NA | |||
| Yes | 0/13 (0) | |||||
| No | 20/63 (32) | |||||
| BM infiltration (histology) | .009 | 0.32 (0.06-1.63) | .17 | |||
| Yes | 9/52 (17) | |||||
| No | 11/24 (46) | |||||
| Clinical stage | .023 | 0.49 (0.02-9.53) | .63 | |||
| Below III | 3/4 (75) | |||||
| III or higher | 17/72 (24) | |||||
| RQ-PCR on BM at diagnosis‡ | <.001 | 11.46 (2.66-49.3) | .001 | |||
| High | 0/19 (0) | |||||
| Intermediate | 3/26 (11) | |||||
| Low | 17/31 (55) | |||||
. | Univariate . | . | Multivariate by logistic analysis . | . | ||
|---|---|---|---|---|---|---|
| Factors . | No. CR/no. total (%) . | P, χ2 . | OR (95% CI) . | P . | ||
| Sex | .46 | 0.80 (0.16-4.17) | .79 | |||
| Male | 14/48 (29) | |||||
| Female | 6/28 (21) | |||||
| Age | .77 | 2.85 (0.23-35.25) | .41 | |||
| 60 y or younger | 17/66 (26) | |||||
| Older than 60 y | 3/10 (30) | |||||
| LDH* | .67 | 1.35 (0.11-16.47) | ||||
| Yes | 3/9 (33) | |||||
| No | 17/64 (27) | |||||
| Bulky disease† | .018 | NA | NA | |||
| Yes | 0/13 (0) | |||||
| No | 20/63 (32) | |||||
| BM infiltration (histology) | .009 | 0.32 (0.06-1.63) | .17 | |||
| Yes | 9/52 (17) | |||||
| No | 11/24 (46) | |||||
| Clinical stage | .023 | 0.49 (0.02-9.53) | .63 | |||
| Below III | 3/4 (75) | |||||
| III or higher | 17/72 (24) | |||||
| RQ-PCR on BM at diagnosis‡ | <.001 | 11.46 (2.66-49.3) | .001 | |||
| High | 0/19 (0) | |||||
| Intermediate | 3/26 (11) | |||||
| Low | 17/31 (55) | |||||
Seventy-six patients (BM sample available at diagnosis) were eligible for univariate and multivariate analysis. Data on 60 patients were analyzed for the multivariate analysis.
NA indicates not applicable.
The LDH value was not available at diagnosis in 3 patients
The presence of bulky disease (13 patients) was automatically dropped from the multivariate analysis model because it perfectly predicts the failure of CR achievement
The numbers of BCL2/IgH+ cells in the BM were defined as being high, when more than one positive cell was detected among 100 normal cells; intermediate, when 1 positive cell was detected among 100 to 1000 normal cells; or low, when 1 positive cell in was detected among 1000 to 100 000 normal cells
Predictive factors of clinical and molecular CR after rituximab
. | Univariate . | . | Multivariate by logistic analysis . | . | ||
|---|---|---|---|---|---|---|
| Factors . | No. CR/no. total (%) . | P, χ2 . | OR (95% CI) . | P . | ||
| Sex | .46 | 0.09 (0.01-0.69) | .02 | |||
| Male | 11/28 (39) | |||||
| Female | 10/15 (67) | |||||
| Age | .95 | 0.22 (0.22-2.58) | .23 | |||
| 60 y or younger | 18/37 (49) | |||||
| Older than 60 y | 3/6 (50) | |||||
| LDH* | .19 | 0.09 (0.003-2.11) | .13 | |||
| Yes | 1/5 (20) | |||||
| No | 18/37 (51) | |||||
| Bulky disease | .34 | 0.37 (0.06-2.37) | .30 | |||
| Yes | 4/11 (36) | |||||
| No | 17/32 (53) | |||||
| BM infiltration (histology) | .52 | 0.32 (0.08-3.30) | .49 | |||
| Yes | 15/32 (47) | |||||
| No | 6/11 (55) | |||||
| RQ-PCR on BM at diagnosis† | .15 | 3.23 (1.01-10.31) | .05 | |||
| High | 4/14 (29) | |||||
| Intermediate | 11/20 (55) | |||||
| Low | 6/9 (67) | |||||
. | Univariate . | . | Multivariate by logistic analysis . | . | ||
|---|---|---|---|---|---|---|
| Factors . | No. CR/no. total (%) . | P, χ2 . | OR (95% CI) . | P . | ||
| Sex | .46 | 0.09 (0.01-0.69) | .02 | |||
| Male | 11/28 (39) | |||||
| Female | 10/15 (67) | |||||
| Age | .95 | 0.22 (0.22-2.58) | .23 | |||
| 60 y or younger | 18/37 (49) | |||||
| Older than 60 y | 3/6 (50) | |||||
| LDH* | .19 | 0.09 (0.003-2.11) | .13 | |||
| Yes | 1/5 (20) | |||||
| No | 18/37 (51) | |||||
| Bulky disease | .34 | 0.37 (0.06-2.37) | .30 | |||
| Yes | 4/11 (36) | |||||
| No | 17/32 (53) | |||||
| BM infiltration (histology) | .52 | 0.32 (0.08-3.30) | .49 | |||
| Yes | 15/32 (47) | |||||
| No | 6/11 (55) | |||||
| RQ-PCR on BM at diagnosis† | .15 | 3.23 (1.01-10.31) | .05 | |||
| High | 4/14 (29) | |||||
| Intermediate | 11/20 (55) | |||||
| Low | 6/9 (67) | |||||
Of 43 patients eligible for analysis, observations were made in 42.
The LDH value was not available at diagnosis in one patient
The amount of BCL2/IgH+ cells in the BM was defined as being high, when more than one positive cell was detected among 100 normal cells; intermediate, when 1 positive cell was detected among 100 to 1000 normal cells; or low, when 1 positive cell in was detected among 1000 to 100 000 normal cells
Correlation between molecular status and clinical outcome
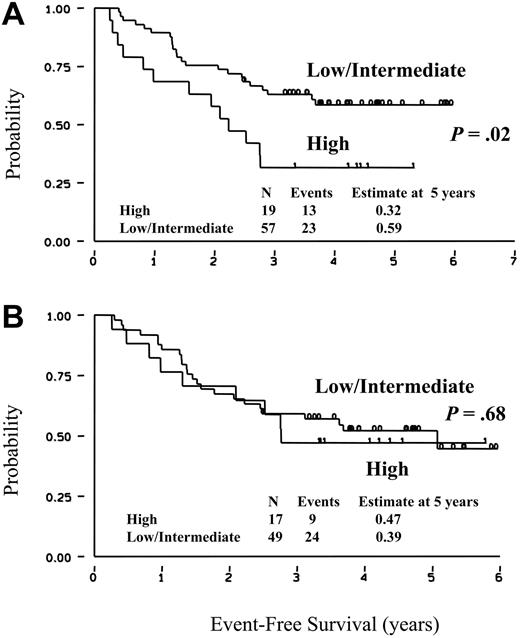
With a median follow-up of 56 months (range, 40-75 months), the OS of these 86 patients is 87% (95% CI, 74%-94%; data not shown). The degree of heterogeneity observed in terms of BCL2/IgH+ cells detectable at diagnosis in the BM and PB prompted us to verify whether this biologic information could be associated with long-term clinical outcomes. The estimated EFS of patients who at diagnosis showed low or intermediate amounts of BCL2/IgH+ cells in the BM is 59% (95% CI, 44%-70%), significantly better than observed in patients showing at diagnosis the highest BM infiltration (32%; CI, 13%-52%; Figure 2A). On the other hand, when the EFS was calculated according to the results obtained by RQ-PCR performed at diagnosis on PB, no significant difference was recorded (Figure 2B). We also analyzed the correlation between BCL2/IgH levels detectable in BM and PB of the 56 patients who had both evaluations performed. Although the statistical significance was not reached due to the low number of patients analyzed, the EFS at 5 years was 53% for patients who at diagnosis had low or intermediate levels of BCL2/IgH+ cells in the BM as compared to 37% of patients showing a high tumor burden. By contrast, the EFS according to BCL2/IgH levels detected in PB was 47% and 39%, respectively. The achievement of a molecular CR was an important predictor of prolonged clinical response. In fact, patients who achieved a PCR- BM status either after CHOP chemotherapy alone or after sequential rituximab administration showed a FFR of 64% (95% CI, 47%-76%), compared to 32% (95% CI, 13%-54%; P < .006) observed in patients who failed to achieve such a molecular response (Figure 3).
EFS according to the amount of BCL2/IgH+ cells. EFS was calculated according to RQ-PCR results obtained at diagnosis on BM (A) or PB (B) samples. High and low/intermediate refer to patients with more or fewer than 1 BCL2/IgH+ cell in 102 normal cells. Patients with a low or intermediate amount of disease were combined in the EFS analysis because their clinical outcome was very similar (53% and 65%, respectively; P = .47).
EFS according to the amount of BCL2/IgH+ cells. EFS was calculated according to RQ-PCR results obtained at diagnosis on BM (A) or PB (B) samples. High and low/intermediate refer to patients with more or fewer than 1 BCL2/IgH+ cell in 102 normal cells. Patients with a low or intermediate amount of disease were combined in the EFS analysis because their clinical outcome was very similar (53% and 65%, respectively; P = .47).
FFR according to minimal residual disease in the BM after treatment. PCR Neg refers to patients achieving a molecular negativity after CHOP (n = 23) or CHOP and rituximab (n = 25). Patients in the PCR Pos curve were all treated with sequential CHOP and rituximab but failed to achieve a molecular remission.
FFR according to minimal residual disease in the BM after treatment. PCR Neg refers to patients achieving a molecular negativity after CHOP (n = 23) or CHOP and rituximab (n = 25). Patients in the PCR Pos curve were all treated with sequential CHOP and rituximab but failed to achieve a molecular remission.
Discussion
In this study, we show that the quantitative evaluation of the BCL2IGH chimeric gene performed at diagnosis in the BM and PB can provide useful information as surrogate marker of the clinical outcome of patients with FL-NHL. The clearance of neoplastic cells from BM and PB obtained either after conventional chemotherapy or after passive immunotherapy using the anti-CD20 chimeric monoclonal antibody rituximab can be accurately quantified. In our experience, CHOP and rituximab are both able to remove approximately 2 logs of tumor infiltration thus explaining why patients with a limited lymphoma infiltration (1 positive cell in 104 normal cell or less) can achieve a molecular remission after CHOP chemotherapy alone. On the other hand, patients showing at diagnosis intermediate or high levels of BM and PB infiltration benefit from the addition of rituximab because they achieve a molecular CR in more than 70% of patients. Our results also confirm the notion that achieving the combined end point of clinical and molecular remission is one of the major goals in the therapy of FL-NHL. Indeed, no matter whether after CHOP alone or after sequential CHOP and rituximab, patients in complete clinical and molecular remission show a significantly longer freedom from disease recurrence. The multivariate analysis significantly documents that an accurate quantification of BCL2/IgH+ cells at diagnosis is an independent predictor of outcome and may be useful to stratify patients for different treatments. Although a simpler clinical parameter, such as the presence of bulky disease at diagnosis, was significantly associated with a lower probability of achieving a complete clinical and molecular remission after CHOP, its prognostic value is limited to only a minority of patients (17% in this study). Unfortunately, flow cytometry of BM cells was not included in the routine diagnostic work-up of this multicenter study and this precluded the possibility of correlating our RQ-PCR results with another quantitative measurement of marrow tumor burden. Although this may represent a limit of our study, a remarkable concordance between morphologic and flow cytometry results has been reported.23 The partial discordance found in some patients who showed low levels of molecularly detectable disease despite microscopically visible tumor has been also observed when flow cytometry was compared to a conventional morphologic evaluation of BM. This could be possibly attributed to a sampling bias, which can occur particularly in cases with a paratrabecular involvement of lymphoma cells.23 Similarly to what reported by others, for reasons not yet clarified, male sex was associated with an inferior clinical outcome.24,25 In keeping with our results, several investigators have recently provided evidence on the value of RQ-PCR analysis in patients undergoing autologous hematopoietic stem cell transplantation. Ladetto and coworkers showed that the evaluation of tumor burden by RQ-PCR in stem cell harvest can predict the effectiveness of ex vivo purging after high-dose chemotherapy.26 Along the same line, it has been shown that the ability of rituximab to eradicate contaminating tumor cells in the graft can contribute to improve the clinical outcome of autologous transplantation.27,28 Moreover, the tumor load of BCL2/IgH+ cells detected in BM or PB samples after autologous or allogeneic transplantation in FL-NHL was also found to positively correlate with the duration of clinical remission.15,17,18 By contrast, Mandigers and coworkers29 reported that quantification of circulating t(14;18)+ cells detectable by RQ-PCR in the PB is of only limited clinical significance in predicting treatment efficacy for patients with FL-NHL treated with conventional chemotherapy. We confirmed in the present study that RQ-PCR analysis performed on PB samples at diagnosis does not allow us to discriminate the long-term EFS of FL-NHL patients. Thus, contrary to BM determinations, sampling only of PB may be inaccurate and lack prognostic value.30 The reasons for this difference are still unknown, although the higher BM disease infiltration that we and others usually found at diagnosis13 may perhaps partially account for the different predictive value of the PB. However, it is tempting to speculate that a higher BM tumor infiltration may also reflect a more general invasive behavior of lymphoma cells. A possible limit of our study relies on the fact that we did not perform a sequence analysis of all the amplified products studied at diagnosis and during the follow-up nor did we compare the amplified DNA obtained from BM or PB with that of pathologic lymph nodes. However, the large cohort of patients analyzed with concordant results both in the BM and PB substantially mitigates the role of this as a source of possible laboratory inconsistencies and confounding effects.31 Because the combination of rituximab with chemotherapy significantly improves the overall response rate, the proportion of CR as well as the time to treatment failure,32,33 questions should be raised as to the most appropriate chemotherapy schedule as first-line treatment in FL-NHL. It is tempting to speculate that the association of rituximab with anthracycline-containing regimens should be recommended particularly for patients who show at diagnosis high levels of BCL2/IgH+ cells in the BM. Therefore, it should be noted that although reasonable at the time of the study, the chemotherapy choice of CHOP is not necessarily standard now and the applicability of our results to other regimens33,34 remains to be confirmed. In addition, our results further support the notion that rituximab should be promptly offered to all patients who fail to achieve a complete clinical and molecular response at the end of front-line therapy. Moreover, the quantitative PCR analysis performed at diagnosis may help in defining patients eligible for studies using high-dose chemotherapy as initial treatment.35
In conclusion, our results support the clinical value of a quantitative evaluation of BCL2/IgH+ cells at diagnosis, which may help to define the probability of response to conventional chemotherapy with or without the addition of rituximab. Our data also confirm that molecular monitoring of minimal residual disease allows an early identification of patients with a remarkably higher risk of disease recurrence. The value of molecular analysis of the BCL2IGH chimeric gene performed at diagnosis on BM samples should be prospectively compared with that of follicular lymphoma international prognostic index (FLIPI), the best and most robust prognostic tool in FL-NHL patients,36 as well as of other innovative molecular predictors.37
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-06-2490.
Supported in part by grants from Associazione Italiana per la Ricerca contro il Cancro (AIRC), Consiglio Nazionale per le Ricerche (CNR; Progetto Oncologia CU 03.00357), Ministero dell'Istruzione Università e Ricerca (MIUR), and Associazione Paolo Belli and Associazione Italiana Lotta alla Leucemia (AIL), sezione Paolo Belli.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal