Abstract
Platelets express ABH antigens, which can adversely effect platelet transfusion recovery and survival in ABH-incompatible recipients. To date, there has been no large, comprehensive study comparing specific donor factors with ABH expression on platelet membranes and glycoconjugates. We studied ABH expression in 166 group A apheresis platelet donors by flow cytometry, Western blotting, and thin layer chromatography relative to donor age, sex, A1/A2 subgroup, and Lewis phenotype. Overall, A antigen on platelet membranes, glycoproteins, and glycosphingolipids was linked to an A1 red blood cell (RBC) phenotype. Among A1 donors, platelet ABH varied significantly between donors (0%-87%). Intradonor variability, however, was minimal, suggesting that platelet ABH expression is a stable, donor-specific characteristic, with 5% of A1 donors typing as either ABH high- or low-expressers. Group A2 donors, in contrast, possessed a Bombay-like phenotype, lacking both A and H antigens. Unlike RBCs, ABH expression on platelets may be determined primarily by H-glycosyltransferase (FUT1) activity. Identification of A2 and A1 low expressers may increase the availability and selection of crossmatched and HLA-matched platelets. Platelets from group A2 may also be a superior product for patients undergoing A/O major mismatch allogeneic progenitor cell transplantation. (Blood. 2005;105:3356-3364)
Introduction
Platelets, like red blood cells (RBCs) and other tissues,1 express ABH antigens on a variety of membrane glycoproteins (GPs) and glycosphingolipids (GSLs).2-8 Clinically, transfusion of ABH-incompatible platelets can be associated with decreased recovery,8-11 shortened survival,10 and an increased incidence and onset of HLA-immune refractoriness.11-13 ABH incompatibility can lead to positive platelet crossmatches,14 acting synergistically with HLA incompatibility to further decrease posttransfusion recovery and survival.11,13 Because of these findings, the American Association of Blood Banks suggests, but does not mandate, transfusion of ABH-compatible platelets when available.15
Although it is clear that ABH incompatibility can adversely effect platelet recovery, the clinical outcome is highly variable between transfusions and individual patients. Risk factors in the recipient can include a group O phenotype and unusually high levels of anti-A and anti-B isohemagglutinins.8,9,12,16-18 Donor and/or platelet factors are less well described but may include storage time,19-21 and Lewis and ABO subtypes.2,4,5,8,22-24 In addition, an ABH-high expresser (ABH-HXP) platelet phenotype has been identified that is specifically linked to transfusion failures.23,24
In this study, we report the first large-scale analysis of individual apheresis platelet donors with regard to identifying donor factors affecting platelet ABH expression. Donor factors examined were age, sex, A1/A2 subgroup, and Lewis phenotype. These were compared with ABH expression on platelet and RBC membranes as determined by flow cytometry, Western blotting, and GSL analysis (Figure 1). In a subset of donors, we examined platelet ABH expression over time. Our results show that platelet ABH expression is a relatively stable, donor-specific characteristic that is influenced by group A subtype, donor age, and sex. Furthermore, the regulation of ABH antigen on platelets differs from RBCs. Our findings have implications for the selection and availability of platelets for ABO-incompatible stem cell transplantation, and HLA and platelet crossmatching.
Analysis of ABH expression in group A platelet donors by RBC serology, flow cytometry, and glycoconjugate analysis.
Analysis of ABH expression in group A platelet donors by RBC serology, flow cytometry, and glycoconjugate analysis.
Materials and methods
Immunologic reagents
Monoclonal antibodies (MoAbs) to blood group A, group B, Lewis a (Lea), and Lewis b (Leb) antigens were purchased from Gamma Biologics (Houston, TX). MoAbs against type 2 chain, H antigen were purchased from Accurate Chemical (MoAb 92FR-A2; San Diego, CA) and Research Diagnostics (MoAb Ery-H/1; Flanders, NJ). Phycoerythrin (PE)-labeled anti-CD41 (anti-GPIIb/IIIa, MoAb P2) was purchased from Immunotech (Westbrook, MA). Anti-GPIIb (CD41b) for Western blotting was purchased from Chemicon (Temecula, CA). Fluorescein (FITC)-labeled Helix pomatia (HPA, anti-A), Ulex europeaus (UEA1, anti-H), Griffonia simplicifolia (GS-1, anti-B), and anti-mouse immunoglobulin were purchased from Sigma (St Louis, MO). Biotinylated UEA1 lectin, biotinylated anti-mouse IgG, anti-mouse IgM, avidin-linked alkaline phosphatase, and alkaline phosphatase substrate for Western blot and high-performance thin layer chromatography (HPTLC)-immunostaining were purchased from Vector Laboratories (Burlingame, CA).
Donor specimens
Whole blood samples (5 mL in EDTA [ethylenediaminetetraacetic acid] anticoagulant) were collected from all group A apheresis platelet donors over a 4-month period at the time of their platelet donation. Samples from group O and group B donors were included as controls. Donor RBCs were typed for ABO, Rh, and Lewis according to manufacturers' instructions. In group A donors, A1/A2 subgroup was determined with the anti-A1 lectin, Dolichus bifloris (Gamma Biologics).15 Donors failing to agglutinate with anti-A1 were considered A2.15
Outdated (6-7 days), single-donor apheresis platelet concentrates for GSL and GP analysis were obtained from the DeGowin Blood Center, The University of Iowa (Iowa City, IA). A total of 109 platelet units were collected over a 3-year period from 72 group A (80 units total), 15 group B, 12 group O, and 2 AB donors. Platelets were isolated by differential centrifugation, washed thrice with ammonium bicarbonate, lyophilized dry, and stored at -40°C as described.25,26 All tissue procurement was in accordance with the institutional investigation review committee.
Flow cytometry
Whole blood samples were centrifuged to obtain platelet-rich plasma, followed by a platelet count (Coulter, Hialeah, FL). For flow cytometry, 106 platelets were incubated with 5 μL PE-labeled anti-CD41 (MoAb P2), 5 μL FITC-labeled HPA (anti-A; 10 μg/mL working dilution), and phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) in a final volume of 100 μL for 30 minutes.25 In 28 donors, platelets were stained in parallel with FITC-UEA1, which recognizes H antigen. After incubation, platelets were washed twice with PBS (1000g, 10 minutes) and resuspended in 400 μL PBS-1% paraformaldehyde. All donor samples were stained and analyzed in duplicate.
Flow cytometry was performed on a Becton Dickinson 440 flow cytometer (San Jose, CA) equipped with an argon laser, counting 10 000 events per sample. FITC and PE spectral overlaps were corrected by electronic compensation. Gating for forward scatter (FS) and orthogonal scatter was based on CD41 staining.25 Data were collected on a VAX station 3200 computer equipped with DESK software (courtesy of Wayne Moore, Stanford University). Results were recorded as the mean percent platelets positive for both CD41 and either HPA (% HPA+) or UEA1 (% UEA1+). In some donors, one-color fluorescence flow cytometry was performed on donor RBCs. RBCs were stained by incubating 50 μL washed RBCs (3% solution in saline) with either FITC-HPA or FITC-UEA1. As before, the results were reported as the percent RBCs positive for HPA or UEA1.
To analyze the effect of platelet age on ABH expression,25,27 platelets were arbitrarily divided, based on FS (channel number), into small (FS < 60), moderate (FS 60-100), large (FS 100-140), and very large platelets (FS 140-220). The distribution (% total CD41+ platelets) and relative ABH expression (CD41+ and HPA+ [or UEA1+]) were determined for each FS population in 60 donors.
Neutral GSL isolation and analysis
GSLs were isolated from single donor apheresis concentrates as described.25 The delipidized platelet protein generated from lipid extraction and filtration was stored at -70°C for Western blot analysis. Neutral GSL from each apheresis sample was resuspended in 500 μL chloroform-methanol 1:1 (C-M, vol/vol).
GSLs were analyzed by high-performance thin layer chromatography (HPTLC) on aluminum-backed HPTLC plates.25,26 Neutral GSL samples were spotted onto HPLTC plates (8-10 μL/lane), followed by development in chloroform-methanol-water 65:25:4 (C-M-W, vol/vol). GSLs were detected by chemical staining with diphenylamine reagent or by HPTLC-immunostaining with blood group-specific MoAbs as described.3,25 All samples were immunostained in duplicate. All samples staining negative for either A, B, or Lewis antigens were repeated at twice the initial GSL concentration.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting
Washed, delipidized platelet membranes (2.4 mg/mL final concentration) were solubilized in SDS-PAGE sample buffer (0.125 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 6.8, 10% SDS) by sonication (30 minutes; Branson Equipment, Shelton, CT) and then heated (100°C, 5 minutes).28 After cooling, 60 μL sample and 15 μL loading solution (50% aqueous glycerol, 0.05% bromophenol blue) were mixed and loaded (25 μL/well) onto precast 4% stacking, 10% to 20% Tris-glycine gel (BioRad Ready-Gel, Hercules, CA). All samples were tested in duplicate and included prestained SDS-PAGE molecular standards (BioRad). Samples were separated by gel electrophoresis,28 followed by electrophoretic blotting onto polyvinylidene difluoride membranes (BioRad; 100 V, 1 hour).24 After washing with PBS, membranes were blocked with PBS-5% nonfat milk (4°C, overnight). Membranes were immunostained for GPIIb (CD41b, AMAC, Westbrook, ME) and ABH using the ABC streptavidin system (Vector Laboratories) described for HPTLC-immunostaining.3,24,25 Gels were retained and stained with Coomassie Brilliant Blue. All Western blot-negative samples were repeated at 80 μg/well. In 45 donors, blots were analyzed by scanning densitometry (Quantiscan; Biosoft, Ferguson, MO).
Statistics
Population studies were reported as the mean and standard deviation (X ± SD). Univariate analysis was performed by chi-square, student t test, Fisher exact test, and Wilcoxon rank sum.29 A P less than .05 was considered statistically significant. Graphic and statistical analyses were performed on KaliedaGraph (Reading, PA) and Epi Info (Centers for Disease Control, Atlanta, GA).
Results
Flow cytometry donor characteristics
A total of 131 donors were analyzed by flow cytometry, including 33 donors with duplicate specimens (Figure 1). The distributions of A1, A2, and Leb phenotypes were consistent with that reported for whites (Table 1).15 There were no significant differences in donor age or sex between A1, A2, Le(b+), and Le (b-) donors.
Lewis, sex, and A1/A2 subgroup on platelet A antigen expression
. | Group A subtype (A1/A2) . | . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor characteristics‡ . | All group A . | P*† . | . | . | A1 . | P*† . | . | . | A2 . | A1vs A2P*† . | ||||||||
| Total no. (%) | 131 (100) | 105 (80) | 26 (20) | — | ||||||||||||||
| Sex, no. (%) | ||||||||||||||||||
| Male | 68 (52) |  NS NS | 55 (81) |  NS NS | 13 (19) | NS | ||||||||||||
| Female | 63 (48) | 50 (79) | 13 (21) | NS | ||||||||||||||
| Age, y, X ± SD† | 39.2 ± 11.9 | 38.7 ± 11.6 | 36.7 ± 10.0 | NS | ||||||||||||||
| Male | 39.3 ± 10.2 |  NS NS | 38.7 ± 9.80 |  NS NS | 36.3 ± 7.50 | NS | ||||||||||||
| Female | 39.4 ± 13.7 | 38.7 ± 13.3 | 39.0 ± 12.3 | NS | ||||||||||||||
| Lewis type, no. (%) | ||||||||||||||||||
| Le (b+) | 84 (64) |  NS NS | 66 (63) |  NS NS | 18 (69) | NS | ||||||||||||
| Le (b−) | 47 (36) | 39 (37) | 8 (31) | NS | ||||||||||||||
| HPA + donors (%) | 100 (76) | 99 (94) | 1 (4) | <.0001 | ||||||||||||||
| No. Le (b+) | 62 (62) |  NS NS | 61 (62) |  NS NS | 1 | — | ||||||||||||
| No. Le (b—) | 38 (38) | 38 (38) | 0 | — | ||||||||||||||
| HPA - donors (%) | 31 (24) | 6 (6) | 25 (96) | <.0001 | ||||||||||||||
| No. Le (b+) | 22 (71) |  NS NS | 5 (83) |  NS NS | 17 (68) | — | ||||||||||||
| No. Le (b−) | 9 (29) | 1 (17) | 8 (32) | — | ||||||||||||||
| %HPA, X ± SD† | 32.1 ± 21.7 | 39.5 ± 17.6 | 2.3 ± 1.9 | <.0001 | ||||||||||||||
| Le (b+) | 28.1 ± 19.5 |  .006 .006 | 35.1 ± 15.8 |  .001 .001 | 2.4 ± 2.1 | <.0001 | ||||||||||||
| Le (b−) | 39.5 ± 23.7 | 47.1 ± 18.1 | 2.2 ± 1.6 | <.0001 | ||||||||||||||
| Female, X ± SD† | 34.8 ± 24.3 | 43.3 ± 19.9 | 2.6 ± 2.2 | <.0001 | ||||||||||||||
| Le (b+) | 29.1 ± 23.0 |  .008 .008 | 43.0 ± 15.1 |  .05 .05 | 2.8 ± 2.4 | <.0001 | ||||||||||||
| Le (b−) | 45.4 ± 22.2 |  .001 .001 |  .0003 .0003 | 51.3 ± 16.0 |  .002 .002 |  .0002 .0002 | 1.9 ± 1.4 | <.0001 | ||||||||||
| Male, X ± SD† | 29.4 ± 19.0 | 36.4 ± 19.9 | 2.1 ± 1.7 | <.0001 | ||||||||||||||
| Le (b+) | 27.7 ± 16.7 | 34.8 ± 10.1 |  .01 .01 | 1.9 ± 1.7 | <.0001 | |||||||||||||
| Le (b−) | 34.2 ± 23.5 | 44.2 ± 17.0 | 2.4 ± 1.9 | <.0001 | ||||||||||||||
| Western, A-GP (%)*§ | 67 (100) |  N = 58∥.000001 N = 58∥.000001 | 33 | 10 | ||||||||||||||
| No. A-GP+ | 53 (79) | 33 | 0 | <.00001 | ||||||||||||||
| No. A-GP− | 14 (21) | 0 | 10 | — | ||||||||||||||
| HPTLC, A-GSL (%)*¶ | 65 (100) | 35 | 10 | — | ||||||||||||||
| No. A-GSL+ | 49 (75) | 33 | 2 | <.00001 | ||||||||||||||
| No. A-GSL− | 16 (25) | 2 | 8 | — | ||||||||||||||
| A-GSL + samples (%)¶ | ||||||||||||||||||
| A-6 and A-7/8 | 40 (82) | 26 | 0 | <.00001 | ||||||||||||||
| A-6 only | 7 (14) | 5 | 2 | .04 | ||||||||||||||
| A-8 only | 2 (4) | 2 | 0 | .03 | ||||||||||||||
. | Group A subtype (A1/A2) . | . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor characteristics‡ . | All group A . | P*† . | . | . | A1 . | P*† . | . | . | A2 . | A1vs A2P*† . | ||||||||
| Total no. (%) | 131 (100) | 105 (80) | 26 (20) | — | ||||||||||||||
| Sex, no. (%) | ||||||||||||||||||
| Male | 68 (52) |  NS NS | 55 (81) |  NS NS | 13 (19) | NS | ||||||||||||
| Female | 63 (48) | 50 (79) | 13 (21) | NS | ||||||||||||||
| Age, y, X ± SD† | 39.2 ± 11.9 | 38.7 ± 11.6 | 36.7 ± 10.0 | NS | ||||||||||||||
| Male | 39.3 ± 10.2 |  NS NS | 38.7 ± 9.80 |  NS NS | 36.3 ± 7.50 | NS | ||||||||||||
| Female | 39.4 ± 13.7 | 38.7 ± 13.3 | 39.0 ± 12.3 | NS | ||||||||||||||
| Lewis type, no. (%) | ||||||||||||||||||
| Le (b+) | 84 (64) |  NS NS | 66 (63) |  NS NS | 18 (69) | NS | ||||||||||||
| Le (b−) | 47 (36) | 39 (37) | 8 (31) | NS | ||||||||||||||
| HPA + donors (%) | 100 (76) | 99 (94) | 1 (4) | <.0001 | ||||||||||||||
| No. Le (b+) | 62 (62) |  NS NS | 61 (62) |  NS NS | 1 | — | ||||||||||||
| No. Le (b—) | 38 (38) | 38 (38) | 0 | — | ||||||||||||||
| HPA - donors (%) | 31 (24) | 6 (6) | 25 (96) | <.0001 | ||||||||||||||
| No. Le (b+) | 22 (71) |  NS NS | 5 (83) |  NS NS | 17 (68) | — | ||||||||||||
| No. Le (b−) | 9 (29) | 1 (17) | 8 (32) | — | ||||||||||||||
| %HPA, X ± SD† | 32.1 ± 21.7 | 39.5 ± 17.6 | 2.3 ± 1.9 | <.0001 | ||||||||||||||
| Le (b+) | 28.1 ± 19.5 |  .006 .006 | 35.1 ± 15.8 |  .001 .001 | 2.4 ± 2.1 | <.0001 | ||||||||||||
| Le (b−) | 39.5 ± 23.7 | 47.1 ± 18.1 | 2.2 ± 1.6 | <.0001 | ||||||||||||||
| Female, X ± SD† | 34.8 ± 24.3 | 43.3 ± 19.9 | 2.6 ± 2.2 | <.0001 | ||||||||||||||
| Le (b+) | 29.1 ± 23.0 |  .008 .008 | 43.0 ± 15.1 |  .05 .05 | 2.8 ± 2.4 | <.0001 | ||||||||||||
| Le (b−) | 45.4 ± 22.2 |  .001 .001 |  .0003 .0003 | 51.3 ± 16.0 |  .002 .002 |  .0002 .0002 | 1.9 ± 1.4 | <.0001 | ||||||||||
| Male, X ± SD† | 29.4 ± 19.0 | 36.4 ± 19.9 | 2.1 ± 1.7 | <.0001 | ||||||||||||||
| Le (b+) | 27.7 ± 16.7 | 34.8 ± 10.1 |  .01 .01 | 1.9 ± 1.7 | <.0001 | |||||||||||||
| Le (b−) | 34.2 ± 23.5 | 44.2 ± 17.0 | 2.4 ± 1.9 | <.0001 | ||||||||||||||
| Western, A-GP (%)*§ | 67 (100) |  N = 58∥.000001 N = 58∥.000001 | 33 | 10 | ||||||||||||||
| No. A-GP+ | 53 (79) | 33 | 0 | <.00001 | ||||||||||||||
| No. A-GP− | 14 (21) | 0 | 10 | — | ||||||||||||||
| HPTLC, A-GSL (%)*¶ | 65 (100) | 35 | 10 | — | ||||||||||||||
| No. A-GSL+ | 49 (75) | 33 | 2 | <.00001 | ||||||||||||||
| No. A-GSL− | 16 (25) | 2 | 8 | — | ||||||||||||||
| A-GSL + samples (%)¶ | ||||||||||||||||||
| A-6 and A-7/8 | 40 (82) | 26 | 0 | <.00001 | ||||||||||||||
| A-6 only | 7 (14) | 5 | 2 | .04 | ||||||||||||||
| A-8 only | 2 (4) | 2 | 0 | .03 | ||||||||||||||
— indicates not applicable.
Chi-square.
Fisher exact test.
Flow cytometry donors only.
A-active platelet glycoproteins by Western blot.
A-active neutral GSLs by HPTLC-immunostaining. A-6 and A-7/8 refer to the 2 major A-GSL bands identified in platelets (Figure 4D).
Chi-square of HPTLC and Western blot results in 58 donors.
A antigen expression on platelets is determined by donor A1/A2 subtype
Membrane expression was determined in 131 group A donors with the anti-A lectin, HPA, and a platelet-specific MoAb, CD41.25 Results were reported as the percent platelets positive for both CD41 and HPA (% HPA+; Figure 2). In group A donors, 80% were HPA+ (range, 8%-87.5%) and 20% typed HPA negative (HPA-). When the results were normalized to A1/A2 subtype, 95% of A1 donors were HPA+ and 95%A2 donors were HPA- (Table 1). Similar results were obtained with an anti-AMoAb, Birma1 (data not shown). Outliers included 1A2 donor (% HPA, 8.5%) and 6 A1 donors (% HPA, <5%). Repeat RBC and platelet phenotyping on separate samples from these 7 donors yielded the same results, excluding the possibility of A1/A2 mistyping or mislabeled specimens.
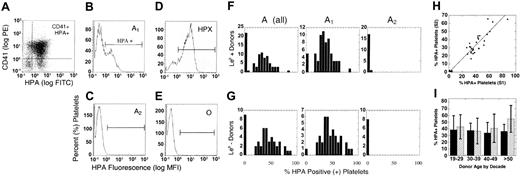
A antigen on platelet membranes by donor, A1/A2 subgroup, age, sex, and Le (b+) or secretor phenotype. (A) Platelets were double stained with PE-CD41, a platelet-specific marker, and FITC-labeled HPA lectin, which recognizes the blood group A antigen. The results were reported as the mean percentage of CD41+ platelets that were also HPA+. Shown are sample histograms from an A1 (B), A2 (C), ABH-high expresser (D, HPX), and group O (E) donors. Horizontal brackets (B-E) indicate HPA+ fluorescence. Cells left of bracketed area were considered HPA-negative. (F-G) HPA staining in all group A, A1, and A2 donors by Le (b+) and Le (b-) phenotype, respectively. (H) The percent HPA+ platelets for the first (S1) and second (S2) samples plotted for each individual donor, where y = 0.5 + 0.34x, R = 0.93. (I) Expression of A antigen on platelet membranes in group A1 donors, relative to donor age and sex. The analysis was restricted to A1 donors only, and reported as percent HPA+ platelets (X ± SD) by decade in female (▨) and male (▪) donors. There was a small but significant increase in HPA staining in female donors after the fifth decade (P = .03).
A antigen on platelet membranes by donor, A1/A2 subgroup, age, sex, and Le (b+) or secretor phenotype. (A) Platelets were double stained with PE-CD41, a platelet-specific marker, and FITC-labeled HPA lectin, which recognizes the blood group A antigen. The results were reported as the mean percentage of CD41+ platelets that were also HPA+. Shown are sample histograms from an A1 (B), A2 (C), ABH-high expresser (D, HPX), and group O (E) donors. Horizontal brackets (B-E) indicate HPA+ fluorescence. Cells left of bracketed area were considered HPA-negative. (F-G) HPA staining in all group A, A1, and A2 donors by Le (b+) and Le (b-) phenotype, respectively. (H) The percent HPA+ platelets for the first (S1) and second (S2) samples plotted for each individual donor, where y = 0.5 + 0.34x, R = 0.93. (I) Expression of A antigen on platelet membranes in group A1 donors, relative to donor age and sex. The analysis was restricted to A1 donors only, and reported as percent HPA+ platelets (X ± SD) by decade in female (▨) and male (▪) donors. There was a small but significant increase in HPA staining in female donors after the fifth decade (P = .03).
ABH-HXP phenotype
Of the A1 donors, 5 (4.8%) were ABH-HXP, defined by percent HPA more than 75% (range, 75.3%-87.5%). As defined by Curtis et al,23 3 donors were type II ABH-HXP, showing a single uniform population of strongly positive platelets (Figure 2D). There were 2 type I donors, defined by a bimodal population of platelets with high and moderate HPA staining. This is similar to other A1 donors, who also showed a bimodal population of positive and negative platelets (Figure 2B). There was no correlation between ABH-HXP and Lewis phenotype, donor age, or sex.23,24
Influence of Lewis blood group type on platelet ABH expression
Very early studies suggested that up to 50% of the ABH antigens on platelets are derived from passively adsorbed ABH-active GSLs present in plasma.22,30 As a consequence, ABH on platelets should reflect donor ABH and Lewis blood types. To test the latter, HPA staining was examined relative to donor Leb or secretor phenotype.15 Among all group A donors, 83% were Le (a-b+), which is slightly higher than average in a white population (70%).15 There was no increase in the Le (b+) phenotype in HPA+ donors (Table 1). More importantly, all group A2 donors were HPA-, regardless of their Lewis phenotype (Figure 2).
Although a secretor phenotype did not determine the presence/absence of ABH on platelet membranes, passive adsorption of ABH-active GSLs could, theoretically, result in higher overall ABH levels.22 Contrary to expectations, mean HPA staining was actually higher among A1, Le(b-) donors (Table 1). This was also true in a subanalysis of only Le (a+b-) donors who, by definition, lack soluble ABH-GSLs in their plasma.15,22 Among A1, Le (b+) donors, there was a disproportionate number of HPA- individuals (5/6); however, the exclusion of these donors did not significantly change the results (% HPA+, 37.8 ± 13.0).
Platelet ABH expression is a stable donor characteristic
Although interdonor variation in platelet ABH expression has been observed by others,4,23,24,31 no data exist relative to ABH expression in individual donors over time. During the course of this study, we obtained duplicate samples in 33 donors, at 4-week minimum intervals. In one donor, 4 separate samples were available. Overall, there was nearly a 1:1 correlation between samples with minimal intersample variability (<7%, Figure 2H). This was particularly evident in our donor with 4 available samples (range, 27%-35% HPA+). An extended subanalysis of HPA staining by forward scatter found no differences in the average age of circulating platelets,25,27 or in the rate of loss of A antigen with platelet senescence,25,32 between A1 donors (data not shown). In summary, the density or relative expression of ABH on platelets appears to be a stable, donor-specific characteristic.
Effect of donor age and sex on platelet ABH expression
Our initial analysis indicated increased ABH expression on platelets from female donors (Table 1). Interestingly, estrogen can modulate glycosyltransferase activity in many tissues,33 and reportedly influences megakaryocytopoiesis and platelet reactivity.34-37 When examined by donor sex and age (Figure 2I), there was a progressive and significant increase in HPA staining on platelets of older female donors (>50 years; P < .05), suggesting a relationship between ABH and falling hormone levels during female menopause.
Correlation between A1/A2 subtype and A antigen on platelet GPs
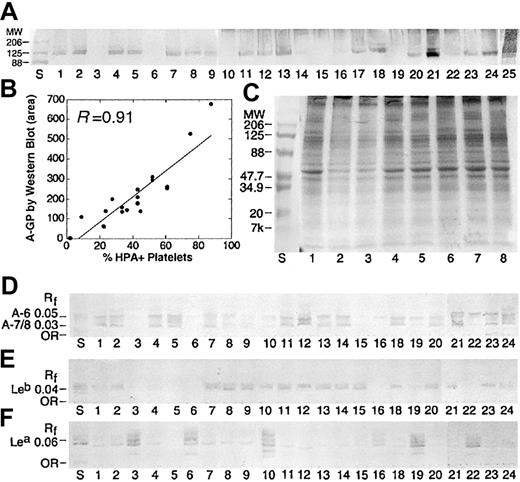
A antigen on platelet GPs (A-GP) was examined in 67 donors by Western blotting. As shown in Table 1, only 79% of donors were Western blot positive, consistent with the distribution of A1/A2 in our population. In 43 individuals, both Western blot and A1/A2 RBC phenotype were available. In A1 donors, there was a 100% concordance between an A1 subtype and A-GP. No A antigen was detected by Western blot in A2 donors. There were 2 major bands, running as a doublet (molecular weight [Mr] ∼ 120, 135 kDa), observed in all A1 donors, consistent with GPIIa, GPIIb, GPIa, and GPIc (Figure 3A).2,4,7 This was confirmed by staining with MoAbs against GPIIb (data not shown). Staining to these 2 bands was particularly strong in 2 ABH-HPX samples (lanes 21 and 25). A weak band (Mr, ∼164 kDa) was also observed in most donors, suggestive of GP1b.4 In many samples, additional bands were observed between 70 to 100 kDa, and could represent ABH expression on GPIIIa, GPIV, and GPV.4,5 A comparison of A antigen by flow cytometry and Western blot was statistically significant (Figure 3B; P < .01, Wilcoxon rank sum).
Blood group A expression on platelet GPs and GSLs is dictated by A1 subtype and is independent of Lewis phenotype. (A) Western blot results for 25 apheresis platelet donors, stained with anti-A (MoAb Birma1). Positive staining was observed in A1 but not A2 individuals. Strong staining was observed in 2 ABH-HPX donors (lanes 21 and 25). (B) The intensity of HPA staining on platelet membranes (% HPA+ platelets) is related to the intensity of GP-A staining by Western blot, as determined by scanning densitometry (area), where y = 46.5 + 6.43x, R = 0.91. (C) Platelet GPs separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Lane numbers refer to the same donor samples shown in panel A. Lane S, molecular weight standards. (D-F) Isolated neutral GSLs from the same donors in panel A, immunostained with anti-A (D), anti-Leb (E), and anti-Lea (F). Solvent C-M-W 65:25:4 (vol/vol). Rf indicates relative mobility.
Blood group A expression on platelet GPs and GSLs is dictated by A1 subtype and is independent of Lewis phenotype. (A) Western blot results for 25 apheresis platelet donors, stained with anti-A (MoAb Birma1). Positive staining was observed in A1 but not A2 individuals. Strong staining was observed in 2 ABH-HPX donors (lanes 21 and 25). (B) The intensity of HPA staining on platelet membranes (% HPA+ platelets) is related to the intensity of GP-A staining by Western blot, as determined by scanning densitometry (area), where y = 46.5 + 6.43x, R = 0.91. (C) Platelet GPs separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Lane numbers refer to the same donor samples shown in panel A. Lane S, molecular weight standards. (D-F) Isolated neutral GSLs from the same donors in panel A, immunostained with anti-A (D), anti-Leb (E), and anti-Lea (F). Solvent C-M-W 65:25:4 (vol/vol). Rf indicates relative mobility.
Correlation between A antigen on platelet GSLs, donor A1/A2, and Lewis phenotype
In addition to GPs, platelets express ABH antigens on GSLs (A-GSL).2,3 In the neutral GSL fraction, platelets express 2 major A-GSL bands, A-6 and A-7/8 (Figure 3D). These 2 bands represent up to 4 separate GSL species, including endogenous type 2 chain GSLs (A-6-2, A-8-2) from megakaryocytes, as well as adsorbed type 1 chain ABH (A-6-1) and Lewis antigens (ALeb, A-7-1) from plasma.1-3,38,39
To examine ABH expression on platelet GSLs, neutral GSLs from 65 donors were separated by HPTLC, followed by immunostaining with an anti-A MoAb, Birma1 (Table 1).3,25,26 Of 65 donors examined, 75% possessed A-GSLs, with most expressing both A-6 and A-7/8. When examined by donor A1/A2 subtype, 95% of A1 donors were A-GSL+, whereas most group A2 donors examined were negative. There was an agreement between Western blot and HPTLC results (Table 1).
There was no correlation between Le (b+) phenotype and A-GSL (P > .50): A Le (b+) phenotype was highly prevalent in both A-GSL-positive (84%) and -negative (77%) donors. As an internal control, we also screened samples for Lewis-GSLs by HPTLC-immunostaining with the same anti-Leb MoAb used for donor typing. As expected, there was a strong correlation between a Le (b+) phenotype and the presence of Leb-GSL (P < .0001). In summary, although platelets are capable of adsorbing type 1 chain GSLs from plasma, platelet ABH-GSLs appear to be predominantly of endogenous or megakaryocyte origin,2,3 whose synthesis is determined by donor A1/A2 subtype.
Summary of A antigen expression on platelet membranes, glycoproteins, and GSLs
A complete data set was available in 36 donors (Table 2). In summary, there was 100% agreement between flow cytometry and Western blot results and 67% agreement with HPTLC. There was also a good correlation between Lea-GSL and Leb-GSL relative to donor Lewis type. As expected, most Le (a-b+) donors expressed small amounts of Lea-GSL (Figure 3F).38,39 Interestingly, there was some correlation between A-7/8 and Lewis type. Specifically, A-7/8 was virtually absent in A1, Le (a+b-) donors, possibly due to an absence of ALeb in these donors (Figure 3D-F, lanes 16, 19, 22). ALeb is the predominant Lewis-active GSL present in the plasma of A1, Lewis (a-b+) individuals.39
Lewis and ABH expression in 36 platelet donors
. | . | . | . | . | . | . | HPTLC-immunostaining . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Donor characteristics . | . | RBC phenotype . | . | . | . | A-GSL . | . | Lewis-GSL . | . | ||||
| Donor . | Sex . | Age, y . | A1/A2 . | Lewis . | Flow, % HPA . | Western, A-GP . | A-6 . | A-7/8 . | Lea . | Leb . | ||||
| 17 | M | 32 | A2 | a− b+ | 4.3 | 0 | +/− | 0 | +/− | ++ | ||||
| 21 | F | 74 | A1 | a− b− | 52 | + | + | + | 0 | 0 | ||||
| 23 | F | 47 | A1 | a− b+ | 47.6 | + | + | 0 | 0 | ++ | ||||
| 24 | M | 45 | A1 | a+ b− | 44 | + | 0 | + | ++ | 0 | ||||
| 27 | M | 35 | A1 | a− b+ | 43 | + | + | + | + | ++ | ||||
| 30 | M | 54 | A1 | a− b+ | 23.7 | + | + | + | + | ++ | ||||
| 35 | F | 27 | A1 | a− b+ | 27.6 | + | + | + | 0 | ++ | ||||
| 38 | M | 35 | A1 | a− b+ | 28.5 | + | + | + | + | ++ | ||||
| 40 | M | 46 | A1 | a− b+ | 33.5 | + | + | + | + | ++ | ||||
| 42 | M | 28 | A1 | a+ b− | 39.5 | + | + | 0 | ++ | 0 | ||||
| 53 | M | 38 | A1 | a− b+ | 16.9 | + | + | + | 0 | 0 | ||||
| 60 | M | 37 | A2 | a− b+ | 3.0 | 0 | 0 | 0 | + | ++ | ||||
| 63 | M | 39 | A1 | a− b+ | 43.1 | + | 0 | 0 | 0 | +/− | ||||
| 64 | M | 21 | A1 | a− b+ | 33.6 | + | + | + | + | ++ | ||||
| 67 | F | 49 | A2 | a+ b− | 1.4 | 0 | 0 | 0 | ++ | 0 | ||||
| 76 | M | 49 | A1 | a− b+ | 35.5 | + | + | + | + | ++ | ||||
| 78 | M | 41 | A1 | a− b+ | 44.7 | + | + | + | +/− | ++ | ||||
| 85 | F | 61 | A1 | a− b+ | 87.5 | + | + | + | 0 | + | ||||
| 88 | M | 35 | A2 | a+ b− | 1.0 | 0 | 0 | 0 | ++ | 0 | ||||
| 95 | M | 39 | A1 | a+ b− | 42.2 | + | + | + | ++ | 0 | ||||
| 98 | M | 42 | A1 | a− b+ | 22.4 | + | + | + | + | ++ | ||||
| 99 | M | 65 | A1 | a+ b− | 22.7 | + | + | 0 | ++ | 0 | ||||
| 102 | F | 21 | A1 | a− b+ | 61.8 | + | + | + | 0 | ++ | ||||
| 104 | M | 27 | A1 | a+ b− | 9.1 | + | + | + | ++ | 0 | ||||
| 107 | M | 48 | A2 | a+ b− | 2.3 | 0 | +/− | 0 | ++ | 0 | ||||
| 108 | F | 33 | A1 | a− b+ | 52 | + | + | + | +/− | ++ | ||||
| 109 | M | 49 | A2 | a− b+ | 4.4 | 0 | 0 | 0 | + | ++ | ||||
| 110 | M | 23 | A1 | a− b+ | 36.6 | + | + | + | + | ++ | ||||
| 112 | F | 44 | A1 | a− b+ | 30.3 | + | 0 | 0 | 0 | + | ||||
| 123 | M | 36 | A1 | a− b+ | 61 | + | + | + | +/− | ++ | ||||
| 125 | M | 29 | A1 | a+ b− | 52.8 | + | + | 0 | ++ | 0 | ||||
| 128 | M | 24 | A2 | a− b+ | <1.0 | 0 | 0 | 0 | +/− | ++ | ||||
| 130 | M | 24 | A1 | a− b+ | 32.3 | + | + | + | +/− | ++ | ||||
| 133 | M | 29 | A1 | a− b+ | 43.3 | + | + | + | 0 | 0 | ||||
| 136 | M | 40 | A1 | a− b+ | 40.0 | + | + | + | +/− | ++ | ||||
| 140 | F | 30 | A1 | a+ b− | 75.3 | + | + | + | ++ | 0 | ||||
| Statistics* | ||||||||||||||
| A1 vs A2 | — | — | — | — | <.0001† | <.00001 | .000009 | .0001 | .09 | .23 | ||||
| Leb + vs Leb | — | — | — | — | .72† | .23 | .63 | .31 | .03 | <.00001 | ||||
. | . | . | . | . | . | . | HPTLC-immunostaining . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Donor characteristics . | . | RBC phenotype . | . | . | . | A-GSL . | . | Lewis-GSL . | . | ||||
| Donor . | Sex . | Age, y . | A1/A2 . | Lewis . | Flow, % HPA . | Western, A-GP . | A-6 . | A-7/8 . | Lea . | Leb . | ||||
| 17 | M | 32 | A2 | a− b+ | 4.3 | 0 | +/− | 0 | +/− | ++ | ||||
| 21 | F | 74 | A1 | a− b− | 52 | + | + | + | 0 | 0 | ||||
| 23 | F | 47 | A1 | a− b+ | 47.6 | + | + | 0 | 0 | ++ | ||||
| 24 | M | 45 | A1 | a+ b− | 44 | + | 0 | + | ++ | 0 | ||||
| 27 | M | 35 | A1 | a− b+ | 43 | + | + | + | + | ++ | ||||
| 30 | M | 54 | A1 | a− b+ | 23.7 | + | + | + | + | ++ | ||||
| 35 | F | 27 | A1 | a− b+ | 27.6 | + | + | + | 0 | ++ | ||||
| 38 | M | 35 | A1 | a− b+ | 28.5 | + | + | + | + | ++ | ||||
| 40 | M | 46 | A1 | a− b+ | 33.5 | + | + | + | + | ++ | ||||
| 42 | M | 28 | A1 | a+ b− | 39.5 | + | + | 0 | ++ | 0 | ||||
| 53 | M | 38 | A1 | a− b+ | 16.9 | + | + | + | 0 | 0 | ||||
| 60 | M | 37 | A2 | a− b+ | 3.0 | 0 | 0 | 0 | + | ++ | ||||
| 63 | M | 39 | A1 | a− b+ | 43.1 | + | 0 | 0 | 0 | +/− | ||||
| 64 | M | 21 | A1 | a− b+ | 33.6 | + | + | + | + | ++ | ||||
| 67 | F | 49 | A2 | a+ b− | 1.4 | 0 | 0 | 0 | ++ | 0 | ||||
| 76 | M | 49 | A1 | a− b+ | 35.5 | + | + | + | + | ++ | ||||
| 78 | M | 41 | A1 | a− b+ | 44.7 | + | + | + | +/− | ++ | ||||
| 85 | F | 61 | A1 | a− b+ | 87.5 | + | + | + | 0 | + | ||||
| 88 | M | 35 | A2 | a+ b− | 1.0 | 0 | 0 | 0 | ++ | 0 | ||||
| 95 | M | 39 | A1 | a+ b− | 42.2 | + | + | + | ++ | 0 | ||||
| 98 | M | 42 | A1 | a− b+ | 22.4 | + | + | + | + | ++ | ||||
| 99 | M | 65 | A1 | a+ b− | 22.7 | + | + | 0 | ++ | 0 | ||||
| 102 | F | 21 | A1 | a− b+ | 61.8 | + | + | + | 0 | ++ | ||||
| 104 | M | 27 | A1 | a+ b− | 9.1 | + | + | + | ++ | 0 | ||||
| 107 | M | 48 | A2 | a+ b− | 2.3 | 0 | +/− | 0 | ++ | 0 | ||||
| 108 | F | 33 | A1 | a− b+ | 52 | + | + | + | +/− | ++ | ||||
| 109 | M | 49 | A2 | a− b+ | 4.4 | 0 | 0 | 0 | + | ++ | ||||
| 110 | M | 23 | A1 | a− b+ | 36.6 | + | + | + | + | ++ | ||||
| 112 | F | 44 | A1 | a− b+ | 30.3 | + | 0 | 0 | 0 | + | ||||
| 123 | M | 36 | A1 | a− b+ | 61 | + | + | + | +/− | ++ | ||||
| 125 | M | 29 | A1 | a+ b− | 52.8 | + | + | 0 | ++ | 0 | ||||
| 128 | M | 24 | A2 | a− b+ | <1.0 | 0 | 0 | 0 | +/− | ++ | ||||
| 130 | M | 24 | A1 | a− b+ | 32.3 | + | + | + | +/− | ++ | ||||
| 133 | M | 29 | A1 | a− b+ | 43.3 | + | + | + | 0 | 0 | ||||
| 136 | M | 40 | A1 | a− b+ | 40.0 | + | + | + | +/− | ++ | ||||
| 140 | F | 30 | A1 | a+ b− | 75.3 | + | + | + | ++ | 0 | ||||
| Statistics* | ||||||||||||||
| A1 vs A2 | — | — | — | — | <.0001† | <.00001 | .000009 | .0001 | .09 | .23 | ||||
| Leb + vs Leb | — | — | — | — | .72† | .23 | .63 | .31 | .03 | <.00001 | ||||
— indicates not applicable.
Significance determined by Chi-square and Fisher exact with the exception of percent HPA-positive platelets.
Student t test.
A2 platelets have a Bombay-like phenotype
On RBCs, the A1/A2 subtypes are characterized by inverse expression of A and H antigen, due to differences in the conversion of H→A by the A1 and A2 glycosyltransferase, respectively (Figure 4D-E).15,40 Given the association between donor A1/A2 subtype and platelet A antigen expression, we tested group A1, A2, and O platelets for H antigen expression with the anti-H lectin, UEA1.
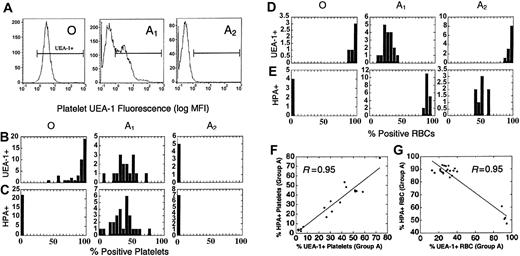
Different regulation of H and A antigen on platelets and RBCs. (A) H antigen expression (FITC-UEA1) on group O, A1, and A2 donors by flow cytometry. (B-C) The distribution of H (B, % UEA1+) and A antigens (C, % HPA+) on platelets in a population of group O, A1, and A2 donors. (D-E) The distribution of H (D, % UEA1+) and A (E, % HPA+) antigens on RBCs in a population of group O, A1, and A2 donors. Note the absence of H and A on A2 platelets, whereas H is increased on A2 RBCs. The relative expression of H and A also differs between A1 RBCs and platelets as well. Horizontal brackets (B-E) indicate UEA1+ fluorescence. Cells left of the bracketed area were considered UEA1-negative. (F-G) Blood group H (% UEA1+) and A (% HPA+) expression in individual group A platelet donors. (F) On platelets, A and H are proportionately coexpressed, where y = 0.15 + 0.93x. (G) Blood groups H and A are inversely expressed on RBCs (y = 103.3-0.53x), with 2 distinct clusters representing A1 and A2 donors.
Different regulation of H and A antigen on platelets and RBCs. (A) H antigen expression (FITC-UEA1) on group O, A1, and A2 donors by flow cytometry. (B-C) The distribution of H (B, % UEA1+) and A antigens (C, % HPA+) on platelets in a population of group O, A1, and A2 donors. (D-E) The distribution of H (D, % UEA1+) and A (E, % HPA+) antigens on RBCs in a population of group O, A1, and A2 donors. Note the absence of H and A on A2 platelets, whereas H is increased on A2 RBCs. The relative expression of H and A also differs between A1 RBCs and platelets as well. Horizontal brackets (B-E) indicate UEA1+ fluorescence. Cells left of the bracketed area were considered UEA1-negative. (F-G) Blood group H (% UEA1+) and A (% HPA+) expression in individual group A platelet donors. (F) On platelets, A and H are proportionately coexpressed, where y = 0.15 + 0.93x. (G) Blood groups H and A are inversely expressed on RBCs (y = 103.3-0.53x), with 2 distinct clusters representing A1 and A2 donors.
As shown in Figure 4A, all group O donors strongly expressed H antigen on platelets (range, 45%-100% UEA1+). Unlike A1 donors, H expression on group O platelets was strongly skewed toward an ABH-HXP-like phenotype, with 85% of donors showing more than 70% UEA1 staining (Figure 4B). Unexpectedly, H and A expression were both elevated in A1 donors, with each showing a similar population mean and distribution. A2 platelets, in contrast, were repeatedly negative for H antigen by UEA1. A2 platelets, therefore, have a Bombay-like phenotype, a rare RBC phenotype negative for A, B, and H antigens due to mutations in the H gene fucosyltransferase (FUT1).15,40
Different regulation of A and H antigens on platelets and RBCs
Given our surprising results with UEA1, we compared A and H expression on RBCs and platelets. As expected, there was an inverse expression of A and H antigens on RBCs, whereas A and H were directly correlated on platelets (Figure 4F-G). We also compared ABH expression on paired RBC and platelet samples from the same donor. Interestingly, there was an inverse relationship between H expression on donor platelets and RBCs (R = 0.93). There was little correlation between A antigen on RBCs and platelets (R = 0.63).
The ABH-HXP phenotype is unrelated to A1int or A1H↑ RBC phenotypes
The surprising correlation between H and A on human platelets suggested the possibility that the ABH-HXP phenotype might be related to the A1 intermediate (A1int) or A1 High H (A1H↑) RBC phenotypes, which possess features of both A1 and A2.41-43 Like A1 RBCs, A1H↑ and A1int RBCs react strongly with anti-A, B, and D biflorus; however, A1H↑ and A1int RBCs also have elevated H expression similar to A2 and other weak A subtypes. The A1int phenotype is particularly noteworthy, having H levels nearly equivalent to those observed on group O RBCs.41 The incidence of A1H↑ and A1int differs among various ethnic populations, ranging from 2.0% to 13.7%.41-43 Like the ABH-HXP phenotype, family studies of A1H donors suggest autosomal dominant inheritance.43
To ascertain whether our ABH-high-expressing platelet donors were of the A1H↑ or A1int phenotype, A and H were examined on RBCs and platelets from an ABH-HXP donor (Table 3). Unlike A1int and A1H↑ phenotypes, H was profoundly decreased on ABH-HXP RBCs. The ABH-HXP phenotype, therefore, is distinct and unrelated to intermediate RBC phenotypes. These results further emphasize a fundamental difference in A/B versus H synthesis on RBCs and platelets, respectively.
ABH-HXP is not related to A1-intermediate or A1-High H RBC phenotype
. | . | Platelets, % positive . | . | RBC, % positive . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | A subtype . | HPA . | UEA1 . | HPA . | UEA1 . | ||
| 10 | A2 | <5 | <5 | 52 | 93 | ||
| 109 | A2 | <5 | <5 | 61 | 89 | ||
| C1 | A2 | <5 | <5 | 51 | 92 | ||
| 50 | A1 | 23 | 31 | 89 | 36 | ||
| 105 | A1 | 53 | 42 | 87 | 25 | ||
| 117 | A1-HPX | 79 | 73 | 87 | 15 | ||
. | . | Platelets, % positive . | . | RBC, % positive . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | A subtype . | HPA . | UEA1 . | HPA . | UEA1 . | ||
| 10 | A2 | <5 | <5 | 52 | 93 | ||
| 109 | A2 | <5 | <5 | 61 | 89 | ||
| C1 | A2 | <5 | <5 | 51 | 92 | ||
| 50 | A1 | 23 | 31 | 89 | 36 | ||
| 105 | A1 | 53 | 42 | 87 | 25 | ||
| 117 | A1-HPX | 79 | 73 | 87 | 15 | ||
Expression on group B and O donors
A small number of group B donors were available for GP (B-GP) and GSL (B-GSL) analysis. B-GPs were detected in 80% of donors by Western blot. Group B-GSL was identified in 90% of donors, independent of Lewis type, and was strongly correlated with B-GP (P < .001). By flow cytometry, only 20% of donors were positive with an anti-B MoAb. Attempts to stain group B platelets with an FITC-labeled anti-B lectin (GS-1) were unsuccessful.
There were 12 group O donors tested by Western blotting. Using 2 different anti-H MoAbs, we detected only weak staining to a single GP (Mr ∼ 200 kDa, not shown). No staining was observed with a biotinylated UEA1 lectin. Weak H staining to group O platelet glycoproteins was also reported by Mollicone et al.7
Discussion
The importance of ABH in platelet compatibility and posttransfusion recovery has been reported in several case reports and clinical studies. Transfusion of ABH-incompatible platelets, even in the presence of HLA matching, can be associated with a decrease in the posttransfusion corrected count increment,8-11,17 increased platelet utilization,12,44 incompatible platelet crossmatches,11,14 HLA alloimmunization,12,13,44 and ABH-specific refractoriness.8-11,16,18,23,24 The impact of ABH incompatibility is determined by patient and donor factors, including the type of ABH mismatch and recipient isohemagglutinin titers.8,9,16-18 Overall, clinical ABH incompatibility is most commonly observed with group A platelets transfused to group O patients, due to increased antigenicity of the A antigen and higher mean anti-A titers.8,9,12,16,17 Repeated transfusions of ABH-incompatible platelets can further exacerbate these effects through immune stimulation, leading to increased isohemagglutinin titers and HLA alloimmunization.12,17
Despite these findings, few studies have examined what factors regulate ABH on donor platelets. This study represents the first large-scale, comprehensive study of platelet donors, correlating ABH expression on platelet membranes, and specific glycoconjugates, to serologic and demographic markers. We chose to focus predominantly on group A platelet donors because (1) A/O major mismatch is more likely to adversely effect the transfusion response8,13 and (2) the higher likelihood of A/O major mismatch transfusion due to distribution of group A (40%) and O (44%) in the population.15
Using a combination of flow cytometry, HPTLC, and Western blotting, we confirmed that donor A1/A2 phenotype is the single greatest predictor of platelet ABH expression. Overall, there was a nearly 100% concordance between an A1 phenotype and A antigen on platelet membranes, GPs, and GSLs. ABH was identified on both GPs and GSLs, independent of secretor status, indicating endogenous synthesis. Furthermore, A antigen on GPs was the predominant determinant of overall antigenicity. In individual donors, ABH expression was relatively consistent over time, suggesting that the relative density of ABH antigens on A1 platelets is a donor-specific, heritable trait. A2 donors, on the other hand, repeatedly typed as “group O compatible” by all 3 methods. The absence of A antigen on group A2 platelets is also observed in other tissues, indicating profound suppression of A2-transferase activity in nonerythroid tissues.45,46
One very surprising finding was the linear coexpression of A and H antigens on platelets—unlike RBCs, in which A and H are inversely expressed. On mature RBCs, ABH levels are determined primarily by A/B-glycosyltransferase activity. This has been verified over the last decade by molecular analysis of weak A and B subtypes, which have consistently linked weak or absent A/B expression, accompanied by increased H, to mutations in the A/B-glycosyltransferase open reading frame.40,47 The latter is not sufficient, however, to explain ABH regulation on platelets, particularly the paradoxical absence of both A and H on A2 platelets. In megakaryocytes and platelets, we believe that ABH levels are primarily determined by H antigen synthesis. This was also suggested by Dunstan,48 who observed clonal regulation of H and A in cultured megakaryocytes and their platelet progeny. It would also support the absence of H and A on A2 platelets, which possess a Bombay-like phenotype. Finally, it provides an alternate etiology for the ABH-HPX platelet phenotype: molecular studies in A1 ABH-HXP donors indicate a consensus A1 allele.23 As shown in Table 3, the highest H expression was observed on ABH-HPX platelets.
How A and H are proportionately coexpressed on platelet membranes is also unclear. Transcriptional coregulation seems unlikely given the differences in FUT1 and A/B-transferase promoters. Whereas FUT1 has 3 distinct promoters directing tissue-specific expression,49 the A/B-transferase promoter is a classic CpG island regulated by methylation and upstream enhancer elements.40,50,51 Coordinated transcription of both genes, therefore, would require a common signaling pathway, present in megakaryocytes but not RBCs, that is genetically linked to the A1, A2, and ABH-HXP subtypes.
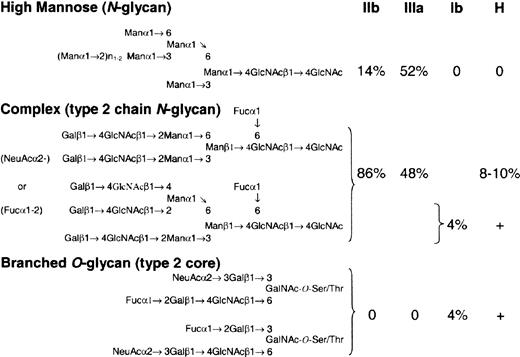
We favor differential expression of FUT1, coupled with synthesis of H-active epitopes that are sterically unfavorable substrates for the A/B-glycosyltransferase. On RBCs, H antigen is synthesized predominantly (85%-90%) on type 2 chain N-glycans, polyglycosylceramides, and GSLs,52 which are readily accessible substrates for the A/B-glycosyltransferase.53 On platelets and megakaryocytes, however, H is expressed on a broad mixture of N-linked and complex O-linked structures. GPIIb/IIIa, the major N-linked GP complex on platelets,54 possesses 11 N-linked glycan sites, of which 5% may terminate in ABH (Figure 5).55 Glycoprotein Ib, the major sialomucin on platelets,54 displays 3 to 4 N-linked60,61 and 50 to 60 O-linked glycans,56-58 including H-active, sialylated branched structures.57 Similar O-linked structures may also be present on GPIV.59 Their branched structure (Figure 5),57 which involves a penultimate oligosaccharide,53 coupled with the relative density of neighboring O-linked structures,56,62,63 may inhibit further modification by the A/B-glycosyltransferase.53 If true, increasing H-transferase activity could manifest as a concurrent rise in both H and A antigen on O- and N-linked structures, respectively (Figure 4F).
H antigen is expressed on a mixture ofN- andO-linked glycans on major platelet GPs. Potential H epitopes on the 3 major platelet GPs: GPIIb, GPIIIa, and GP1b. On GPs IIb and IIIa, there is a mixture of high mannose, biattenary, and triattenary type 2 chain N-glycans.55 It is estimated that 8% to 10% of biattenary and triattenary structures may terminate in an H-active epitope, which could be further modified to A and B antigens in group A and B individuals, respectively. Based on the number of GPIIb/IIIa molecules per platelet,54 this could equal approximately 100 000 ABH epitopes/platelet. GPIb, a sialomucin, has limited N-glycans but is rich in O-linked carbohydrate, which comprises 60% of the molecular weight of the molecule.54 Most of the O-linked carbohydrate exists as a disialo, branched core 2 oligosaccharide.56-58 A minor component (4%) is a monosialo, fucosylated derivative with H-like activity57 that could number nearly 75 000 H epitopes/platelet. Similar O-glycan structures may exist on GPIV.59 Because of their branched structure, and the density of O-glycan on GPIb molecule, these H-like epitopes may be sterically inaccessible for further modification by the A/B glycosyltransferase. As a consequence, increases in H-fucosyltransferase activity could result in parallel increases in H and A antigen on O- and N-linked glycans, respectively.
H antigen is expressed on a mixture ofN- andO-linked glycans on major platelet GPs. Potential H epitopes on the 3 major platelet GPs: GPIIb, GPIIIa, and GP1b. On GPs IIb and IIIa, there is a mixture of high mannose, biattenary, and triattenary type 2 chain N-glycans.55 It is estimated that 8% to 10% of biattenary and triattenary structures may terminate in an H-active epitope, which could be further modified to A and B antigens in group A and B individuals, respectively. Based on the number of GPIIb/IIIa molecules per platelet,54 this could equal approximately 100 000 ABH epitopes/platelet. GPIb, a sialomucin, has limited N-glycans but is rich in O-linked carbohydrate, which comprises 60% of the molecular weight of the molecule.54 Most of the O-linked carbohydrate exists as a disialo, branched core 2 oligosaccharide.56-58 A minor component (4%) is a monosialo, fucosylated derivative with H-like activity57 that could number nearly 75 000 H epitopes/platelet. Similar O-glycan structures may exist on GPIV.59 Because of their branched structure, and the density of O-glycan on GPIb molecule, these H-like epitopes may be sterically inaccessible for further modification by the A/B glycosyltransferase. As a consequence, increases in H-fucosyltransferase activity could result in parallel increases in H and A antigen on O- and N-linked glycans, respectively.
In summary, we have confirmed the importance of donor A1/A2 subgroup in platelet ABH expression. Because group A2 platelets are group O compatible, they could increase the available inventory of group O platelets from 44% to 52%.15 The latter would increase the number of apheresis platelets available for crossmatching, which requires ABO compatibility between donor and recipient.14 It would also benefit selection and survival of HLA-matched platelets, which are also effected by ABH incompatibility.8,11-13 Additional ABH low expressers could be identified by flow cytometry. In this study, 10% to 20% of A1 donors are potentially compatible with group O patients due to absent/low A expression (% HPA < 15%). These findings agree with Heal et al,14 who showed that nearly 40% of group A and 80% of group B platelet donors were crossmatch compatible with group O patients. Conversely, ABH-HXP platelets should be reserved for ABH-identical recipients.24
Finally, platelets from group A2 donors (and A1 low expressers) should be considered for patients undergoing A/O ABH major-mismatch allogeneic progenitor cell transplantations. Because of the risks of hemolysis, pure RBC aplasia, and delayed engraftment,15,64-66 these patients require provision of blood products compatible with both donor and recipient.15 This is particularly problematic for platelets because of the need to consider both plasma and platelet compatibilities. This inherent conflict may contribute to increased platelet utilization, morbidity, and mortality in ABO-mismatched transplants, particularly in those patients receiving nonmyeloablative conditioning.64,67-69 It is noteworthy that both the number of platelet transfusions and the transfusion of incompatible plasma are linked to an overall increase in treatment-related toxicity, including multiorgan dysfunction and early death.67-69 In A/O-mismatched transplants, this conflict can be resolved by transfusion of A2 platelets, which are compatible with both the recipient (group O platelets) and donor (group A plasma). Future studies to test the benefit of A2 platelets in allogeneic progenitor cell transplantation should be explored.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-08-3080.
Supported by a grant from the National Blood Foundation.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ronald Strauss, Kathy Walker, and the staff of the DeGowin Blood Bank for their help and assistance in collecting samples used in this study; Theresa Duling for technical assistance in flow cytometry analysis; and Elizabeth Horn Walker for technical assistance in figure design and composition.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal