Abstract
The zebrafish is an attractive vertebrate model for genetic studies of development, apoptosis, and cancer. Here we describe a transgenic zebrafish line in which T- and B-lymphoid cells express a fusion transgene that encodes the zebrafish bcl-2 protein fused to the enhanced green fluorescence protein (EGFP). Targeting EGFP-bcl-2 to the developing thymocytes of transgenic fish resulted in a 2.5-fold increase in thymocyte numbers and a 1.8-fold increase in GFP-labeled B cells in the kidney marrow. Fluorescent microscopic analysis of living rag2-EGFP-bcl-2 transgenic fish showed that their thymocytes were resistant to irradiation- and dexamethasone-induced apoptosis, when compared with control rag2-GFP transgenic zebrafish. To test the ability of bcl-2 to block irradiation-induced apoptosis in malignant cells, we compared the responsiveness of Myc-induced leukemias with and without EGFP-bcl-2 expression in living transgenic zebrafish. T-cell leukemias induced by the rag2-EGFP-Myc transgene were ablated by irradiation, whereas leukemias in double transgenic fish expressing both Myc and EGFP-bcl-2 were resistant to irradiation-induced apoptotic cell death. The forward genetic capacity of the zebrafish model system and the ability to monitor GFP-positive thymocytes in vivo make this an ideal transgenic line for modifier screens designed to identify genetic mutations or small molecules that modify bcl-2-mediated antiapoptotic pathways. (Blood. 2005;105:3278-3285)
Introduction
Apoptosis is a critical mechanism for regulating homeostasis and tissue remodeling during development. Thus, it is not surprising that many of the molecular components and pathways regulating this process have been conserved throughout evolution.1-4 Apoptosis is also essential for hematopoietic cell development in vertebrates.5,6 For example, regulated programmed cell death is critical for the maintenance of hematopoietic stem cell numbers7,8 and for normal T-lymphocyte development.9-12 Failure of lymphoid cells to undergo cell death can contribute to oncogenic transformation, and in fact, the suppression of apoptosis through key mutations in one or more regulatory pathways is often a critical component of malignant transformation.13-16 Although many of the molecular pathways that regulate apoptosis are shared among vertebrate and nonvertebrate organisms, evolution has led to increased complexity in the pathways regulating programmed cell death in higher organisms. Unlike worms and flies, vertebrates have multiple antiapoptotic and proapoptotic Bcl-2 family members.17 Thus, it is not surprising that loss-of-function studies in mice have demonstrated tissue-specific roles for many of these proteins, and in other cases have suggested redundant functions due to the expression of more than one family member.9,10,18-23 Given the remarkable complexity associated with the control of apoptosis in vertebrates, new insights may be gained from studying cell death pathways in vertebrate forward-genetic model systems, such as the zebrafish.
Vertebrate evolution has resulted in the development of new organ systems; thus, regulation of cell death pathways within vertebrate-specific tissue types cannot be directly studied in invertebrate species. Although Drosophila have blood cells, they do not have the same blood cell lineages as vertebrates and many of the tissues that regulate vertebrate blood formation are absent in flies,24 including the thymus and marrow. Thus, the regulation of cell death pathways in T and B cells cannot be studied directly in genetically tractable invertebrate models. By contrast, zebrafish have conserved genetic programs underlying vertebrate blood development,25 and like their mammalian counterparts, fish have both T and B cells.26-28 Finally, many of the genes that regulate apoptosis in the zebrafish appear to be conserved in mammals.29 Zebrafish homologs of bad,30 bax,29 bcl-xL,31 mcl-1,32 caspase-3,33 caspase-8, and noxa have been identified; however, few studies have addressed the ability of the zebrafish proteins to suppress or induce apoptosis in vivo.
Here we report the identification and functional analysis of the zebrafish bcl-2 homolog. We show that a chimeric fusion protein between zebrafish bcl-2 and the enhanced green fluorescent protein (EGFP) is able to block irradiation- and dexamethasone-induced apoptosis when targeted to the lymphocytes. Transgenic rag2-EGFP-bcl-2 fish have increased numbers of thymocytes and B cells when compared with control fish. Finally, Myc-induced leukemias can be ablated by γ-irradiation, but EGFP-bcl-2-expressing leukemias are resistant to irradiation-induced apoptosis. Thus, bcl-2-mediated suppression of apoptosis in thymocytes is remarkably conserved in zebrafish compared with mammals. Studies in the zebrafish may lead to new insights into the mechanisms by which B and T lymphocytes undergo cell death during lymphocyte development.
Materials and methods
Animals
Cloning of the zebrafish bcl-2 gene
A degenerate polymerase chain reaction (PCR) strategy was used to isolate a fragment of the zebrafish bcl-2 cDNA. RNA was extracted from 6-, 24-, and 96-hour-old embryos (Trizol; Invitrogen, Carlsbad, CA) and cDNA fragments were generated by reverse transcription. Degenerate PCR primers (forward: GAYGGIGTIAAYTGRGGIMGIAT; reverse: CAICCICCRTTITCYTGIATCCA) amplified a 167-nucleotide fragment, which was cloned into pGEMT-easy (Promega, Madison, WI) and sequenced. A full-length clone was obtained by screening an embryo cDNA library (RZPD). Protein sequence comparisons were completed using GCG and the Jotun Hein algorithm in the MEGALIGN program of the DNA STAR sequence analysis software package (DNASTAR, Madison, WI).
Transgene construction
The zebrafish bcl-2 open reading frame was amplified by PCR and cloned into the EGFP-C1 vector (Clontech, Palo Alto, CA). The forward primer contained a SalI enzyme site and altered the methionine start site of bcl-2, changing the amino acid to a leucine. The reverse primer contained the bcl-2 translation stop sequence and a HindIII site. The PCR-purified fragment was digested with SalI and HindIII and subsequently cloned in the XhoI and HindIII digest sites of the EGFP-C1 vector. Clones containing inserts were sequenced fully.
In order to place the EGFP-bcl-2 fusion gene into the rag2 promoter-containing vector, we again used a PCR-mediated cloning strategy. The forward primer contained a BamHI site upstream of the EGFP Kozak sequence and the reverse primer contained the bcl-2 translation stop sequence followed by a HindIII site. The PCR fragment was digested with BamHI and HindIII, and ligated into the rag2 promoter-containing vector using the same restriction digest sites.36 The rag2-EGFP-bcl-2 construct was sequenced to verify that PCR did not introduce point mutations.
The rag2-EGFP-bcl-2 vector was digested with ClaI and BamHI, releasing the EGFP-bcl-2 coding sequence fragment, which was subsequently cloned into the pCS2+ vector using these same restriction digest sites.
RNA rescue experiments
In vitro transcription reactions were used to generate GFP and EGFP-bcl-2 RNA. Specifically, the pCS2+GFP or pCS2+EGFP-bcl-2 vectors were digested with NotI, phenol/chloroform extracted, and ethanol precipitated. Linearized DNA was used in in vitro transcription reactions using SP6 RNA polymerase. RNA was diluted to 200 ng/μL and injected into embryos at the 1-cell stage of development. To quantitate developmental apoptosis, uninjected and GFP-positive injected embryos were analyzed for terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-X nick-end labeling (TUNEL staining, performed as described in Liu et al37 and Cole and Ross38 ) at 16 hours postfertilization (hpf), and the number of apoptotic cells per embryo was determined using microscopic analysis (n = 12 per group). Photographic images were obtained using a Leica MZ FLIII dissecting microscope (1.5 × lens, Leica Microsystems, Bannockburn, IL), a 3CCD camera (model DC330E, Dage-MTI, Michigan City, IN), and the Openlab imaging software (Improvision, Lexington, MA). Openlab images were converted to tiff files, and Adobe Photoshop (Adobe Systems, San Jose, CA) was subsequently used for creation of publication quality images.
For irradiation experiments, uninjected and GFP-positive injected embryos were selected at 14 hpf and irradiated with 15 Gy. Irradiated and unirradiated embryos were analyzed by TUNEL staining at 20 hpf. Because of the high number of irradiation-induced TUNEL-positive cells in the anterior of the embryos, apoptotic cells from the region of the tail below the plane of the lower boundary of the yolk were counted. A square root transformation was applied to each data point to stabilize variance and was subsequently used to determine whether groups differed in total number of apoptotic cells per embryo (Student t test). P values for these comparisons have been doubled to address issues of multiple comparison. Additionally, a Wilcoxin rank sum analysis was completed to show that qualitative assessment of total irradiation-induced apoptotic cells contained within each embryo differed between GFP-injected and EGFP-bcl-2 injected fish.
Generation of stable transgenic lines
Immunocytochemistry and RNA in situ hybridization on paraffin-embedded sections
Fluorescence-activated cell sorting (FACS) and reverse transcriptase (RT)-PCR analysis
Blood cells from the kidney and thymus from transgenic rag2-GFP or rag2-EGFP-bcl-2 fish were analyzed by FACS on the basis of forward and side scatter and GFP fluorescence.39,40
GFP-positive and GFP-negative FACS-sorted blood cell populations were obtained from the kidney and thymus of rag2-GFP and rag2-EGFP-bcl-2 transgenic fish. RNA was extracted with Trizol (GibcoBRL), treated with DNAseI to eliminate residual genomic DNA, and used in reverse transcription reactions with or without reverse transcriptase. cDNA samples were diluted (1:1, 1:10, 1:100, and 1:1000) and used in semiquantitative PCR with primers specific for lck, IgLC3, rag2, and β-actin. PCR primers and cycling conditions have been described previously.39
T- and B-cell ablation by γ-irradiation
Six-day-old rag2-GFP and rag2-EGFP-bcl-2 transgenic embryos were administered a whole-body dose of γ-irradiation (15 Gy from a 137Cs source) and analyzed for GFP expression within the thymus at 1, 2, and 3 days after treatment. Adult rag2-GFP and rag2-EGFP-bcl-2 transgenic fish were administered a whole-body dose of γ-irradiation (20 Gy from a 137Cs source) and analyzed for GFP expression within the thymus at 1, 2, 3, and 4 days after irradiation. Irradiation doses of 20 Gy to 23 Gy were chosen because these doses are sublethal and have been shown to be immune-ablative in adult zebrafish (David Traver and Leonard I. Zon, unpublished, 2004).39 Irradiated adult rag2-EGFP-bcl-2 and rag2-GFP transgenic fish were killed 1, 2, 3, and 4 days after treatment, fixed, and sectioned. Paraffin-embedded sections of the thymus were analyzed by hematoxylin and eosin staining to confirm that loss of GFP expression in the rag2-GFP transgenic thymus resulted from elimination of T cells.
Adult rag2-GFP and rag2-EGFP-bcl-2 transgenic fish were administered a whole-body dose of γ-irradiation (20 Gy) and GFP-labeled lymphocytes were analyzed for changes in annexin V staining (Annexin V-APC; BD Pharmingen, San Diego, CA). Thymocytes and whole-kidney marrow were obtained from rag2-GFP and rag2-EGFP-bcl-2 transgenic fish and placed in ice-cold 0.9x phosphate-buffered saline (PBS). Following filtration over a 40-μ filter, GFP-positive cells were analyzed for annexin V staining before γ-irradiation and 24 hours after irradiation treatment (20 Gy).
T-cell ablation by dexamethasone
Five-day-old rag2-GFP and rag2-EGFP-bcl-2 transgenic embryos were treated with dexamethasone (250 μg/mL [1% ethanol] and 100 μg/mL [0.4% ethanol]) or with ethanol vehicle alone (1% or 0.4%) in egg water (60 mg/L instant ocean in distilled water containing methylene blue). Fish were analyzed 3 and 4 days after treatment for T-cell ablation as detected by loss of GFP fluorescence in the thymus.
EGFP-bcl-2 blocks irradiation-induced death in Myc-induced tumors
Two classes of Myc-induced T-cell acute lymphoblastic leukemias (T-ALLs) were generated: (1) GFP-labeled or (2) GFP-labeled and expressing the zebrafish bcl-2 protein. GFP-labeled T-ALLs were obtained from the rag2-EGFP-mMyc stable transgenic line described previously,34 whereas bcl-2-expressing T-ALLs were created by injecting heterozygous rag2-EGFP-bcl-2 transgenic fish with the rag2-mMyc construct at the one-cell stage of development.
Leukemic fish were isolated at 4 to 6 weeks of development and grown in isolation tanks to facilitate growth. Leukemic cells were harvested and injected into irradiated recipient fish (23 Gy, administered 2 to 3 days before transplantation). Irradiated recipients underwent transplantation with 1 × 106 leukemic blasts. Following infiltration into regions adjacent to the site of intraperitoneal injection (2 to 6 weeks after transplantation), leukemic transplant fish were irradiated (23 Gy) and monitored for loss of lymphoblasts as determined by fluorescent microscopy. Photographs were taken before and 3 days after irradiation treatment. In total, 3 leukemias from each class were analyzed.
Results
The zebrafish bcl-2 protein is highly evolutionarily conserved
The zebrafish bcl-2 protein shares a high level of amino acid similarity with other BCL-2 family members, particularly within the BH1-BH4 functional domains (Supplemental Figure 1A-B; see the Supplemental Figure link at the top of the online article on the Blood website). Phylogenetic analysis places the zebrafish gene with other BCL-2 homologues (Supplemental Figure 1C), whereas the previously identified bcl-xL-like protein 1 (zfBLP1) is most similar to other BCL-xL homologues, indicating that we have likely identified the bcl-2 orthologue in the zebrafish (GenBank accession no. AY695 820).
EGFP-bcl-2 blocks developmentally regulated and irradiation-induced apoptosis
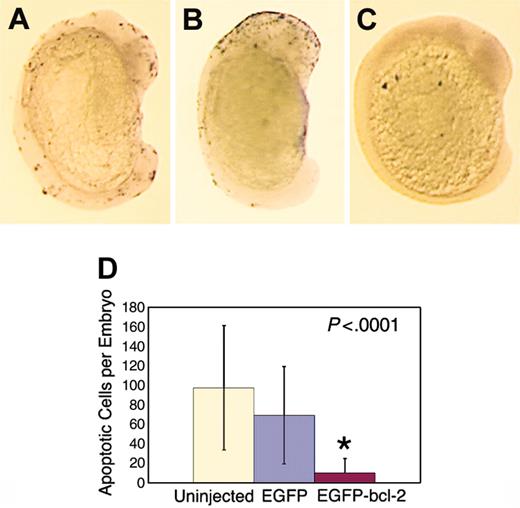
In order to determine if zebrafish bcl-2 has similar functions as the mammalian antiapoptotic BCL-2 family members, we injected embryos at the one-cell stage of development with either GFP or EGFP-bcl-2 RNA and analyzed embryos for number of TUNEL-positive apoptotic cells. Injection of RNA at the one-cell stage of development resulted in GFP expression within the embryo by 12 hpf (data not shown), confirming that both the GFP and EGFP-bcl-2 proteins were expressed following microinjection. EGFP-bcl-2-expressing embryos had significantly fewer apoptotic cells than uninjected or GFP-expressing control fish (n = 12 per treatment group) when analyzed by whole-mount TUNEL staining at 16 hpf (P = .000 004 and P = .000 04, respectively; Figure 1).
The EGFP-bcl-2 fusion protein blocks developmentally regulated apoptosis at 16 hpf. TUNEL staining of an uninjected embryo (A) or embryos injected with GFP (B) or EGFP-bcl-2 RNA (C). (A-C) Lateral view of embryos at 16 hpf, dorsal to the left. TUNEL-positive apoptotic cells denoted by black staining. (D) Quantification of apoptotic cells in embryos injected with EGFP-bcl-2 RNA (red bar) when compared with uninjected control (beige bar) or GFP-injected control fish (blue bar). The asterisk denotes a significant difference between EGFP-bcl-2-injected (n = 12) and both GFP-injected (n = 12; P = .000 04) and uninjected fish (n = 12; P = .000 04). There were no differences in the mean number of apoptotic cells between the 2 control groups (P = .22). Error bars (1+/- standard deviation). Images A-C photographed at 2×.
The EGFP-bcl-2 fusion protein blocks developmentally regulated apoptosis at 16 hpf. TUNEL staining of an uninjected embryo (A) or embryos injected with GFP (B) or EGFP-bcl-2 RNA (C). (A-C) Lateral view of embryos at 16 hpf, dorsal to the left. TUNEL-positive apoptotic cells denoted by black staining. (D) Quantification of apoptotic cells in embryos injected with EGFP-bcl-2 RNA (red bar) when compared with uninjected control (beige bar) or GFP-injected control fish (blue bar). The asterisk denotes a significant difference between EGFP-bcl-2-injected (n = 12) and both GFP-injected (n = 12; P = .000 04) and uninjected fish (n = 12; P = .000 04). There were no differences in the mean number of apoptotic cells between the 2 control groups (P = .22). Error bars (1+/- standard deviation). Images A-C photographed at 2×.
Irradiation-induced apoptosis is also suppressed by EGFP-bcl-2 expression during development. GFP-positive embryos, as well as uninjected control embryos, were irradiated (15 Gy) at 14 hpf and analyzed by whole-mount TUNEL staining at 20 hpf. EGFP-bcl-2-expressing embryos had fewer apoptotic cells than GFP-expressing or uninjected control fish (n = 12 per treatment group) following irradiation treatment (P = .0006 and P = .0002, respectively; Supplemental Figure 2). In fact, the expression of EGFP-bcl-2 reduced the numbers of apoptotic cells to levels equivalent to those of uninjected, unirradiated wild-type embryos (Supplemental Figure 2E). Taken together, these results indicate that the EGFP-bcl-2 fusion protein inhibits apoptosis and that its expression can be monitored by fluorescent microscopic analysis.
EGFP-bcl-2-expressing thymocytes are not ablated by irradiation or dexamethasone treatment
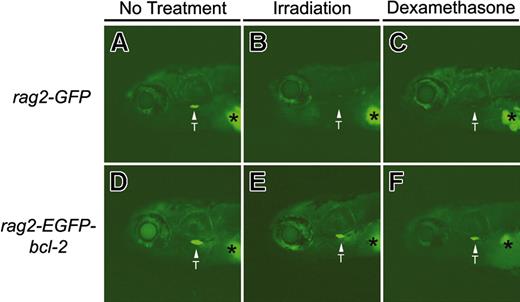
To test whether zebrafish thymocytes that overexpress bcl-2 are susceptible to irradiation- and chemical-induced apoptosis, we generated rag2-EGFP-bcl-2 and rag2-GFP transgenic fish and treated them with both irradiation and dexamethasone. Because thymocyte development can be easily monitored by fluorescent microscopic analysis in larval and adult rag2-GFP and rag2-EGFP-bcl-2 fish, T-cell ablation can be discerned by loss of externally visible GFP-positive cells within the thymi of living fish.39 Larval rag2-GFP and rag2-EGFP-bcl-2 fish were either irradiated (15 Gy, 6 days postfertilization [dpf]) or treated with dexamethasone (100 μg/mL, 5 dpf) and analyzed for GFP fluorescence within the thymus. Both treatments led to the loss of fluorescent-labeled thymocytes in rag2-GFP transgenic larvae (Figure 2A-C). All 8 rag2-GFP fish irradiated at 6 dpf exhibited a complete loss of GFP-labeled thymocytes by 8 dpf (Figure 2B). By contrast, rag2-EGFP-bcl-2 fish retained GFP-labeled thymocytes following γ-irradiation (n = 8; Figure 2E). Similar results were observed in 10-week-old adult fish, with loss of GFP-expressing thymocytes in rag2-GFP fish by 4 days after irradiation treatment, whereas T cells within the thymus of rag2-EGFP-bcl-2 transgenic fish survived (Figure 3). Seven of 8 rag2-GFP transgenic fish treated with dexamethasone (100 μg/mL) lost GFP-labeled thymocytes by 8 dpf (Figure 2C). By contrast, all rag2-EGFP-bcl-2 larvae retained GFP-labeled thymocytes (n = 8 per treatment; Figure 2F), even after treatment with 250 μg/mL dexamethasone.
Thymocytes from larval 8-day-old rag2-EGFP-bcl-2 transgenic fish are resistant to irradiation- and dexamethasone-induced apoptosis. Fluorescent microscopic analysis of 8-day-old rag2-GFP transgenic fish without treatment (A), 2 days after irradiation (15 Gy; B), or 3 days after dexamethasone treatment (250 μg/mL; C). Eight-day-old rag2-EGFP-bcl-2 transgenic fish without treatment (D), 2 days after irradiation (E), or 3 days after dexamethasone treatment (F). Location of the thymus is denoted by arrows and labeled (T). Asterisk denotes autofluorescence of the swim bladder. Fish oriented with anterior to the left and dorsal to the top.
Thymocytes from larval 8-day-old rag2-EGFP-bcl-2 transgenic fish are resistant to irradiation- and dexamethasone-induced apoptosis. Fluorescent microscopic analysis of 8-day-old rag2-GFP transgenic fish without treatment (A), 2 days after irradiation (15 Gy; B), or 3 days after dexamethasone treatment (250 μg/mL; C). Eight-day-old rag2-EGFP-bcl-2 transgenic fish without treatment (D), 2 days after irradiation (E), or 3 days after dexamethasone treatment (F). Location of the thymus is denoted by arrows and labeled (T). Asterisk denotes autofluorescence of the swim bladder. Fish oriented with anterior to the left and dorsal to the top.
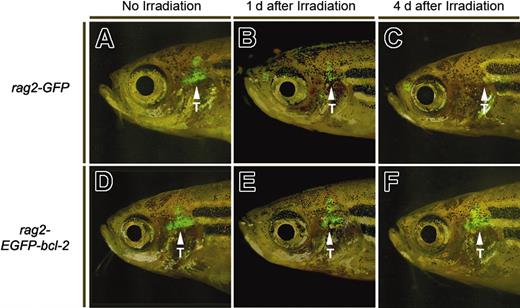
Thymocytes from 10-week-old rag2-EGFP-bcl-2 transgenic fish are resistant to irradiation-induced apoptosis. Fluorescent microscopic analysis of rag2-GFP transgenic fish without γ-irradiation (A) or 1 day or 4 days after irradiation treatment (20 Gy; B and C, respectively). rag2-EGFP-bcl-2 transgenic fish without γ-irradiation (D) or 1 or 4 days after irradiation treatment (E and F, respectively). Location of the thymus is denoted by arrows and labeled (T). Fish oriented with anterior to the left and dorsal to the top.
Thymocytes from 10-week-old rag2-EGFP-bcl-2 transgenic fish are resistant to irradiation-induced apoptosis. Fluorescent microscopic analysis of rag2-GFP transgenic fish without γ-irradiation (A) or 1 day or 4 days after irradiation treatment (20 Gy; B and C, respectively). rag2-EGFP-bcl-2 transgenic fish without γ-irradiation (D) or 1 or 4 days after irradiation treatment (E and F, respectively). Location of the thymus is denoted by arrows and labeled (T). Fish oriented with anterior to the left and dorsal to the top.
To determine whether the loss of irradiated thymocytes from rag2-GFP transgenic fish was due to apoptosis, we irradiated 10-week-old rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish (20 Gy). Twenty-four hours later, GFP-positive thymocytes were analyzed for apoptosis as determined by annexin V staining and FACS analysis (Supplemental Figure 3). Irradiated rag2-GFP fish contained 79.2% annexin V-positive thymocytes whereas only 40.5% of irradiated EGFP-bcl-2-expressing thymocytes stained positive for annexin V. Even without treatment, rag2-GFP fish contained more annexin V-positive thymocytes than rag2-EGFP-bcl-2 transgenic fish (48.4% ± 3.8% and 39.8 ± 2.5%, respectively; P = .03).
The complete loss of thymocytes in adult rag2-GFP transgenic fish treated with γ-irradiation was confirmed by hematoxylin and eosin staining of paraffin-embedded sections (n = 2 per time point, 20 Gy). Thymus size and lymphocyte number were greatly decreased in rag2-GFP fish by 1 day after irradiation, and T cells within the thymus were completely lost by 4 days after treatment. By contrast, histologic analysis revealed that EGFP-bcl-2-expressing thymocytes are retained in the thymus at 1, 2, 3, and 4 days after irradiation treatment (data not shown). These experiments indicate that the EGFP-bcl-2 transgene protects developing zebrafish thymocytes from apoptotic cell death.
EGFP-bcl-2-expressing B cells are not ablated by irradiation
To determine if B cells from rag2-EGFP-bcl-2 transgenic fish are also protected from irradiation-induced apoptosis, we irradiated adult rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish (20 Gy), and 24 hours later, GFP-positive B cells from whole-kidney marrow were analyzed for apoptosis by annexin V staining followed by FACS analysis. In the absence of γ-irradiation, the relative percentage of dying cells was not significantly different between the 2 transgenic lines (rag2-GFP, 75.3% ± 8.3%; rag2-EGFP-bcl-2, 73.9% ± 4.3%; n = 4; P = .77). By contrast, irradiated rag2-GFP fish demonstrated a significant increase in apoptotic B cells (94.1% ± 4.6%) when compared with rag2-EGFP-bcl-2 fish (79.6% ± 4.7%; n = 4 per treatment; P = .002).
Expression of EGFP-bcl-2 in the lymphoid compartment results in increased T- and B-cell populations
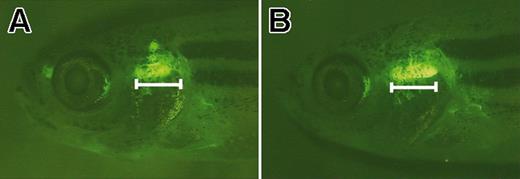
Fluorescent microscopic analysis showed that the thymus was enlarged in rag2-EGFP-bcl-2 transgenic fish when compared with rag2-GFP control fish (Figure 4). The increase in thymus size was accounted for by an increase in total number of thymocytes. Dissected thymi from 10-week-old rag2-EGFP-bcl-2 fish had increased numbers of thymocytes when compared with rag2-GFP control fish (3.4 million ± 1.8 million compared with 1.4 million ± 0.5 million; n = 6; P = .02).
The thymus is expanded in rag2-EGFP-bcl-2 transgenic fish. Fluorescent microscopic analysis of 52-day-old rag2-EGFP-bcl-2 transgenic fish having enlarged thymi (B) when compared with age- and sex-matched rag2-GFP fish (A). Bar denotes length of the ventral-most portion of the thymus in rag2-EGFP-bcl-2 fish. Fish oriented with anterior to the left and dorsal to the top.
The thymus is expanded in rag2-EGFP-bcl-2 transgenic fish. Fluorescent microscopic analysis of 52-day-old rag2-EGFP-bcl-2 transgenic fish having enlarged thymi (B) when compared with age- and sex-matched rag2-GFP fish (A). Bar denotes length of the ventral-most portion of the thymus in rag2-EGFP-bcl-2 fish. Fish oriented with anterior to the left and dorsal to the top.
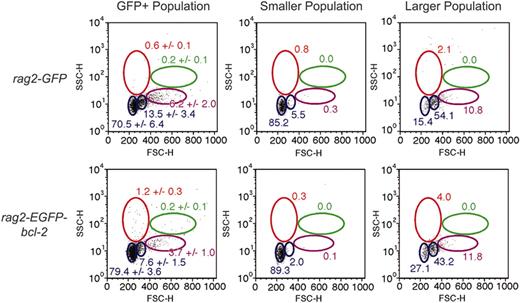
T-cell development in rag2-EGFP-bcl-2 fish was also perturbed. Previously, we identified 2 distinct populations of T cells in the thymus based on size.39 FACS analysis indicated that rag2-EGFP-bcl-2 fish have increased numbers of small lymphocytes when compared with rag2-GFP fish (79.4% ± 3.6% and 70.5% ± 6.4%, respectively; n = 6; P = .02) and a concomitant decrease in large T cells (Figure 5). Taken together, these results suggest that T-cell development may be perturbed in rag2-EGFP-bcl-2 fish, with T cells possibly arresting at a stage during which cell division is suppressed or at a stage of development marked by decreased cell size.
EGFP-bcl-2 alters T-cell development when expressed in thymocytes. FACS analysis of GFP-positive thymus cell populations from 10-week-old rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish. Gated populations of erythrocytes (red), lymphocytes (blue), granulocytes and monocytes (green), and blood cell precursors (purple) are outlined in color. Populations of cells within each gate are described as percentages of total live cells (1 SD; n = 6). Total GFP-positive populations (left column) and sorted, reanalyzed cell populations gated (middle and right columns). Cell size is represented by forward scatter (FSC), and granularity by side scatter (SSC).
EGFP-bcl-2 alters T-cell development when expressed in thymocytes. FACS analysis of GFP-positive thymus cell populations from 10-week-old rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish. Gated populations of erythrocytes (red), lymphocytes (blue), granulocytes and monocytes (green), and blood cell precursors (purple) are outlined in color. Populations of cells within each gate are described as percentages of total live cells (1 SD; n = 6). Total GFP-positive populations (left column) and sorted, reanalyzed cell populations gated (middle and right columns). Cell size is represented by forward scatter (FSC), and granularity by side scatter (SSC).
The kidney is the site of definitive hematopoiesis in adult zebrafish and where B-cell development occurs.25,26,39 Since the rag2-promoter is expressed by both thymocytes and by B cells in adult zebrafish kidney marrow, we reasoned that B-cell development might be altered in rag2-EGFP-bcl-2 transgenic fish. B cells in the kidney cannot be visualized by fluorescent microscopy in undissected living fish because the kidney is positioned deep within the animal. Thus, we used FACS analysis and cell counts to assess if B-cell populations were altered in the rag2-EGFP-bcl-2 kidney (Figure 6). Although total cell counts were similar between rag2-EGFP-bcl-2 and rag2-GFP fish (0.74 million ± 0.26 million compared with 0.55 million ± 0.14 million; n = 6; P = .19), the lymphoid population of the kidney was significantly increased in rag2-EGFP-bcl-2 fish when compared with rag2-GFP control fish (34.4% ± 6.2% and 26.3% ± 4.7%; n = 6; P = .03; Figure 6). Increased numbers of total lymphoid cells were accompanied by an increase in GFP-expressing lymphoid cells in rag2-EGFP-bcl-2 transgenic fish when compared with rag2-GFP control fish (20.0% ± 5.8% and 11.0% ± 3.6%; n = 6; P = .009; Figure 6).
EGFP-bcl-2 induces B-cell hyperplasia in the kidney when expressed in developing rag2-positive lymphoid cells. FACS analysis of kidney marrow cell populations from 10-week-old rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish. Gated populations of erythrocytes (red), lymphocytes (blue), granulocytes and monocytes (green), and blood cell precursors (purple) are outlined in color. Populations of cells within each gate are described as percentages of total live cells (1 SD; n = 6). Total cell populations (left column) and GFP gated populations (middle and right columns). Cell size is represented by forward scatter (FSC), and granularity by side scatter (SSC).
EGFP-bcl-2 induces B-cell hyperplasia in the kidney when expressed in developing rag2-positive lymphoid cells. FACS analysis of kidney marrow cell populations from 10-week-old rag2-GFP and rag2-EGFP-bcl-2 transgenic zebrafish. Gated populations of erythrocytes (red), lymphocytes (blue), granulocytes and monocytes (green), and blood cell precursors (purple) are outlined in color. Populations of cells within each gate are described as percentages of total live cells (1 SD; n = 6). Total cell populations (left column) and GFP gated populations (middle and right columns). Cell size is represented by forward scatter (FSC), and granularity by side scatter (SSC).
FACS-sorted GFP-positive kidney marrow cell populations were analyzed by RT-PCR to confirm that rag-positive cell populations were B cell in origin. As described previously for the rag2-GFP transgenic line,39 GFP-positive cells from rag2-EGFP-bcl-2 transgenic zebrafish expressed IgLC3, rag1, and rag2, but failed to express the T-cell-specific kinase, lck (data not shown). Taken together, these results indicate that B-cell populations are expanded in the kidney of rag2-EGFP-bcl-2 transgenic fish.
bcl-2 expression blocks irradiation sensitivity of Myc-induced T-cell leukemias
Myc-induced leukemias in the mouse require suppression of apoptosis, which usually occurs by either loss of p53 or ARF, or up-regulation of Mdm2.41 However, it is not known if these transforming mechanisms are present in zebrafish T-ALLs. In order to assess whether the p53 DNA damage pathway is intact in Myc-induced T-ALL in the zebrafish, we irradiated fish that underwent transplantation with leukemic cells from our rag2-EGFP-mMyc stable transgenic fish (described in Langenau et al34 ). In response to 20 Gy γ-irradiation, leukemias were ablated by 3 days after treatment (Figure 7A-C). Because bcl-2 functions downstream of p53, we reasoned that the expression of EGFP-bcl-2 in Myc-induced leukemias would confer resistance to irradiation-induced cell death. By 3 days after treatment, EGFP-bcl-2-expressing leukemic cells were retained within the fish that underwent transplantation (Figure 7D-F).
Myc-induced leukemias undergo apoptosis following irradiation, whereas Myc-induced leukemias that overexpress EGFP-bcl-2 do not. Leukemias from stable transgenic rag2-EGFP-mMyc (A) and rag2-EGFP-bcl-2 embryos injected with rag2-mMyc (D) were transplanted into irradiated recipient fish (B-C, E-F). After local infiltration of leukemic cells into the abdomen adjacent the site of injection (B, E), fish were treated with γ-irradiation (23 Gy) and analyzed 3 days after treatment (C, F). Anterior to the left and dorsal toward the top. Panels B, C, E, and F show abdominal views of transplant recipient fish. IR, irradiation.
Myc-induced leukemias undergo apoptosis following irradiation, whereas Myc-induced leukemias that overexpress EGFP-bcl-2 do not. Leukemias from stable transgenic rag2-EGFP-mMyc (A) and rag2-EGFP-bcl-2 embryos injected with rag2-mMyc (D) were transplanted into irradiated recipient fish (B-C, E-F). After local infiltration of leukemic cells into the abdomen adjacent the site of injection (B, E), fish were treated with γ-irradiation (23 Gy) and analyzed 3 days after treatment (C, F). Anterior to the left and dorsal toward the top. Panels B, C, E, and F show abdominal views of transplant recipient fish. IR, irradiation.
Discussion
The mechanisms that regulate apoptosis are remarkably conserved across the animal kingdom; however, few studies have tested whether the molecules regulating this process in the zebrafish are functionally conserved. Previous studies have shown that fusion of GFP with Bcl-2 family members does not alter function, half-life, or cellular distribution.42,43 Thus, we cloned the zebrafish bcl-2 gene and fused it to the green fluorescent protein. The primary benefit of tagging bcl-2 with the GFP fluorophore is that cells expressing this transgene can be easily detected by fluorescent microscopy, FACS analysis, and immunohistochemistry. For example, RNA injection of Xenopus embryos with GFP-X11 (an antiapoptotic bcl-2 family member) results in both fluorescence detection within the embryo and in the suppression of cell death within Rohon-Beard sensory neurons.44 Functional assays using RNA-injected zebrafish embryos indicated that the EGFP-bcl-2 fusion protein can suppress normal developmentally regulated apoptosis as well as fancd2-37 and irradiation-induced apoptosis. Many zebrafish mutant lines have been described that exhibit a perturbation of development and loss of specific organ systems, often associated with increased apoptosis in affected cell types. EGFP-bcl-2 RNA rescue experiments, such as those performed in the context of fancd2 knockdown,37 can indicate whether apoptosis contributes to, or is the result of, a mutant phenotype.
Zebrafish thymocytes can be ablated by either irradiation or dexamethasone treatment39 ; however, it is unknown if these processes can be inhibited by disrupting the apoptotic machinery within the zebrafish thymocyte. For example, irradiation-induced apoptosis can be suppressed by either loss of p53 or the overexpression of Bcl-2 in mouse thymocytes.45,46 In contrast, dexamethasone-induced apoptosis cannot be inhibited in p53-deficient thymocytes.45 However, bcl-2 overexpression can suppress dexamethasone-induced cell death.46,47 These experiments indicate that the molecular mechanisms initiating apoptosis in T cells can be markedly different depending on the source and type of cell damage. Our experiments show that thymocytes from larval rag2-EGFP-bcl-2 fish survive following irradiation or dexamethasone treatment, whereas thymocytes from rag2-GFP zebrafish larvae die in response to either treatment. Additional experiments using p53-deficient zebrafish embryos will be required to test whether apoptotic responses to either dexamethasone or irradiation are mediated by the p53 pathway.
To confirm that T-cell loss was due to apoptosis following γ-irradiation, we analyzed thymocytes from transgenic adult fish for annexin V staining following irradiation treatment. Thymocytes from rag2-GFP fish exhibited increased numbers of annexin V-positive cells at 24 hours following γ-irradiation. By contrast, the number of apoptotic thymocytes did not change following γ-irradiation of rag2-EGFP-bcl-2 transgenic zebrafish. Similarly, immature B cells residing in the kidney are protected from irradiation-induced apoptosis when EGFP-bcl-2 is overexpressed, indicating that bcl-2 can block apoptotic responses in zebrafish lymphoid cells.
In order to assess whether bcl-2 expression perturbs T-cell homeostasis, we analyzed GFP-positive T-cell populations by FACS analysis to determine if small and large T-cell populations were altered within the thymus. As in the mouse, only a subset of thymocytes from transgenic rag2-EGFP-bcl-2 fish appear to be protected from cell death. For example, there was a significant increase in the number of small thymocytes in rag2-EGFP-bcl-2 fish when compared with the rag2-GFP transgenic fish (79.4% ± 3.6% vs 70.5% ± 6.4%; P = .02). This 8% increase in small thymocytes correlates with the 8% increase in total thymocyte survival observed in the rag2-EGFP-bcl-2 transgenic fish, suggesting that small thymocytes may be preferentially protected from cell death by the EGFP-bcl-2 transgene or that bcl-2 overexpression may significantly alter thymocyte homeostasis. Similar results have been documented in the mouse. For example, Lck-Bcl-2 transgenic mice do not have increased thymus size or altered thymic architecture, but have altered distribution of cortical thymocyte subsets including increased numbers of CD3HI/TCRHI and mature CD8+ thymocyte subpopulations when compared with nontransgenic littermate control animals.46 Similarly, Bim deficiency or overexpression of bcl-2 in vav-bcl-2 transgenic mice leads to perturbation of T-cell homeostasis, causing a 2- to 3-fold increase in pro-T cells (CD4-/CD8-) and mature single-positive T cells (CD4+ and CD8+) and a subsequent decrease in double-positive (CD4+/CD8+) thymocyte number.12,48
Not only is the relative percentage of small thymocytes altered in rag2-EGFP-bcl-2 fish, but the total lymphoid cell number in both the thymus and kidney marrow differs as well. In fact, the thymus is enlarged in rag2-EGFP-bcl-2 fish, having a 2.5-fold increase in total thymocyte number than when compared with rag2-GFP transgenic fish. Although total numbers of kidney marrow cells are not significantly different between transgenic rag2-EGFP-bcl-2 and rag2-GFP fish, B-cell populations within the kidney of rag2-EGFP-bcl-2 fish are expanded. As we observed in our rag2-EGFP-bcl-2 zebrafish transgenic model, E mu-Bcl-2 mice have expanded B-cell populations within the marrow, due to increased numbers of small resting IgM/IgD+ B cells.49 In marked contrast to mouse transgenic experiments,47,49 bcl-2 overexpression within zebrafish thymocytes leads to an increase in thymus size and subsequent increase in total cell number. Thus, it appears that the levels of Bcl-2 expression and/or the timing of its expression during T-cell development greatly influence Bcl-2 effects on thymocyte number and homeostasis.
Although Bcl-2 overexpression in B and T cells leads to malignant transformation in mice and humans, no rag2-EGFP-bcl-2 transgenic fish have developed lymphoma or leukemia. Even following irradiation treatment at 3 to 4 weeks of age, rag2-EGFP-bcl-2 fish failed to develop lymphoma 6 months after treatment (D.M.L. and A.T.L., unpublished data, 2004). By contrast, BCL-2 overexpression can induce follicular lymphoma in human patients who have translocations that place the BCL-2 gene in close proximity to the immunoglobulin enhancer regions, t(14;18),13 and mice expressing the human minitransgene for the t(14;18) develop follicular hyperplasia, which in some instances, progresses to diffuse large-cell lymphoma after a long latency.14 Finally, Bcl-2 overexpression in T cells can also induce malignancy.15 For example, Lck-Bcl-2 transgenic mice develop mature CD4+ lymphomas with the mean latency to onset of 18 months. Taken together, these experiments show that BCL-2 overexpression can be a transforming event in T- and B-cell malignancies in mice and humans.
Promoter effects may be responsible for the lack of tumor development in our zebrafish transgenic model. Because rag-1 and rag-2 expression are regulated in distinct waves during lymphoid development and because the rag promoter is turned off following receptor rearrangement,50,51 rag expression is absent in more mature lymphoid cells. Thus, if mature lymphoid cells are the target for oncogenic transformation, then lymphomas would not develop. For example, T- and B-cell lymphomas similar to those observed in Lck-Bcl-2 and E mu-Bcl-2 transgenic mice would not be observed using the rag2-EGFP-bcl-2 transgenic zebrafish model. In mice, T-cell malignancy is accompanied by accumulation of CD4+ T cells and in B-cell malignancy by IgM/IgD+ B cells. A second possibility that may account for why we have not observed leukemia or lymphoma in our rag2-EGFP-bcl-2 transgenic zebrafish is that the latency of disease may be very long. Very few of these transgenic fish have been raised past 1 year of age. Given that B- and T-cell lymphomas in Bcl-2 transgenic mice take a long time to develop (mean latency of 17 months and 18 months, respectively),14,15 it is possible that our rag2-EGFP-bcl-2 fish may still develop disease over time.
Myc-induced ALL in the mouse requires suppression of apoptosis, which commonly results from loss of ARF or p53, or up-regulation of Mdm-2,41,52 and Myc and Bcl-2 have been shown to collaborate in leukemogenesis.53 Given that many of these transforming events act by inhibiting the p53-mediated DNA-damage response pathway, we reasoned that Myc-induced T-ALLs in the zebrafish might be refractory to irradiation treatment. GFP-labeled Myc tumors could be ablated by irradiation treatment, suggesting that the p53 pathway is likely to be intact in these tumors. Because cycling T-lymphoma cells from p53-/- mice undergo apoptosis after irradiation or genotoxic drug treatment and because it is likely that p53 is not the only mediator of apoptosis provoked by DNA damage,54 we sequenced the zebrafish p53 gene locus, but failed to identify any p53 mutations in Myc-induced T-ALLs (n = 13; D.M.L., Hui Feng, S.B., J.P.K., J.L.K., A.T.L., manuscript under review, 2005). These results indicate that mutation of p53 is not a frequent event in T-cell transformation in the zebrafish model. In contrast, EGFP-bcl-2-expressing Myc-induced leukemias fail to undergo apoptosis following irradiation treatment, as has been reported in mouse T-cell lymphomas that overexpress Bcl-2.54 Given that the teleost fishes lack the ARF locus,55 it is possible that Myc up-regulation may not induce apoptosis through the p53 pathway, as has been observed in mice and humans.41,56-58 Further studies will be required to determine the extent and role that apoptosis plays in transformation induced by Myc in zebrafish T-ALL.
Because the zebrafish is amenable to forward genetic screens, the rag2-EGFP-bcl-2 transgenic zebrafish model may be useful for the identification of genetic suppressors of bcl-2. For example, a larval screen based on loss of GFP-labeled thymocytes following irradiation or dexamethasone treatment would likely identify mutations that synergize with these T-cell ablative agents to elicit cell death within EGFP-bcl-2-expressing lymphoid cells. Mutations that allow T cells to undergo irradiation-induced apoptosis even in the presence of the EGFP-bcl-2 transgene would be potential targets for the development of small-molecule inhibitors. Because many BCL-2-overexpressing tumors are resistant to chemotherapeutics and irradiation treatment,59-61 developing drugs that sensitize BCL-2-expressing cells to apoptosis would be an important step forward in the treatment of these cancers. In addition to genetic screens, the rag2-EGFP-bcl-2 fish may be useful in chemical screens designed to identify drugs that disrupt bcl-2 function. Finally, use of our EGFP-bcl-2 transgenic fish may lead to new insights into the conserved role of bcl-2 family members in lymphocyte maturation and function and in the onset of T- and B-cell malignancy.
Prepublished online as Blood First Edition Paper, December 23, 2004; DOI 10.1182/blood-2004-08-3073.
Supported by the National Institutes of Health (CA-68484, A.T.L.; CA-06516, J.L.K.), a National Institutes of Health Program Training grant in Molecular Hematology (5T32HL07 623-17; C.J.), and a fellowship from the Lady Tata Memorial Trust T.P.). D.M.L. is an National Science Foundation predoctoral fellow. S.B. is a Lymphoma Research Foundation Fellow.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Y. Yang and A.A. Ferrando for expert technical assistance, D.S. Neuberg and R. Santiago for help with statistical analysis, and J. Vinokur, G. Kourkoulis, and W. Saganic for fish care and husbandry.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal