Abstract
Vaso-occlusion is a hallmark of sickle cell disease. Agonist-induced activation of sickle red blood cells (SS RBCs) promotes their adhesion to vascular proteins, potentially contributing to vasoocclusion. Previously, we described a cyclic adenosine monophosphate (cAMP)-dependent increase in SS RBC adhesion to laminin. Here, we investigated whether Rap1, a small guanosine triphosphatase (GTPase) known to promote integrin-mediated adhesion in other cells, was involved in this signaling pathway. We found that agonists known to induce cAMP signaling promoted the GTP-bound, active state of Rap1 in SS RBCs. The cAMP-dependent exchange factor Epac (exchange protein directly activated by cAMP) is a likely upstream activator of Rap1, since Epac is present in these cells and the Epac-specific cAMP analog 8CPT-2-Me (8-(4-cholorophenylthio)-2′-O-methyl-cAMP) activated Rap1 and promoted SS RBC adhesion to laminin. This 8CPT-2-Me-stimulated adhesion was integrin independent, since it was insensitive to RGD peptide or antibodies against the only known integrin on SS RBCs, α4β1. However, this adhesion was completely inhibited by either a soluble version of basal cell adhesion molecule/Lutheran (BCAM/LU) or a BCAM/LU adhesion-blocking anti-body. Surprisingly, 8CPT-2-Me-activated Rap1 did not promote SS RBC adhesion to a known α4β1 ligand, vascular cell adhesion molecule 1 (VCAM-1). These results demonstrate that Epac-induced Rap1 activation in SS RBCs promotes BCAM/LU-mediated adhesion to laminin. Thus, Epac-mediated Rap1 activation may represent an important signaling pathway for promoting SS RBC adhesion. (Blood. 2005;105:3322-3329)
Introduction
Recurrent, painful vaso-occlusive crises are a hallmark of sickle cell disease. A likely contributor to vasoocclusion is the propensity of sickle red blood cells (SS RBCs) to adhere to proteins in the vasculature. We recently determined that SS RBC adhesiveness is promoted by intracellular signaling events leading to receptor-mediated adhesion via either the integrin α4β1 or the immunoglobulin (Ig) superfamily adhesion receptor basal cell adhesion molecule/Lutheran (BCAM/LU).1,2 However, the signaling pathways leading to adhesion of SS RBCs are not well understood. One signaling molecule that may be responsible for promoting adhesion in SS RBCs is the small guanosine triphosphatase (GTPase) receptor-associated protein 1 (Rap1).
Rap1 is a close relative of Ras. There are 2 known isoforms of Rap1, Rap1a and Rap1b, which are 95% identical in amino acid sequence.3 Like other small G proteins, Rap1 is active when GTP-bound and inactive when guanosine diphosphate (GDP)-bound. Guanine nucleotide exchange factors (GEFs) promote the exchange of GDP for GTP, thus activating Rap1. GTPase-activating proteins (GAPs) activate the intrinsic GTPase activity of Rap1, resulting in the hydrolysis of bound GTP to GDP, thus inactivating Rap1. Several GEFs can activate Rap1 and are themselves activated by a diverse array of signaling pathways. One class of GEFs is exchange proteins activated by cAMP (Epac's), which activate Rap1 upon binding cyclic adenosine monophosphate (cAMP). Epac's are widely expressed and are emerging as an important class of cAMP effectors.4 The downstream effectors of Rap1 are largely unknown. However, it has been demonstrated that Rap1 promotes the activation of integrin adhesion receptors, leading to cellular adhesion. Integrins known to be activated by Rap1 include αIIbβ3, α5β1, αLβ2, αMβ2, and α4β1.3 A role for Rap1 in activating other classes of adhesion receptors is not well characterized.
Rap1 is present in a number of cell types, including hematopoietic cells. Previous studies have shown that Rap1 is abundant in both platelets and white blood cells (WBCs) and can contribute to integrin-mediated signaling in both cell types.5,6 However, neither the presence of Rap1 nor its signaling capacity in RBCs has been examined.
SS RBCs represent a unique system for the study of signal transduction-mediated adhesion. Circulating SS RBCs are less mature relative to normal RBCs and are consequently highly reactive signaling cells. Moreover, the youngest population of RBCs found in patients with sickle cell disease (SCD) is known to express only one integrin, α4β1, and adhesion can be mediated through this receptor as well as through the nonintegrin, Ig superfamily receptor BCAM/LU.1,7-9 Signaling leading to enhanced SS RBC adhesion in the vasculature may contribute to the vasoocclusive crises experienced by patients with SCD. Recent studies in our laboratory have demonstrated that SS RBCs exhibit agonist-induced increases in cell adhesion to the extracellular matrix proteins thrombospondin and laminin via intracellular signaling events.1,10 Signaling in SS RBCs can occur via a variety of pathways, one major pathway being mediated by cAMP.1,10 A recent study confirmed that an immature population of RBCs that was intermediate in maturity between reticulocytes and fully mature SS RBCs was responsible for cAMP-dependent, BCAM/LU-mediated SS RBC adhesion to laminin.1 Although SS RBCs are activated by signaling pathways that, in other cells, are also known to activate Rap1, neither the presence nor the potential role of Rap1 in promoting SS RBC adhesion has ever been examined.
In this study, we find that Rap1 is present and can be activated in SS RBCs by cAMP, most likely via the Rap1 GEF, Epac. Although Rap1 can activate adhesion in a diverse array of cells via a variety of integrins, very few studies address Rap1 signaling leading to adhesion via nonintegrin receptors.11 We further demonstrate that SS RBC adhesion to the extracellular matrix protein laminin is promoted by Rap1 via the Ig superfamily receptor BCAM/LU, providing evidence for a new paradigm in Rap1 adhesive signaling as well as elucidating a novel signaling pathway that promotes SS RBC adhesion.
Patients, materials, and methods
Materials
Isoproterenol, forskolin, Zwittergent 3-16, and IBMX (3-isobutyl-1-methylxanthine) were obtained from Calbiochem (La Jolla, CA). Dibutyryl cAMP was obtained from Sigma Aldrich (St Louis, MO). The 8CPT-2-Me (8-(4-Cholorophenylthio)-2′-O-methyl-cAMP) was obtained from Axxora USA (San Diego, CA). RGD and RGE peptides (RGDW) and (RGEW) were synthesized and purified via high-performance liquid chromatography (HPLC) at the University of North Carolina Protein Chemistry Laboratory (Chapel Hill, NC). The LDV and control peptides (EILDV and EILEVPST) as well as the 4N1K peptide (kRFYVVMWKk) were obtained from SynPep Corporation (Dublin, CA). Adhesion-blocking antibodies for the α4 (mouse antihuman clone 6S6) and β1 (mouse antihuman clone 46) integrin subunits were obtained from Chemicon International (Temecula, CA). The BCAM/LU adhesion-blocking antibody (goat antihuman-derived recombinant human BCAM extracellular domain epitope) was obtained from R&D Systems (Minneapolis, MN).
Red blood cell preparation
This study was conducted with the approval of The University of North Carolina (UNC)-Chapel Hill institutional review board, and informed consent from each patient was obtained in accordance with the Declaration of Helsinki. SS RBCs were obtained from patients with sickle cell anemia at the UNC Comprehensive Sickle Cell Center during clinic visits. Normal (AA) RBCs were obtained from healthy donors. Blood was obtained by venipuncture into 0.13 M sodium citrate and subjected to centrifugation at 150g for 15 minutes at room temperature to isolate the RBCs from plasma and platelets. The plasma, buffy coat, and top layer of RBCs were removed by aspiration to minimize WBC and platelet contamination. RBCs were then washed 3 times in CGS buffer (1.29 mM sodium citrate; 3.33 mM glucose; 124 mM sodium chloride, pH 7.2). Cells were resuspended in phosphate-buffered saline (PBS) and subjected to centrifugation at 400g for 10 minutes. For all pharmacologic assays, a 10% hematocrit was prepared by suspending 200 μL of packed cells per mL of perfusion media (Hanks balanced salt solution [HBSS], 1.25 mM CaCl2 · 2H20, 0.811 mM MgSO4, 5.37 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.34 mM Na2PO4, and 5.5 mM D-glucose; Sigma Aldrich; supplemented with 0.3% bovine serum albumin [BSA], phenol red, and 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4]). The cells were then counted in a Coulter counter (Beckman Coulter, Miami, FL) and the RBC concentration was adjusted to 1 × 109 cells/mL. The diluted cells were treated with 25 μL/mL of anti-CD45 antibody-conjugated magnetic beads (Dynal, Lake Success, NY) for 5 minutes with shaking to remove any remnant WBCs. The cell suspension was placed against a magnet, and the cell suspension was separated from the beads for use in the assays. For the flow adhesion assay, a 1% hematocrit was prepared by suspending 30 μL of cells in 1.5 mL of perfusion media and, if necessary, this suspension was diluted 1:1 in HBSS to form a 0.5% hematocrit.
White blood cell preparation
WBCs were prepared by obtaining buffy coats from separated whole blood and layering them on top of a 1.0770-1.0800 Ficoll-sodium diatrizoate solution (ICN Biomedicals, Aurora, OH). The layered suspension was centrifuged for 25 minutes at 400g. The WBCs were removed, washed in PBS, and then suspended in perfusion media. The cells were counted on a hemacytometer and adjusted to a concentration of 2 × 107 cells/mL.
GST-RalGDS-RBD precipitation of GTP-bound Rap1
Glutathione-S-transferase RalGDS Ras-binding domain (GST-RalGDS-RBD) beads were generated and GTP-bound Rap was precipitated from RBC or WBC lysates as previously described.12 Briefly, suspensions at a concentration of 1 × 109 RBCs/mL were treated with various agonists. At each time point, a 500-μL aliquot of cells was added to 500 μL of cold 2 × lysis buffer (50 mM HEPES, 150 mM NaCl, 100 mM NaF, 20 mM β-glycerophosphate, 1% deoxycholate, 1:100 protease inhibitor cocktail III; Calbiochem), mixed, and placed immediately on ice. WBCs were lysed in 2 × lysis buffer on ice for 20 minutes. For loading controls, an aliquot of lysate was removed prior to bead addition and blotted for protein phosphatase 2A (PP2A) with PP2A-specific antibody (mouse antihuman, clone 46; BD Biosciences Pharmingen, San Jose, CA). A bead volume of 15 μL was added to the lysates and incubated for 1 hour at 4°C. The beads were separated from the lysate via centrifugation, protein was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and active Rap1 was detected by immunoblotting with a Rap1-specific antibody (mouse antihuman, clone 3; BD Biosciences Pharmingen).
Western blotting for αIIb in platelet and RBC lysate
RBCs were prepared and lysed as described in “Red blood cell preparation.” Platelets were obtained by centrifugation of platelet-rich plasma at 800g. The platelet pellet was then lysed in 3 mL of the 2 × lysis buffer used to lyse RBCs as described in “Red blood cell preparation.” Lysate from RBCs and platelets, respectively, was then separated on a 6% SDS-PAGE gel, and αIIb was detected by blotting with a rabbit antihuman αIIb-specific antibody.
Detection of Epac in SS RBCs
SS RBC lysate was prepared as described under “Western blotting.” The lysate was combined with a corresponding amount of 3 × Laemmli sample buffer under reducing conditions and boiled 5 minutes at 95°C.13 The lysate was then quickly centrifuged to remove any debris. The samples were separated via a 6% SDS-PAGE gel at 120 V for 2 hours, and the proteins were subsequently transferred to a polyvinylidene fluoride (PVDF) membrane for 1 hour at 5 V. The membrane was blocked in Tris-buffered saline/Tween 20 (TBST) with 5% BSA and then probed with an anti-Epac1 antibody (rabbit antihuman polyclonal; Upstate, Lake Placid, NY) or a rabbit antihuman Rap1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), which served as a control. The blot was developed with Western Lightening chemiluminescent reagent (PerkinElmer Life Sciences, Boston, MA). The molecular weight (MW) of the protein was calculated from the Western blot by measuring the migration distance of the molecular weight markers, graphing the log MW versus migration distance, and then interpolating based on the migration distance of the Epac protein band.
Flow adhesion assay
RBC adhesion to extracellular matrix proteins was measured in a parallel plate flow chamber as previously described.1 Briefly, 0.75 μg of purified laminin (Sigma Aldrich; Chemicon International; and Gibco BRL, Grand Island, NY) in PBS or 3 μg of recombinant human vascular cell adhesion molecule 1 (VCAM-1; R&D Systems) was immobilized in identical wells formed by a silicon gasket pressed into a 35-mm polystyrene tissue culture dish via incubation overnight at 4°C. Laminin from Sigma Aldrich was used in 2 of the 4 RGD experiments comprising Figure 5A and one of the 5 α4 and β1 adhesion-blocking antibody experiments comprising Figure 5B, due to a temporary lack of availability of anti-α5 chain 4C7 antibody-purified laminin from other sources. Laminin from Chemicon International and Gibco BRL provided the most consistent results, most likely because they are enriched for laminins 10 and 11 via purification with the 4C7 antibody, and laminin from these suppliers was used for all other experiments.14,15 It has been previously shown that SS RBCs adhere only to α5 chain-containing laminins, which include laminins 10 and 11.16 A 0.5% or 1% hematocrit of RBCs (1 mL) in perfusion media with the reagents used for each specific treatment condition was flowed across the laminin- or VCAM-1-coated wells at a flow rate of 1.0 mL per minute and a constant shear stress of 1 dyne/cm2. The indicated antibody, peptide, inhibitor, and 8CPT-2-Me concentrations were maintained throughout the experiment. The adherent cells were washed for 3 minutes in perfusion media and were counted directly by light microscopy from 4 different representative areas, averaged, and expressed as adherent cells/mm2. Due to patient-to-patient variability in the level of baseline adhesion that could be due to such factors as hydroxyurea treatment of some patients,17 baseline adhesion was normalized to 1 and the treatment conditions were compared with the baseline value as a fold increase in adhesion. Any occasional adherent WBCs were excluded from the analysis.
Rap1 activation does not promote SS RBC adhesion to laminin via the α4β1 integrin. (A) SS RBC adhesion to laminin stimulated via the Epac/Rap1 pathway is not RGD-dependent. SS RBCs in perfusion media were treated with 100 μM 8CPT-2-Me for 20 minutes or with 1 mM of either RGDW or RGEW peptide for 30 minutes before the 20-minute 8CPT-2-Me treatment. While still in the presence of the indicated reagents, the cells were then flowed over chambers coated with 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 4 separate experiments. (B) SS RBC adhesion to laminin is not dependent on the LDV sequence. SS RBCs were untreated or pretreated with 1 mM EILDV peptide or 1 mM EILEVPST peptide for 30 minutes. The SS RBCs and the SS RBC/peptide mixture were then treated with 100 μM 8CPT-2-Me for 20 minutes and flowed over 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 3 separate experiments. (C) The α4β1 integrin does not mediate SS RBC adhesion to laminin. SS RBCs in perfusion media either were not pretreated or were preincubated with either 1 μg/mL α4 and β1 integrin subunit-blocking antibodies or an equivalent concentration of IgG control antibody for 30 minutes and then 100 μM 8CPT-2-Me was added to the RBC/antibody mixture for 20 minutes. The SS RBCs, still in the presence of antibody and 8CPT-2-Me, were flowed across chambers coated with 0.75 μg immobilized laminin. Results are expressed as mean ± SE from 5 separate experiments. (D) Rap1 activation via Epac does not promote adhesion to the α4β1 selective substrate VCAM-1. Cells were treated with either 100 μM 8CPT-2-Me or 100 μM 4N1K peptide for 20 minutes. The SS RBCs, still in the presence of the indicated pharmacologic agents, were then flowed over chambers coated with 3 μg of immobilized VCAM in a flow adhesion assay. Results shown are combined data ± SE from 2 separate experiments.
Rap1 activation does not promote SS RBC adhesion to laminin via the α4β1 integrin. (A) SS RBC adhesion to laminin stimulated via the Epac/Rap1 pathway is not RGD-dependent. SS RBCs in perfusion media were treated with 100 μM 8CPT-2-Me for 20 minutes or with 1 mM of either RGDW or RGEW peptide for 30 minutes before the 20-minute 8CPT-2-Me treatment. While still in the presence of the indicated reagents, the cells were then flowed over chambers coated with 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 4 separate experiments. (B) SS RBC adhesion to laminin is not dependent on the LDV sequence. SS RBCs were untreated or pretreated with 1 mM EILDV peptide or 1 mM EILEVPST peptide for 30 minutes. The SS RBCs and the SS RBC/peptide mixture were then treated with 100 μM 8CPT-2-Me for 20 minutes and flowed over 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 3 separate experiments. (C) The α4β1 integrin does not mediate SS RBC adhesion to laminin. SS RBCs in perfusion media either were not pretreated or were preincubated with either 1 μg/mL α4 and β1 integrin subunit-blocking antibodies or an equivalent concentration of IgG control antibody for 30 minutes and then 100 μM 8CPT-2-Me was added to the RBC/antibody mixture for 20 minutes. The SS RBCs, still in the presence of antibody and 8CPT-2-Me, were flowed across chambers coated with 0.75 μg immobilized laminin. Results are expressed as mean ± SE from 5 separate experiments. (D) Rap1 activation via Epac does not promote adhesion to the α4β1 selective substrate VCAM-1. Cells were treated with either 100 μM 8CPT-2-Me or 100 μM 4N1K peptide for 20 minutes. The SS RBCs, still in the presence of the indicated pharmacologic agents, were then flowed over chambers coated with 3 μg of immobilized VCAM in a flow adhesion assay. Results shown are combined data ± SE from 2 separate experiments.
Preparation of soluble BCAM/LU protein
The extracellular domain of the BCAM/LU receptor was cloned, expressed in 293 cells, and purified as previously described.1 Briefly, a cDNA construct was generated from the extracellular domain of full-length Lutheran by polymerase chain reaction (PCR), subcloned into the pcDNA3.1/V5-His-TOPO expression vector (Invitrogen, Carlsbad, CA), and transfected into 293 cells with lipofectin (Life Technologies, Gaithersburg, MD). The 293 cells were adapted to 293 serum-free medium (SFM) that contained 0.5 mg/mL of geneticin, and the secreted recombinant Lutheran protein was purified with the Xpress protein purification system (Invitrogen).
Preparation of protein sample for mass spectrometry
Rap protein was obtained by precipitation with a 60-μL bead volume of GST-RalGDS-RBD beads in 1 mL RBC lysate made from 500 μL of packed RBCs in 2 × lysis buffer (50 mM HEPES, 150 mM NaCl, 1% Zwittergent 3-16) for 1 hour at 4°C. The sample was then washed 6 times in 0.1% PBS and reconstituted in 120 μL 0.1% PBS. Rap protein was cleaved from the GST-RalGDS-RBD beads by incubation of the suspension with 14 U thrombin for 2 hours at room temperature. The beads were collected with a quick centrifugation and the supernatant was digested in 100 mM ammonium bicarbonate. Trypsin was prepared by adding a 20-μL aliquot of Promega buffer (Promega, Madison, WI; 50 mM acetic acid) to 20 μg of trypsin. A 2-μL aliquot of this solution (containing 2 μg of trypsin) was added to each sample tube. Samples were digested overnight at 37°C with slow agitation, lyophilized, and stored at -80°C. Immediately prior to analysis, the lyophilized samples were reconstituted with 20 μL at a 5:95 acetonitrile-water ratio (0.1% formic acid).
Liquid chromatography-tandem mass spectrometry of Rap peptides
A 6.4-μL aliquot of each digest was analyzed by liquid chromatography-tandem mass spectrometry (LC/MS/MS) on a Waters/Micromass API US quadrupole time-of-flight (Q-tof) mass spectrometer (Waters, Milford, MA), interfaced to Waters CapLC. The HPLC system was equipped with a 5mm × 80 nm internal diameter C18 P3 trapping column and a 75 μ id C18 PepMap analytical column (Dionex Corporation, Sunnyvale, CA). Spectra were acquired in the “survey” mode, where an MS survey scan is acquired first, followed by MS/MS scans on parent ions meeting a preselected intensity threshold. For these experiments, the intensity threshold was set to “1” (the minimum allowable). MS spectra were acquired over the mass range 400 to 1900, and MS/MS spectra were acquired over the mass range 50 to 1900, at a scan rate of 1 second/scan. The Waters/Micromass ProteinLynx software (version 1.1) was used to create tabulated MS/MS spectra (peak lists) from the raw data. These peak lists were input into a Mascot database searching program,18 which matches the observed spectrum with those from a theoretical digest of all of the proteins in the database.19 The MS/MS data matched fragmentation data from a theoretical tryptic digest of Rap1a and Rap1b with a confidence level of P less than .05.
Results
Determination of the presence of Rap1 in RBCs
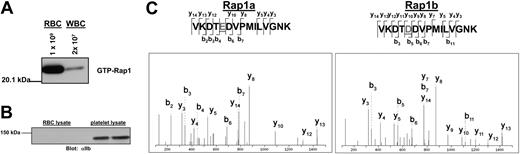
Although Rap1 is abundant in platelets and WBCs, its existence in RBCs has never been examined. To confirm that Rap1 exists in RBCs, active Rap1 was precipitated with GST-RalGDS-RBD-conjugated beads, which bind selectively to the GTP-associated form of Rap1.20 Rap1 was then detected on Western blots with a Rap1-specific antibody. As shown in Figure 1A, RBCs exhibited a robust basal Rap1 activation signal. While contamination of our RBC preparations by platelets was generally undetectable, a low level of contamination by WBCs was possible. We therefore examined the preparation microscopically and found the WBC contamination levels to be less than 0.01%. To determine whether Rap1 from contaminating WBCs contributes to the observed Rap1 signal, a concentration of WBCs corresponding to 2% contamination, an approximately 200-fold overestimate of the contamination level, was examined for active Rap1. WBCs contain predominantly Rap1a, which is readily detected by the antibody used for the Western blot.21 However, a minimal signal was observed (Figure 1A), making it apparent that the observed RBC signal is in fact derived predominantly from RBCs. Additionally, to insure that there was minimal platelet contamination in the RBC preparation, RBC lysate was probed for the αIIb integrin subunit, which is expressed abundantly on the surface of platelets. As shown in Figure 1B, no platelet contamination was apparent in the RBC preparation, although the αIIb signal was abundant in platelet lysate. Moreover, no αIIb signal could be observed in the RBC preparation after overexposure of this Western blot (data not shown), further indicating that there is no detectable platelet contamination.
Rap1 is present in a pure fraction of red blood cells. (A) WBC contamination does not contribute to the observed Rap1 signal. RBCs were counted with a Coulter cell counter and diluted to 1 × 109 cells/mL. WBCs were counted microscopically on a hemacytometer and adjusted to 2 × 107 cells/mL (representing a 2% contamination level). The cells were lysed and subjected to a GST-RalGDS-RBD pull-down assay. Rap1 was detected by Western blotting with a Rap1-specific antibody. (B) Platelet contamination is not detectable in the RBC preparation. RBC and platelet lysates were probed by Western blotting with an αIIb-specific antibody as described in “Patients, materials, and methods.” (C) Both Rap1a and Rap1b are present in RBCs. Shown are MS/MS spectra corresponding to peptides from Rap1a and Rap1b obtained from tryptic digestion of Rap protein from a GST-RalGDS-RBD pull-down of RBCs.
Rap1 is present in a pure fraction of red blood cells. (A) WBC contamination does not contribute to the observed Rap1 signal. RBCs were counted with a Coulter cell counter and diluted to 1 × 109 cells/mL. WBCs were counted microscopically on a hemacytometer and adjusted to 2 × 107 cells/mL (representing a 2% contamination level). The cells were lysed and subjected to a GST-RalGDS-RBD pull-down assay. Rap1 was detected by Western blotting with a Rap1-specific antibody. (B) Platelet contamination is not detectable in the RBC preparation. RBC and platelet lysates were probed by Western blotting with an αIIb-specific antibody as described in “Patients, materials, and methods.” (C) Both Rap1a and Rap1b are present in RBCs. Shown are MS/MS spectra corresponding to peptides from Rap1a and Rap1b obtained from tryptic digestion of Rap protein from a GST-RalGDS-RBD pull-down of RBCs.
To provide additional confirmation that Rap1 is present in RBCs and to determine which of the Rap1 isoforms are present, a sample of Rap1 precipitated from RBCs was trypsin digested and subjected to analysis by tandem mass spectrometry. Peptides corresponding to both Rap1a and Rap1b, respectively, were detected (Figure 1C).
Rap1 activation in SS RBCs is promoted via cAMP signaling
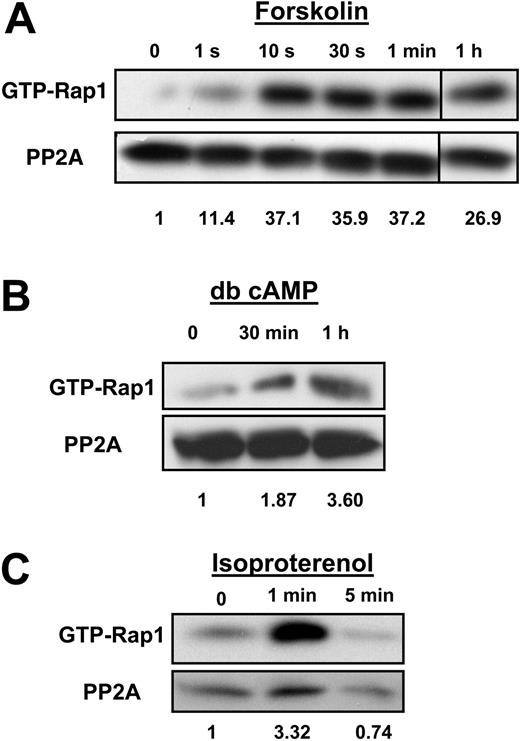
To understand Rap1 function in SS RBCs, it is important to identify the pathway by which Rap1 becomes activated. A common upstream activator of Rap1 in a number of cell types is cAMP.22,23 Also, our recent studies have shown that that cAMP production in SS RBCs enhances SS RBC adhesion to laminin in a significant subset of patients (46% of the SCD patients studied).1 We therefore speculated that cAMP production might promote Rap1 activation in these cells. We found that cAMP pathway-specific agonists promote Rap1 activation in SS RBCs. Treatment of cells with forskolin, a direct activator of adenylyl cyclase, which stimulates cAMP production, in conjunction with the phosphodiesterase inhibitor IBMX, promoted rapid activation of Rap1 that was sustained for at least 1 hour (Figure 2A). The onset of Rap1 activation corresponded closely to the onset of cAMP production caused by forskolin treatment of SS RBCs that has been previously reported in multiple SCD patients.1 SS RBCs treated with the cell-permeable cAMP analog dibutyryl (db) cAMP also exhibited Rap1 activation (Figure 2B). The activation was less rapid, most likely due to the time needed for dibutyryl cAMP to diffuse into the cell.24 Treatment of cells with the β2 adrenergic receptor agonist isoproterenol promoted activation of Rap1 within 1 minute (Figure 2C). The onset of Rap1 activation corresponded closely to the time previously reported for the β-adrenergic receptor agonist epinephrine to promote cAMP production in SS RBCs from multiple patients.1 Thus, cAMP pathway-specific agonists promote Rap1 activation in SS RBCs.
Rap1 is activated by the cAMP pathway in SS RBCs. (A) Forskolin stimulates Rap1 in SS RBCs. Cells were pretreated with 200 μM IBMX for 30 minutes to inhibit phosphodiesterase activity. The cells were subsequently treated with 80 μM forskolin for the time points shown. After lysis, GTP-bound Rap1 was precipitated with GST-RalGDS-RBD beads and detected with a Rap1-specific antibody by Western blotting. The numbers shown below the blot are relative densitometry values obtained by taking a ratio of the densitometry value obtained from the Rap1 band to its corresponding PP2A loading control band. Values were then normalized to the zero time point. Densitometry for panels B and C was also analyzed in this manner. The 1-hour time point is from the same Western blot as the other time points. Data are representative of experiments from 3 different patient samples. (B) Dibutyryl (db) cAMP activates Rap1. SS RBCs were treated with 200 μM db cAMP for the indicated time points. GTP-bound Rap1 was detected as in panel A. Data are representative of experiments from 5 different patient samples. (C) Rap1 is activated by isoproterenol in SS RBCs. Cells were treated with 100 μM isoproterenol for the time points indicated. After lysis, GTP-bound Rap1 was detected as in panel A. Data are representative of experiments from 5 different patient samples.
Rap1 is activated by the cAMP pathway in SS RBCs. (A) Forskolin stimulates Rap1 in SS RBCs. Cells were pretreated with 200 μM IBMX for 30 minutes to inhibit phosphodiesterase activity. The cells were subsequently treated with 80 μM forskolin for the time points shown. After lysis, GTP-bound Rap1 was precipitated with GST-RalGDS-RBD beads and detected with a Rap1-specific antibody by Western blotting. The numbers shown below the blot are relative densitometry values obtained by taking a ratio of the densitometry value obtained from the Rap1 band to its corresponding PP2A loading control band. Values were then normalized to the zero time point. Densitometry for panels B and C was also analyzed in this manner. The 1-hour time point is from the same Western blot as the other time points. Data are representative of experiments from 3 different patient samples. (B) Dibutyryl (db) cAMP activates Rap1. SS RBCs were treated with 200 μM db cAMP for the indicated time points. GTP-bound Rap1 was detected as in panel A. Data are representative of experiments from 5 different patient samples. (C) Rap1 is activated by isoproterenol in SS RBCs. Cells were treated with 100 μM isoproterenol for the time points indicated. After lysis, GTP-bound Rap1 was detected as in panel A. Data are representative of experiments from 5 different patient samples.
Role of the exchange factor Epac in cAMP-mediated stimulation of Rap1
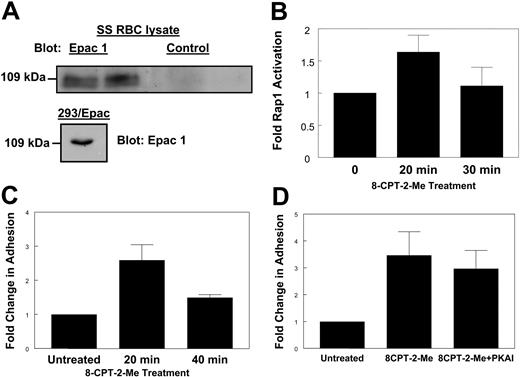
A guanine nucleotide exchange factor known to activate Rap1 in response to cAMP is Epac.25 Since cAMP appears to cause Rap1 activation in SS RBCs, we asked whether Epac was involved in this activation. Because the presence of Epac in SS RBCs has never been examined, we probed SS RBC lysates with an antibody known to be efficient for detecting Epac on Western blots.26 A band at approximately 110 kDa, where Epac is typically observed, was apparent upon probing either SS RBC lysate or lysate from HEK293 cells stably overexpressing Epac26 with the Epac-specific antibody but not with a control antibody (Figure 3A). This indicates the presence of Epac in SS RBCs.
Epac contributes to Rap1 activation in SS RBCs. (A) Epac is present in SS RBCs. SS RBC lysate or HEK293/Epac lysate was separated by SDS-PAGE. The proteins were then transferred to PVDF membrane and Western blotted with a rabbit anti-human Epac1 antibody (left and bottom) or a rabbit control antibody (right). Duplicate lanes are shown. (B) Treatment with an Epac-specific cAMP analog stimulates Rap1. SS RBCs were treated with 100 μM 8CPT-2-Me at the indicated time points. The cells were lysed, and GTP-bound Rap1 was detected as in Figure 1A. Densitometry values were calculated as described in Figure 2A. Results are expressed as mean ± SE from 4 separate experiments. (C) Stimulation of Rap1 via Epac promotes cellular adhesion to laminin. SS RBCs were treated with 100 μM 8CPT-2-Me at the times indicated. While still in the presence of 100 μM 8CPT-2-Me, the cells were then flowed over chambers coated with 0.75 μg laminin in a flow adhesion assay. Adhesion was quantified as described in “Patients, materials, and methods.” Results are expressed as mean ± SE from 1 of 3 similar experiments. (D) Inhibition of PKA has no effect on 8CPT-2-Me-stimulated adhesion. SS RBCs were untreated or pretreated with 87 nM PKAI for 1 hour and then 100 μM 8CPT-2-Me was added in with the PKAI for 20 minutes. The cells, while still in the presence of these pharmacologic agents, were flowed across chambers coated with 0.75 μg laminin in a flow adhesion assay. Adhesion was quantified as described in “Patients, materials, and methods.” Results are expressed as mean ± SE from 4 separate experiments.
Epac contributes to Rap1 activation in SS RBCs. (A) Epac is present in SS RBCs. SS RBC lysate or HEK293/Epac lysate was separated by SDS-PAGE. The proteins were then transferred to PVDF membrane and Western blotted with a rabbit anti-human Epac1 antibody (left and bottom) or a rabbit control antibody (right). Duplicate lanes are shown. (B) Treatment with an Epac-specific cAMP analog stimulates Rap1. SS RBCs were treated with 100 μM 8CPT-2-Me at the indicated time points. The cells were lysed, and GTP-bound Rap1 was detected as in Figure 1A. Densitometry values were calculated as described in Figure 2A. Results are expressed as mean ± SE from 4 separate experiments. (C) Stimulation of Rap1 via Epac promotes cellular adhesion to laminin. SS RBCs were treated with 100 μM 8CPT-2-Me at the times indicated. While still in the presence of 100 μM 8CPT-2-Me, the cells were then flowed over chambers coated with 0.75 μg laminin in a flow adhesion assay. Adhesion was quantified as described in “Patients, materials, and methods.” Results are expressed as mean ± SE from 1 of 3 similar experiments. (D) Inhibition of PKA has no effect on 8CPT-2-Me-stimulated adhesion. SS RBCs were untreated or pretreated with 87 nM PKAI for 1 hour and then 100 μM 8CPT-2-Me was added in with the PKAI for 20 minutes. The cells, while still in the presence of these pharmacologic agents, were flowed across chambers coated with 0.75 μg laminin in a flow adhesion assay. Adhesion was quantified as described in “Patients, materials, and methods.” Results are expressed as mean ± SE from 4 separate experiments.
To determine if Epac signaling was contributing to Rap1 activation in SS RBCs, we used the Epac-selective cAMP analog 8CPT-2-Me. This cAMP analog is the product of rational design and has been well characterized to bind and activate Epac without causing activation of protein kinase A (PKA).27 SS RBCs were treated with a concentration of 8CPT-2-Me known to promote maximal activation of Rap1.28 Treatment with 8CPT-2-Me promoted robust Rap1 activation as shown in Figure 3B, providing evidence that Epac is involved in the cAMP/Rap1 pathway in SS RBCs. The onset of Rap1 activation occurred at 20 minutes, as shorter time points did not show Rap1 activation (data not shown). There was slight activation of Rap1 under basal conditions. This basal activation of Rap1 has been observed in other cell types and its cause is unknown.11,29 RBC samples from 48% of sickle cell patients (12 of 25) were consistently responsive to 8CPT-2-Me. This corresponds closely to the 46% of patients found to be responsive to cAMP-specific agonists demonstrated in a previous study.1 Therefore, for subsequent assays, the 8CPT-2-Me responder population of patients was used to further characterize the Rap1 signaling pathway and its effects on SS RBC adhesion. We introduced this selection bias in order to better isolate and understand this potentially adhesive signaling pathway in SS RBCs.
Role of Rap1 in promoting SS RBC adhesion to laminin
Previous studies have shown that SS RBC treatment with epinephrine, forskolin, and dibutyryl cAMP promotes adhesion to laminin.1 Since Rap1 is activated by the Epac-specific cAMP analog 8CPT-2-Me, and since elevated cAMP is known to increase SS RBC adhesion to laminin, we asked whether 8CPT-2-Me treatment also increased SS RBC adhesion to laminin via Epac activation.1 Indeed, SS RBCs treated with 8CPT-2-Me exhibited increased adhesion to immobilized human laminin in a flow adhesion assay with a time course corresponding to the onset of Rap1 activation in these cells (Figure 3C). The 8CPT-2-Me treatment did not promote RBC adhesion to laminin in healthy patients (AA RBCs; data not shown). To insure that any observed adhesion to laminin via 8CPT-2-Me was not due to PKA activation by 8CPT-2-Me, RBCs were treated with 8CPT-2-Me in the presence of the PKA inhibitor, 14-22 amide (PKAI), which has previously been shown to inhibit PKA-dependent SS RBC adhesion to laminin.1 As shown in Figure 3D, PKAI treatment induced a small but statistically insignificant decrease in SS RBC adhesion to laminin (P > .05). Thus, the adhesion is dependent on Epac/Rap1 signaling and not on PKA signaling.
Role of Epac and Rap1 expression levels in 8CPT-2-Me-induced responses
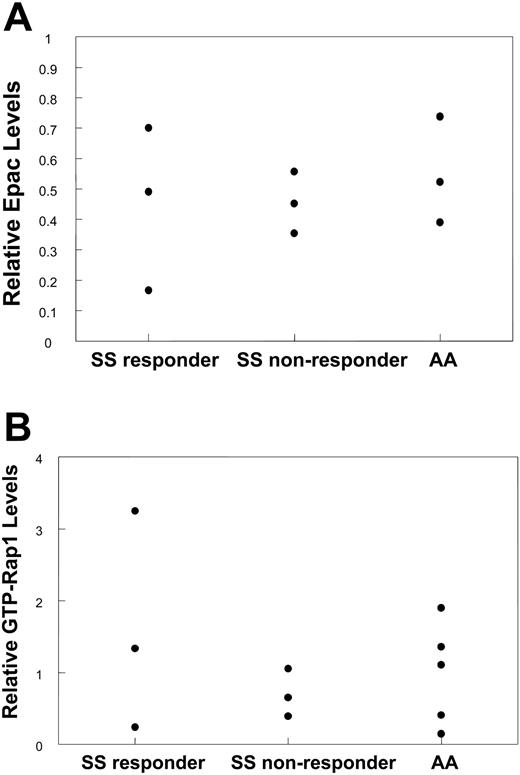
One possible explanation for the difference in response to 8CPT-2-Me among SS responder and nonresponder populations as well as in healthy (AA) persons is that the levels of Epac and/or Rap1 expression may be different in these populations. To explore this possibility, RBC lysate samples were probed for Epac. As shown in Figure 4A, there were no significant differences in Epac levels between SS responder, SS nonresponder, and AA RBCs, indicating that the level of Epac expression does not have a bearing on the response to 8CPT-2-Me. To determine if basal levels of Rap1 activation have an effect on the propensity to respond to 8CPT-2-Me, the basal Rap1 activation levels were compared between these populations. Figure 4B shows that Rap1 levels do not affect the response to laminin, as SS responders with relatively high and very low levels of Rap1 both respond to 8CPT-2-Me.
Response to 8CPT-2-Me is not dependent on Epac or Rap1 protein levels in SS RBCs. (A) Response to 8CPT-2-Me is not dependent on the levels of Epac protein. An equivalent concentration of RBC lysate was derived from 9 previously characterized donors relative to 8CPT-2-Me-induced RBC adhesion to laminin (3 SS responders, 3 SS nonresponders, and 3 AAs). The lysate was separated on a 6% SDS polyacrylamide gel under reducing conditions, transferred to a PVDF membrane, and blotted for Epac1 with an Epac1 monoclonal antibody. PP2A was used as a loading control. Relative Epac protein levels were determined by taking a ratio of the densitometry value obtained from the Epac sample to the densitometry value from the corresponding PP2A loading control and plotted for each patient. (B) Response to 8CPT-2-Me is independent of basal GTP-Rap1 levels. RBC lysates from 11 patients (3 SS responders, 3 SS nonresponders, and 5 AAs) of equivalent RBC concentration were assayed for GTP-Rap1 with GST-RalGDS-RBD beads. Rap1 was then detected on a Western blot with a Rap1-specific antibody, with PP2A as a loading control. Relative Rap1 values were obtained by densitometry as described in Figure 2A. The relative GTP-Rap1 value obtained for each patient is represented as a dot.
Response to 8CPT-2-Me is not dependent on Epac or Rap1 protein levels in SS RBCs. (A) Response to 8CPT-2-Me is not dependent on the levels of Epac protein. An equivalent concentration of RBC lysate was derived from 9 previously characterized donors relative to 8CPT-2-Me-induced RBC adhesion to laminin (3 SS responders, 3 SS nonresponders, and 3 AAs). The lysate was separated on a 6% SDS polyacrylamide gel under reducing conditions, transferred to a PVDF membrane, and blotted for Epac1 with an Epac1 monoclonal antibody. PP2A was used as a loading control. Relative Epac protein levels were determined by taking a ratio of the densitometry value obtained from the Epac sample to the densitometry value from the corresponding PP2A loading control and plotted for each patient. (B) Response to 8CPT-2-Me is independent of basal GTP-Rap1 levels. RBC lysates from 11 patients (3 SS responders, 3 SS nonresponders, and 5 AAs) of equivalent RBC concentration were assayed for GTP-Rap1 with GST-RalGDS-RBD beads. Rap1 was then detected on a Western blot with a Rap1-specific antibody, with PP2A as a loading control. Relative Rap1 values were obtained by densitometry as described in Figure 2A. The relative GTP-Rap1 value obtained for each patient is represented as a dot.
Lack of integrin dependence of Epac/Rap mediated adhesion to laminin
Since Rap1 promotes integrin-mediated adhesion of several cell types,28,30 and since laminin contains an integrin-binding RGD sequence, we asked whether the adhesion of SS RBCs promoted by Rap1 was mediated by this sequence.31,32 However, 8CPT-2-Me-stimulated adhesion to laminin was not affected by treatment with an RGD peptide, suggesting that the adhesion to laminin is not integrin dependent (Figure 5A). The concentration of RGD peptide used here was previously shown by us to block α4β1 integrin-mediated adhesion of SS RBCs to immobilized thrombospondin under flow conditions.2
In addition to containing an RGD sequence, laminin also contains putative integrin-binding LDV sequences. Since α4β1 can adhere to proteins in an LDV-dependent manner, we next sought to determine if the observed adhesion to laminin was LDV dependent. A concentration of LDV peptide was used that was previously shown to block α4β1-mediated adhesion of SS RBCs to VCAM-1 under flow conditions.2 Pretreatment of 8CPT-2-Me-stimulated SS RBCs with a fibronectin-derived LDV peptide did not block adhesion to laminin (Figure 5B). This indicates that 8CPT-2-Me-stimulated adhesion to laminin does not occur via the laminin LDV sequence.
The only known integrin on SS RBCs is α4β1,8 which can bind to substrates in both an RGD-dependent and -independent manner.33,34 While α4β1 is not a known receptor for laminin, we further explored its potential role by preincubating cells with α4 and β1 integrin subunit antibodies that have been shown previously to block α4β1-mediated adhesion of SS RBCs under flow conditions.2 These antibodies did not block 8CPT-2-Me-stimulated adhesion to laminin, providing further evidence that the observed adhesion was not integrin dependent, or specifically, α4β1 dependent (Figure 5C).
Although α4β1 appeared not to be the receptor mediating SS RBC adhesion to laminin, it was still possible that Rap1 activation could promote α4β1-mediated adhesion to an authentic α4β1 substrate. It was also important to confirm that the SS RBCs being studied contained functional α4β1 such that the lack of integrin-mediated adhesion being observed could not be attributed to a lack of ability of the α4β1 integrin on these SS RBCs to mediate adhesion. Therefore, adhesion of SS RBCs to the α4β1-selective cell adhesion molecule VCAM-1 was examined in a flow adhesion assay.2 Treatment with 8CPT-2-Me did not promote adhesion to an immobilized, soluble version of VCAM-1. However, these cells did adhere to VCAM-1 in response to a positive control, the 4N1K peptide derived from thrombospondin, which is known to promote α4β1-mediated SS RBC adhesion to VCAM-1 via activation of CD47 on these cells (Figure 5D).2
Adhesion to laminin via Epac/Rap1 is mediated by the BCAM/LU receptor
Since the 8CPT-2-Me-induced adhesion to laminin appears to occur independent of integrins, we explored the possibility that Rap1 could promote adhesion via a nonintegrin-dependent mechanism. A candidate receptor for mediating SS RBC adhesion to laminin is the BCAM/LU receptor, a member of the Ig superfamily of receptors, recently shown to be the major receptor mediating cAMP-stimulated SS RBC adhesion to laminin.1 A soluble form of BCAM/LU was used to determine if it could compete for SS RBC binding. Blockage of immobilized laminin with a soluble form of the BCAM/LU receptor abrogated 8CPT-2-Me-stimulated adhesion to laminin (Figure 6A). Additionally, preincubation of immobilized laminin with another member of the Ig superfamily, a soluble form of VCAM-1, did not affect adhesion promoted by 8CPT-2-Me (Figure 6B). To provide additional confirmation that the stimulated adhesion was being mediated via BCAM/LU, SS RBCs were preincubated with a BCAM/LU function-blocking antibody. As shown in Figure 6C, treatment with the BCAM/LU blocking antibody, but not with a control IgG antibody, almost completely inhibited adhesion stimulated by 8CPT-2-Me. Taken together, these data demonstrate that BCAM/LU is the major receptor by which the Epac/Rap1 pathway in SS RBCs promotes adhesion to laminin.
Rap1 promotes adhesion to laminin via the BCAM/LU receptor. (A) Rap1 promotes adhesion to laminin via the BCAM/LU receptor. SS RBCs were treated with 100 μM 8CPT-2-Me for 20 minutes and then flowed over chambers coated with either 0.75 μg laminin or 0.75 μg laminin blocked by precoating the immobilized laminin with 3 μg soluble BCAM/LU in 75 μL of PBS for 3 hours at 37°C in a flow adhesion assay. Results shown are expressed as mean ± SE from 2 separate experiments. (B) Soluble VCAM does not block adhesion to laminin. Immobilized laminin (0.75 μg) either was not precoated or precoated with 50 μg/mL soluble VCAM-1. SS RBCs were treated with 100 μM 8CPT-2-Me for 20 minutes. The cell suspension, while still in the presence of 8CPT-2-Me at the aforementioned concentration, was flowed across 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 2 separate experiments. (C) The BCAM/LU receptor mediates adhesion to laminin stimulated via Epac/Rap1. SS RBCs were either untreated or preincubated with either 25 μg/mL BCAM adhesion-blocking antibody or an equivalent concentration of IgG control antibody for 1 hour. The RBCs or RBC/antibody mixture were then treated with 100 μM 8CPT-2-Me for 20 minutes and, while still in the presence of antibody and 8CPT-2-Me at the aforementioned concentrations, flowed over channels coated with 0.75 μg laminin in a flow adhesion assay. Results shown are expressed as mean ± SE from 4 separate experiments.
Rap1 promotes adhesion to laminin via the BCAM/LU receptor. (A) Rap1 promotes adhesion to laminin via the BCAM/LU receptor. SS RBCs were treated with 100 μM 8CPT-2-Me for 20 minutes and then flowed over chambers coated with either 0.75 μg laminin or 0.75 μg laminin blocked by precoating the immobilized laminin with 3 μg soluble BCAM/LU in 75 μL of PBS for 3 hours at 37°C in a flow adhesion assay. Results shown are expressed as mean ± SE from 2 separate experiments. (B) Soluble VCAM does not block adhesion to laminin. Immobilized laminin (0.75 μg) either was not precoated or precoated with 50 μg/mL soluble VCAM-1. SS RBCs were treated with 100 μM 8CPT-2-Me for 20 minutes. The cell suspension, while still in the presence of 8CPT-2-Me at the aforementioned concentration, was flowed across 0.75 μg laminin in a flow adhesion assay. Results are expressed as mean ± SE from 2 separate experiments. (C) The BCAM/LU receptor mediates adhesion to laminin stimulated via Epac/Rap1. SS RBCs were either untreated or preincubated with either 25 μg/mL BCAM adhesion-blocking antibody or an equivalent concentration of IgG control antibody for 1 hour. The RBCs or RBC/antibody mixture were then treated with 100 μM 8CPT-2-Me for 20 minutes and, while still in the presence of antibody and 8CPT-2-Me at the aforementioned concentrations, flowed over channels coated with 0.75 μg laminin in a flow adhesion assay. Results shown are expressed as mean ± SE from 4 separate experiments.
Discussion
Our results demonstrate that Rap1 is present and activated in response to agonists in SS RBCs and contributes to their adhesion to laminin, a protein present in both the blood vessel wall and in SS patient plasma.35 Hence, Rap1 may be an important mediator of signaling leading to vasoocclusion. Tandem mass spectrometry data reveal that peptides corresponding to both Rap1a and Rap1b are present, indicating that both Rap1 isoforms exist in RBCs. Rap1 in SS RBCs can be activated by the agonists isoproterenol, forskolin, and db cAMP, implicating a cAMP-dependent mechanism of Rap1 activation in these cells. The role of cAMP in Rap1 activation has gained increased attention with the discovery of Epac, a cAMP-dependent Rap1 GEF, and development of the pharmacologic tool 8CPT-2-Me, a cAMP analog that has been well characterized to specifically bind and activate Epac but not PKA.27 Western blotting of SS RBC lysate confirmed the presence of Epac in these cells. This is the first evidence that Epac is present in RBCs. By use of the analog 8CPT-2-Me, we found that Epac contributes to cAMP-induced activation of Rap1 in SS RBCs. Additionally, 8CPT-2-Me-induced Rap1 activation promotes SS RBC adhesion to the extracellular matrix protein laminin that was not reduced by PKA inhibition. Since SS RBCs are anucleate, making transfection impossible, treatment with 8CPT-2-Me is the only readily available means of studying Epac function in these cells.
Only a subset of patients (48%) responds to 8CPT-2-Me. Patient variability, which manifests as “responder” and “nonresponder” SCD patient populations to particular cAMP-specific agonists, has been previously described.1 We demonstrate that the levels of Epac and/or Rap1 in RBCs have no bearing on whether or not there is a response to 8CPT-2-Me. Thus, the factors that cause a certain population of SS RBCs to respond to this treatment are likely complex and multifactorial. As is the case with many other chronic diseases, this may be a reflection of the broad range of clinical manifestations and severity observed with SCD, which may be due to such issues as complex genetic backgrounds and/or other clinical factors.
Another possibility may be that the levels of downstream effectors between Rap1 and the BCAM/LU receptor are different in these 2 populations. Thus, the SCD patients who respond to 8CPT-2-Me have all of the signaling components in place to mount an adhesive response via the BCAM/LU receptor. In the nonresponder populations, some or all of these signaling components may be missing, making an adhesive response impossible.
There has been some conjecture that the vasoocclusive manifestations of SCD are due to the high number of circulating reticulocytes found in SCD patients. However, previous studies have demonstrated that the reticulocytes are not the RBC population involved in cAMP-dependent SS RBC adhesion to laminin.1 In fact, when the fold increase in adhesion to laminin in response to epinephrine, via cAMP signaling, was plotted against the reticulocyte count of each corresponding patient, no correlation was observed.1 Further, an increase in the number of reticulocytes within individual patients did not cause an increased epinephrine-induced adhesive response to laminin.1 In addition, persons with other hematologic disorders that cause high reticulocyte counts do not necessarily experience vasoocclusive events like those observed in SCD. It was therefore concluded that the responsive, adhesive SS RBC population was at an intermediate stage of development relative to the immature reticulocytes and more mature erythrocytes.1 As RBCs mature, they lose their signaling capacity, with fully mature RBCs having very little signaling capability. While reticulocytes per se do not appear to cause the increase in epinephrine- or cAMP-stimulated adhesion, the overall population of RBCs in patients with SCD is younger. Thus, SS RBCs still retain more signaling capacity than a population of more mature AA RBCs and this makes the cells more likely to be responsive to agonists.
In addition, the vasculature of persons with SCD tends to be extensively damaged. This damage exposes extracellular matrix proteins, including laminin, to flowing blood, providing a substrate for the adhesive SS RBCs. This, in conjunction with the hypercoagulant and proinflammatory state in which SCD patients typically exist, provides an environment that is far more amenable to cellular adhesion and vasoocclusion than would typically be found in normal human physiology.
An additional reason that SS RBCs exhibit more adhesion to laminin compared with AA RBCs may be due to differences in the expression of the BCAM/LU protein on the cell surface. A previous study revealed that SS RBCs contain 67% more BCAM/LU than AA RBCs.36 This substantial increase in the amount of BCAM/LU could contribute to the increased adhesive potential of SS RBCs.
SS RBCs provide a unique system for studying Rap1-promoted cellular adhesion, as they contain only one integrin, α4β1.8,9 Since laminin contains an RGD sequence,32 the potential existed for Rap1-stimulated adhesion to laminin to be RGD dependent and integrin dependent. Although α4β1-mediated adhesion is not necessarily RGD dependent, it is in some instances. For example, we have shown that α4β1-mediated adhesion of SS RBCs to thrombospondin is RGD dependent and another study has demonstrated that α4β1-mediated adhesion of B cells to fibronectin is RGD dependent.2,34 However, SS RBC adhesion to laminin via Rap1 was not inhibited by an RGD peptide. Also, incubation of SS RBCs with the LDV peptide, another sequence in laminin that is potentially recognized by integrins, had no effect on Rap1-promoted adhesion to laminin. In addition, incubation of SS RBCs with α4 and β1 integrin subunit adhesion-blocking antibodies did not affect the stimulated adhesion, confirming that α4β1 was not the Rap1-stimulated receptor responsible for mediating the adhesion to laminin.
Upon elimination of α4β1 as the Rap1-stimulated receptor, we next considered the Ig superfamily member, BCAM/LU, as it has been shown to mediate cAMP-dependent SS RBC adhesion to laminin.1 Since both a soluble form of BCAM/LU and a BCAM/LU function-blocking antibody abrogated the adhesion to laminin promoted via the Epac/Rap1 pathway, it appears that Rap1 in these cells promotes adhesion via a nonintegrin adhesion receptor. This is the first time that Rap1 has been shown to promote adhesion via an Ig superfamily member. However, it is not the first time that Rap1 has been linked to an integrin-independent adhesive process. There is evidence that Rap1 may be involved in cell-cell contact by modulating adherens junctions. In a recent study, it was reported that Rap1 is localized in adherens junctions and is involved in the localization of adherens junctions within cells.37 Another study demonstrated that Rap1 may be involved in cell-cell adhesion mediated by E-cadherin.11
Surprisingly, we found that Epac-mediated Rap1 activation did not induce α4β1-mediated adhesion to a known α4β1 substrate, VCAM-1, even though adhesion via this integrin could be activated by another signaling pathway. This finding spawns the question of why a nonintegrin adhesion receptor is activated by Rap1 to the exclusion of an integrin, when integrins, including α4β1, are known to be activated by Rap1 signaling.38 The answer to this question may well exist in the least well-understood aspect of Rap1 signaling: the downstream pathways by which Rap1 signals to adhesion receptors. It is reasonable to assume that the signaling molecules downstream of Rap1 responsible for activating integrins would be different from the signaling molecules responsible for activating other classes of adhesion receptors.
Because Rap1 has been studied intensively in the integrin field, less attention has been paid to Rap1-mediated activation of adhesion via other adhesion receptor families. Since BCAM/LU is a member of the Ig superfamily of adhesion receptors, it is possible that Rap1 may also promote adhesion via other Ig superfamily members.
A previous study in our laboratory demonstrated that the cAMP-dependent adhesion of SS RBCs to laminin via BCAM/LU was PKA dependent.1 In this study, we demonstrate that BCAM/LU-mediated adhesion to laminin promoted by Epac/Rap1 signaling occurs independent of PKA. Thus, it appears that cAMP signaling can promote adhesion to laminin via BCAM/LU through 2 divergent signaling pathways. The observation that adhesion promoted by epinephrine, which can signal via both PKA and Epac, can be blocked via PKA inhibition suggests that these pathways may synergize. Thus, the potential exists for both pathways to act individually or in concert to promote an adhesive, vasoocclusive pathology. The relative contributions of these signaling pathways to promoting SS RBC adhesion and vasoocclusion could be a subject of future investigation. Obtaining a better understanding of adhesive signaling in SS RBCs with regard to Rap1 as well as other pathways may lead to more targeted approaches for preventing and treating vasoocclusion.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-07-2881.
Supported by the National Institutes of Health RR0046 to the UNC General Clinical Research Center, 1-RO1-HL67440-01 (L.V.P.) HL58939 (M.J.T., L.V.P.) and HL63409 (M.J.T.).
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Eugene Orringer, Dell Strayhorn, and Susan Jones at the UNC Comprehensive Sickle Cell Center and Shantres Clark in the Parise laboratory for their assistance with obtaining patient blood samples. We thank Julia Brittian for the 4N1K peptide and technical advice. The protein identification work was performed at the UNC Michael Hooker Proteomics Core facility. We thank Christine Eyler at Duke University for purified BCAM/LU. We would also like to thank Xiaodong Cheng at University of Texas, Galveston for the HEK293/Epac lysate.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal