Abstract
Using specific antibodies against isoforms of WAVE (WASP [Wiskott-Aldrich syndrome protein] family Verprolin-homologous protein, also called Scar), we demonstrated that human platelets express all 3 isoforms. With the use of an in vitro pull-down technique, the src homology 3 (SH3) domain of insulin receptor substrate p53 (IRSp53) precipitated WAVE2 from platelet lysates more efficiently than did profilin I. The opposite was true for WAVE1, and neither precipitated WAVE3, suggesting that WAVE isoforms have different affinities to these ligands, while the SH3 domain of abl binds to all 3 isoforms. The 3 WAVE isoforms were distributed in the actin-rich Triton X-100–insoluble pellets following platelet aggregation induced by thrombin receptor–activating peptide. We also found that all 3 WAVE isoforms are substrates for calpain in vivo and in vitro. Although portions of these 3 isoforms were commonly distributed in the actin- and actin-related protein 2 and 3 (Arp2/3)–rich edge of the lamellipodia in spreading platelets, only WAVE2 remained in the cell fringe following detergent extraction or fixation of the cells. Finally, by mass spectrometry, we found that the proteins, which reportedly interact with WAVE/Scars, are present in platelets. These data suggest that the 3 WAVE isoforms exhibit common and distinct features and may potentially be involved in the regulation of actin cytoskeleton in platelets.
Introduction
Reorganization of cortical actin filaments plays a critical role in cell movement and pattern formation.1,2 WASP (Wiskott-Aldrich syndrome protein), N-WASP, and WAVE (WASP family Verprolin-homologous protein, or Scar), called WASP family proteins, are likely to regulate cortical actin filament reorganization in response to extracellular stimuli, although not all of these proteins have been linked to actin polymerization.1,2 Each of these proteins has a verprolin-homology (V) domain, cofilin-homology (C) domain, and an acidic (A) region at the C-terminus, which are necessary to enhance the intrinsic actin polymerization activity of the actin-related protein 2 and 3 (Arp2/3) complex.1-9
Platelets are essential for normal hemostasis and have an unusually high actin content (0.5 mM).10,11 In suspension, platelets are rapidly transformed from disks to spheres with spiny protrusions upon stimulation by agonists such as adenosine diphosphate, collagen, and thrombin.10-15 This “shape change” is inhibited by cytochalasins, which prevent actin polymerization.10-15 Platelets also adhere onto various surfaces and rapidly spread, a process that is also inhibited by cytochalasins. Thus, actin polymerization is pivotal in the rapid morphologic transformation of platelets in the initial phase of hemostasis. Among the WASP and WAVE family proteins, platelets express WASP, which becomes strongly tyrosine phosphorylated upon stimulation by collagen or following cross-linking of CD32, a low-affinity Fc receptor for immunoglobulin G (IgG).16 However, despite the very small size of platelets from patients with WAS, these small platelets undergo shape changes in suspensions and upon surfaces that are similar to normal platelets.17,18 The presence of N-WASP in platelets is controversial. It has previously been shown that platelets did not express measurable amounts of N-WASP, which, similar to WASP, is a CDC42 effector.19 However, a more recent report suggested that platelets express very small amounts of N-WASP.20 These observations are consistent with those of Hartwig et al13 who had provided evidence earlier that, for platelets in suspension, Rac rather than Cdc42 is essential for actin polymerization. However, both Rac and Cdc42 are reportedly involved in the development of lamellipodia in spreading platelets.21 The critical role of the Arp2/3 complex in the regulation of the actin cytoskeleton in platelets has been recognized.14,15 Thus, the investigation of the proteins that may connect guanosine triphosphate loading of Rac with Arp2/3 complex activation should greatly contribute to our understanding of the regulation of the cytoskeleton in platelets. Because the ubiquitously expressed WAVE2 is activated downstream of Rac, leading to the formation of lamellipodia in fibroblasts,2,3,22 we examined which isoforms of WAVEs are expressed in human platelets. We show here that platelets express all 3 isoforms and that they have common and distinct features.
Materials and methods
Washed platelets
Blood from healthy volunteers, after obtaining written informed consent, was drawn by venipuncture into 1/10 volume of 3.8% (wt/vol) trisodium citrate and gently mixed. Alternatively, buffy coat, provided by the Hokkaido Red Cross Blood Center (Sapporo, Japan), was used instead of whole blood. Washed human platelets were prepared as described previously16 and suspended in a modified N-2 hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–Tyrode buffer (129 mM NaCl, 8.9 mM NaHCO3, 0.8 mM KH2PO4, 2 mM KCl, 0.8 mM MgCl2, 5.6 mM dextrose, and 10 mM HEPES, pH 7.4) at a concentration of 3 × 108 cells/mL with apyrase (2 U/mL) at 37°C.
Reagents
Polyclonal anti-WAVE1, -WAVE2, and -WAVE3 antibodies were prepared in rabbits immunized with the basic regions of human WAVEs (WAVE1, amino acids 180-246; WAVE2, amino acids 180-241; WAVE3, amino acids 181-246) expressed in Escherichia coli. Each antiserum was purified with the appropriate antigen-immobilized column. As the amino acid sequences of WAVE1 to WAVE3 are highly conserved between human and mouse,1,2 anti-WAVE1 and -WAVE2 antibodies recognize mouse WAVE1 and WAVE2, respectively, and these antibodies were used to confirm lack of WAVE1 or WAVE2 from WAVE1- or WAVE2-null embryonic fibroblasts.23 A polyclonal antibody against WAVE3 (amino acids 211-223) and an anticortactin monoclonal antibody were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-Arp3 was produced in the laboratory of L.M.M. and used as a reporter of the Arp2/3 complex.24 An anti-WASP (503) polyclonal antibody was used as previously described.16 An anti-WASP monoclonal antibody for immunostaining and blotting and an anti-WIP (WASP-interacting protein) polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–Abi-1 monoclonal antibodies (clones 4E2 and 1G9) were from Biodesign (Saco, ME) and MBL (Nagoya, Japan), respectively. Thrombin, thrombin-receptor activating peptide (TRAP; SFLLRNPNQKYEPF), bovine serum albumin (BSA) protein standard, Arg-Gly-Asp-Ser (RGDS), fibronectin, fibrinogen, anti-FLAG (Five NH2-terminally deleted epitope-tagged) M2, M2-conjugated agarose, control IgG1, and other reagents were obtained from Sigma (St Louis, MO). Alexa-labeled secondary antibodies, and Alexa-conjugated or unconjugated phalloidin were purchased from Molecular Probes (Eugene, OR).
Immunoprecipitation and immunoblotting
Platelets in suspension (0.5 mL) were lysed by the addition of an equal amount of lysis buffer (15 mM HEPES, 150 mM NaCl, 1 mM phenylmethanesulfonyl fluoride, 10 mM EGTA (ethylene glycol tetraacetic acid), 1 mM sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 (vol/wt), pH 7.4). Immunoprecipitation and immunoblotting using the electrochemiluminescence methods were performed as previously described.16
GST binding assays
Glutathione S-transferase–CT10 regulator of kinase like (GST-CrkL)–amino terminal Src homology 3 (SH3) fusion protein and GST-SH3 of c-abl were gifts from Dr Brian J. Druker (Oregon Health Sciences University, Portland, OR) and Dr Ann Marie Pendergast (Duke University Medical Center, Durham, NC), respectively. Production of GST-fusion proteins and binding experiments using cell lysates were performed as previously described.25 GST-IRSp53 (insulin receptor substrate p53) and GST–profilin I were prepared as previously described.22,26 The GST-fusion proteins were isolated from sonicated bacterial lysates with the use of glutathione Sepharose beads. Coomassie Brilliant Blue (CBB)–stained gels were used to normalize for the expression of the various GST fusion proteins.
Isolation of platelet cytoskeleton
The Triton X-100–insoluble cytoskeleton was isolated as previously described.27 An equal amount of lysis buffer was added to the platelet suspensions to solubilize the platelets. After 5 minutes on ice, the lysate was centrifuged at 10 000g. The resulting pellet was washed twice in washing buffer. For one-dimensional sodium dodecyl sulfate (SDS) electrophoresis, the Triton X-100–insoluble pellets were solubilized in SDS sample buffer. The supernatant was diluted with an equal volume of 2 × concentrated SDS sample buffer.
In vitro cleavage of WAVEs by calpain
Lysis of cells and subsequent precipitation of WAVEs by GST-fusion proteins or an antibody were performed as described previously.25 Precipitates were washed twice with a reaction buffer (NaCl 150 mM, MgCl2 5 mM, MnCl2 5 mM, Na3VO4 1 mM, and HEPES 10 mM, pH 7.4) followed by in vitro cleavage with μ-calpain, purchased from Calbiochem (San Diego, CA), as previously described.28
Localization of WAVEs in spread platelets
Platelets from diluted platelet-rich plasma were allowed to spread on glass for 1 hour at 37°C and fixed in 3.7% paraformaldehyde. Following treatment with 100 mM tris(hydroxymethyl)aminomethane–HCl (pH 7.4) for 15 minutes, the cells were permeabilized in 0.15% Triton X-100 for 1 minute. In some experiments, fixation and permeabilization were performed simultaneously as described previously.14 Alternatively, spread platelets were fixed in 4% paraformaldehyde in PHEM buffer (60 mM 1,4-piperazinediethanesulphonic acid; pH 6.9, 25 mM HEPES [pH 6.9], 10 mM EGTA, 2 mM MgCl2, and protease inhibitors) containing a 1/50 dilution of Alexa-conjugated phalloidin or unlabeled phalloidin with 0.25% Triton for 20 minutes at room temperature. The fixed samples were blocked with Block Ace (Snow Brand, Tokyo, Japan) for 30 minutes. The samples were incubated with primary and secondary antibodies and subjected to extensive washing. Images were taken with an inverted confocal laser scanning microscope (Zeiss LSM 510; Carl Zeiss, Jena, Germany) with a 100 × oil objective lens and processed by Adobe Photoshop version 7.0 (Adobe, San Jose, CA).
Ectopic expression of WAVEs and interacting proteins in Cos7 cells
The full-length cDNA3,29 of human WAVE/Scars (WAVE1 [Genbank Accession no. D87459 as KIAA0269], WAVE2 [AB026542], and WAVE3 [AB026543]) was subcloned into FLAG-tagged pBluescript and then constructed in the pEF-BOS vector.29,30 Human Nck-associated protein 1/HEM-2 (Nap1; GenBank Accession no. AB011159: KIAA0587) and cytoplasmic FMRP (fragile X mental retardation protein) interacting protein 1/p140Sra-1 (Sra-1; GenBank Accession no. XM_03 922; KIAA0068) cDNAs were kindly provided by Dr T. Nagase (Kazusa DNA Research Institute, Chiba, Japan), and amplified by polymerase chain reaction (PCR). Resulting fragments were ligated into pFLAG-CMV-6c vector (Sigma, St Louis, MO), to be FLAG-tagged at the NH2 terminus. Nucleotide sequences were confirmed for all these plasmids after their construction. To express proteins, Cos7 cells were transfected with 2 μg recombinant plasmid of each one of these proteins using Lipofectamine 2000 (GIBCO-BRL, Grand Island, NY). The cells were cultured in Dulbecco Modified Eagle medium supplemented with 10% fetal bovine serum. After 24 hours, the cells were harvested with the lysis buffer as described16 for immunoprecipitation or pull-down assay.
Quantification of WAVE/Scars in platelets
We purified FLAG-tagged WAVE/Scar 1 to WAVE/Scar-3 proteins, expressed in Cos7 cells, using anti-FLAG M2 agarose. The proteins and serially diluted BSA standard were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) electrophoresis. Following Coomassie Brilliant Blue (CBB) staining, the gels were dried and scanned by a densitometer, and the WAVE/Scar proteins were quantified by the National Institutes of Health (NIH) Image software (Bethesda, MD). These proteins were serially diluted and used as standards for quantitative immunoblotting of platelet lysates. In a similar fashion, actin concentration in platelet lysates was measured by using the same BSA standard. By this method, we determined the amount of WAVE/Scar proteins in platelet lysates in relation to a known amount of actin. The molecular weights of WAVE proteins were calculated according to the published amino acid sequences. FLAG-tagged WAVE proteins have higher molecular weights than endogenous proteins because of the tag (1013 Da). Platelets express β-actin and γ-actin.10,11 Since their molecular weights are similar, we used that of β-actin. Assuming that the concentration of actin in platelets is 0.5 mM,10,11 we used this value to calculate the molar concentration of endogenous WAVE proteins.
MALDI-TOF/MS
From platelet lysates, we precipitated proteins that bind to the SH3 domain of abl, expressed in bacteria as a GST-fusion protein. Following digestion by trypsin, the bound proteins were analyzed by Matrix-assisted Laser Desorption/Ionization time-of-flight mass spectrometry (MALDI-TOF/MS) using a Voyager-DE/STR (Applied Biosystems, Foster City, CA). The proteins were identified by comparison between the molecular weights determined by MALDI-TOF/MS and the theoretical peptide masses derived from the proteins registered in the NCBInr protein sequence database.31
Results
All 3 WAVE isoforms are present in platelets
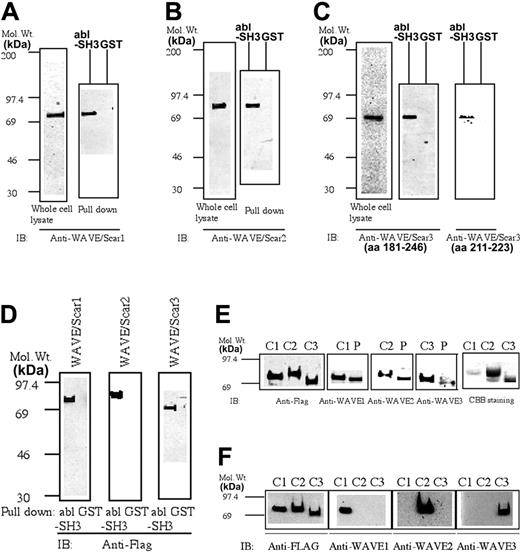
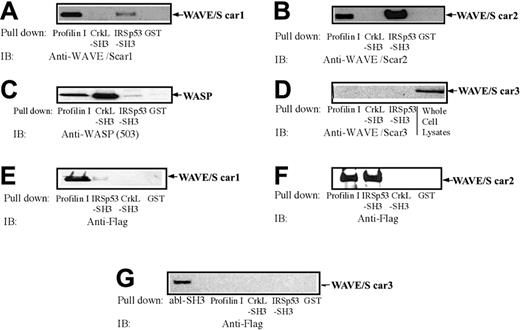
The blots shown in Figure 1A-C demonstrate that each antibody against 1 of the 3 isoforms of WAVE recognizes a single band in platelet lysates. The relative molecular weights of platelet-associated WAVE1, WAVE2, and WAVE3 were approximately 80, 85, and 70 kDa, respectively. However, in most experiments, WAVE1 and WAVE2 migrated very closely in the gel. It was reported that WAVE1, WAVE2, and WAVE3 commonly bind to the SH3 domain of abl protein, although actual data for WAVE2 and WAVE3 have not been provided.32 In agreement with this report, the GST-fused SH3 domain of abl, but not GST, precipitated FLAG-tagged WAVE1, WAVE2, and WAVE3, expressed in Cos7 cells (Figure 1D). Platelet-associated WAVE1, WAVE2, and WAVE3 were also specifically precipitated by the GST-fused SH3 domain of c-abl but not GST (Figure 1A-C). We also used the Cos7 whole-cell lysates expressing similar amounts of FLAG-tagged WAVE1, WAVE2, and WAVE3 (Figure 1E, far left panel) as a standard to compare the relative levels of expressions of WAVE1 with WAVE3 in platelets. Using this standard, we found that WAVE1 and WAVE2 are expressed at similar quantities in platelets, while WAVE3 is expressed at a relatively low level (Figure 1E). It should be noted that FLAG-tagged WAVEs migrated slightly slower than their platelet counterparts because of FLAG-tag (1013 Da). The concentration of actin in platelets is 0.5 mM.10,11 We purified FLAG-tagged WAVE1 to WAVE3 (Figure 1E, far right panel), which was used for more accurate quantification of platelet WAVE1 to WAVE3. By quantitative immunoblotting and determination of actin content in the platelet lysates (see “Materials and methods” for detail), the concentration of WAVE1, WAVE2, and WAVE3 was estimated to be approximately 3.4, 2.4, and 0.15 μM (mean of 3 separate determinations which differ within 20% of each other), respectively. These values were comparable to those of major actin regulators like gelsolin (12 μM), profilin (0.5 μM), or vasodilator-stimulated phosphoprotein (VASP; 5 μM) (reviewed in Fox10 and Bearer et al11 ). We also confirmed that anti-WAVE1 only recognizes WAVE1 but not WAVE2 and WAVE3, expressed in Cos7 cells (Figure 1F). To confirm further that the isoform-specific antibodies recognize distinct molecules, we tested the binding of other ligands to WAVEs by pull-down assays. WAVEs have a well-documented, proline-rich domain that is a potential docking site for profilin and SH3-containing proteins.1-3 CrkL has 1 amino terminal SH2 domain followed by 2 SH3 domains. We have previously reported that WASP was precipitated by the amino terminal SH3 domain of CrkL but not the SH2 domain or GST alone.25 By pull-down assay, WAVE1 was more efficiently precipitated by GST–profilin I than by the SH3 domain of IRSp53 (Figure 2A). The opposite was true for WAVE2; the SH3 domain of IRSp53 precipitated more WAVE2 than did GST–profilin I (Figure 2B). Neither WAVE1 nor WAVE2 was precipitated by the amino terminal SH3 domain of CrkL-GST or GST alone, while the SH3 domain of CrkL precipitated WASP as reported previously. None of the GST fusion proteins tested, except the SH3 domain of abl, precipitated WAVE3 from platelet lysates (Figures 1C and 2D), while the SH3 domain of IRSp53 precipitated WASP less efficiently than GST–profilin I, similar to WAVE1 (Figure 2C).
The presence of WAVE1, WAVE2, and WAVE3 in human platelets. (A-C) Whole cell lysates from platelets (7.5 × 106 cells) or the precipitates eluted from glutathione Sepharose, which had been immobilized with GST or GST-SH3 of abl and then incubated with platelet lysates, were examined for the presence of WAVE1 (A), WAVE2 (B), or WAVE3 (C), as indicated by SDS-PAGE electrophoresis followed by immunoblotting. For WAVE3, 2 polyclonal antibodies were used (see “Materials and methods”). (D) Cos7 cells were transfected with FLAG-tagged WAVE1 to WAVE3 and lysed. The cell lysates (500 μg) were incubated with glutathione Sepharose, which had been immobilized with GST or GST-SH3 of abl. The presence of WAVE1 to WAVE3 was examined as in panels A to C by using an anti-FLAG monoclonal antibody (M2). (E) Whole cell lysates from Cos7 cells expressing similar amounts of FLAG-tagged WAVE1 to WAVE3 or whole-cell lysates from platelets (7.5 × 106 cell) were subjected to SDS-PAGE electrophoresis, followed by immunoblotting as indicated. (Far right panel) FLAG-tagged WAVE proteins were purified with anti-FLAG M2–conjugated agarose, subjected to SDS-PAGE electrophoresis, and stained by CBB. (C1-3 indicates lysates from Cos7 cells expressing FLAG-tagged WAVE1-WAVE3; P, platelet whole-cell lysates). (F) The lysates (20 μg/lane) of Cos7 cells expressing WAVE1 to WAVE3 (C1-3) were subjected to SDS-PAGE, followed by immunoblotting with anti-FLAG (far left panel) or anti-WAVE1 to -WAVE3 as indicated. Mol. Wt. indicates molecular weight in kilodaltons.
The presence of WAVE1, WAVE2, and WAVE3 in human platelets. (A-C) Whole cell lysates from platelets (7.5 × 106 cells) or the precipitates eluted from glutathione Sepharose, which had been immobilized with GST or GST-SH3 of abl and then incubated with platelet lysates, were examined for the presence of WAVE1 (A), WAVE2 (B), or WAVE3 (C), as indicated by SDS-PAGE electrophoresis followed by immunoblotting. For WAVE3, 2 polyclonal antibodies were used (see “Materials and methods”). (D) Cos7 cells were transfected with FLAG-tagged WAVE1 to WAVE3 and lysed. The cell lysates (500 μg) were incubated with glutathione Sepharose, which had been immobilized with GST or GST-SH3 of abl. The presence of WAVE1 to WAVE3 was examined as in panels A to C by using an anti-FLAG monoclonal antibody (M2). (E) Whole cell lysates from Cos7 cells expressing similar amounts of FLAG-tagged WAVE1 to WAVE3 or whole-cell lysates from platelets (7.5 × 106 cell) were subjected to SDS-PAGE electrophoresis, followed by immunoblotting as indicated. (Far right panel) FLAG-tagged WAVE proteins were purified with anti-FLAG M2–conjugated agarose, subjected to SDS-PAGE electrophoresis, and stained by CBB. (C1-3 indicates lysates from Cos7 cells expressing FLAG-tagged WAVE1-WAVE3; P, platelet whole-cell lysates). (F) The lysates (20 μg/lane) of Cos7 cells expressing WAVE1 to WAVE3 (C1-3) were subjected to SDS-PAGE, followed by immunoblotting with anti-FLAG (far left panel) or anti-WAVE1 to -WAVE3 as indicated. Mol. Wt. indicates molecular weight in kilodaltons.
In vitro binding of GST fusion proteins to WAVEs and WASP. (A-D) Platelet lysates prepared without stimulation were subjected to GST pull-down assay. The precipitates eluted from glutathione Sepharose, which had been immobilized with GST, GST–profilin I, GST-SH3 domain of IRSp53, or GST-SH3 (the amino terminal SH3) of CrkL, were equally divided and examined for the presence of WAVE1 (A), WAVE2 (B), WASP (C), or WAVE3 (D) as indicated by immunoblotting. For WAVE3 (D), whole-cell lysates from platelets (7.5 × 106 cells) were also subjected to SDS-PAGE followed by immunoblotting. (E-G) Cos7 cells were transfected with FLAG-tagged WAVE1 (E), WAVE2 (F), or WAVE3 (G) and lysed. The cell lysates (500 μg) were incubated with glutathione Sepharose, which had been immobilized with GST, GST-SH3 of abl, GST–profilin I, GST-SH3 domain of IRSp53, or GST-SH3 (the amino terminal SH3) of CrkL as indicated. The presence of WAVE1 to WAVE3 was examined by using an anti-FLAG monoclonal antibody (M2).
In vitro binding of GST fusion proteins to WAVEs and WASP. (A-D) Platelet lysates prepared without stimulation were subjected to GST pull-down assay. The precipitates eluted from glutathione Sepharose, which had been immobilized with GST, GST–profilin I, GST-SH3 domain of IRSp53, or GST-SH3 (the amino terminal SH3) of CrkL, were equally divided and examined for the presence of WAVE1 (A), WAVE2 (B), WASP (C), or WAVE3 (D) as indicated by immunoblotting. For WAVE3 (D), whole-cell lysates from platelets (7.5 × 106 cells) were also subjected to SDS-PAGE followed by immunoblotting. (E-G) Cos7 cells were transfected with FLAG-tagged WAVE1 (E), WAVE2 (F), or WAVE3 (G) and lysed. The cell lysates (500 μg) were incubated with glutathione Sepharose, which had been immobilized with GST, GST-SH3 of abl, GST–profilin I, GST-SH3 domain of IRSp53, or GST-SH3 (the amino terminal SH3) of CrkL as indicated. The presence of WAVE1 to WAVE3 was examined by using an anti-FLAG monoclonal antibody (M2).
WAVE3 uniquely failed to bind to GST–profilin I or the SH3 domain of IRSp53 (Figure 2D). Given these differences, we performed similar experiments using lysates from Cos7 cells expressing FLAG-tagged WAVE1 to WAVE3 (Figure 2E-G). The SH3 domain of IRSp53 efficiently precipitated WAVE2, while only a small amount of WAVE1 was precipitated (Figure 2E,F). WAVE3 failed to bind to GST or GST-fusion proteins except the SH3 domain of abl. These clear differences in ligand binding properties of WAVE1, WAVE2, and WAVE3 establish that each anti-WAVE antibody recognizes a distinct molecule, and none of the bands recognized by these antibodies is WASP, a ligand for the amino terminal SH3 domain of CrkL.
Translocation of WAVEs following platelet aggregation
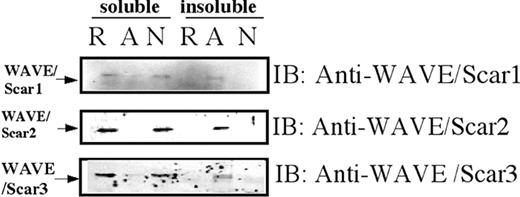
We next examined whether WAVEs translocate to the Triton X-100–insoluble pellets in activated platelets, which represent the operationally defined actin cytoskeleton.10,27 We found that all 3 WAVE isoforms translocated to the TRAP-activated actin cytoskeleton, depending upon platelet aggregation (Figure 3). These data suggest that these 3 isoforms may be involved in the organization of the actin cytoskeleton following platelet aggregation.
Association of WAVE proteins with Triton X-100–insoluble pellets. Platelets were lysed with Triton X-100–EGTA buffer before (R for resting) or after stimulation by TRAP (A for aggregation) or TRAP in the presence of RGDS (N for no aggregation). Lysates were separated by high-speed centrifugation into soluble fractions (soluble) and insoluble (insoluble) pellets. Proteins from each fraction were separated by SDS-PAGE electrophoresis and immunoblotted with anti-WAVE antibodies as indicated.
Association of WAVE proteins with Triton X-100–insoluble pellets. Platelets were lysed with Triton X-100–EGTA buffer before (R for resting) or after stimulation by TRAP (A for aggregation) or TRAP in the presence of RGDS (N for no aggregation). Lysates were separated by high-speed centrifugation into soluble fractions (soluble) and insoluble (insoluble) pellets. Proteins from each fraction were separated by SDS-PAGE electrophoresis and immunoblotted with anti-WAVE antibodies as indicated.
WAVEs are substrates for calpain in vitro and in vivo
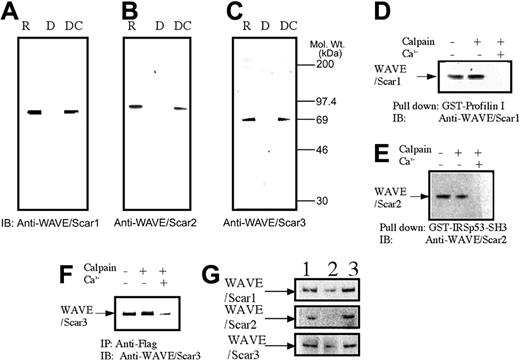
As WASP is a substrate for calpain in platelets,16,20 we asked whether the same was true for WAVEs. When the platelets were treated with dibucaine, known to induce calpain activation in platelets,16,33,34 we observed a significant loss of immunoreactive proteins that interacted with anti-WAVEs (Figure 4A-C, lane 2). Under the same or similar conditions, we and others previously reported that calpain and their substrates are also cleaved.16,33,34 Inhibition of calpain activation by adding calpeptin, a calpain inhibitor,35 resulted in the inhibition of cleavage of all 3 WAVE isoforms (Figure 4A-C, lane 3). Next, we asked whether calpain cleaves WAVE1 and WAVE2 in vitro.
Calpain-mediated cleavage of WAVEs. (A-C) Platelets were treated with calpeptin (20 μM) (lane 3) or 0.1% dimethyl sulfoxide (vehicle for calpeptin; lanes R = resting and D = dibucaine) for 15 minutes. The cells were then lysed in 1% SDS, before or 15 minutes after the addition of dibucaine (1 mM) or dibucaine + calpeptin. The proteins from 1.5 × 106 cells were separated on SDS-PAGE. After transferring to the nitrocellulose membrane, the membrane was probed with isoform-specific anti-WAVE antibodies. Lane R indicates resting cells; lane D, dibucaine-treated cells; and lane DC, calpeptin- and dibucaine-treated cells. (D-F) In vitro cleavage of WAVEs. WAVE1 or WAVE 2 was precipitated from platelets either by GST–profilin I (for WAVE1) or GST-IRSp53 (for WAVE2) as indicated. FLAG-tagged WAVE3 was expressed in Cos7 cells and immunoprecipitated by anti-FLAG monoclonal antibody. The precipitates were untreated or incubated with purified μ-calpain in the presence or absence of calcium ion as indicated. After denaturing in SDS, WAVE1 (D), WAVE2 (E), and WAVE3 (F) were detected by immunoblotting. (G) Cleavage of WAVEs during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After the addition of EGTA (5 mM) and ethylenediaminetetraacetic acid (5 mM), platelets were lysed by boiling in SDS sample buffer. WAVEs were detected by immunoblotting as described in panels A to C. Lane 1 indicates resting platelets; lane 2, thrombin stimulation of platelets for 30 minutes with stirring; and lane 3, thrombin stimulation for 30 minutes without stirring.
Calpain-mediated cleavage of WAVEs. (A-C) Platelets were treated with calpeptin (20 μM) (lane 3) or 0.1% dimethyl sulfoxide (vehicle for calpeptin; lanes R = resting and D = dibucaine) for 15 minutes. The cells were then lysed in 1% SDS, before or 15 minutes after the addition of dibucaine (1 mM) or dibucaine + calpeptin. The proteins from 1.5 × 106 cells were separated on SDS-PAGE. After transferring to the nitrocellulose membrane, the membrane was probed with isoform-specific anti-WAVE antibodies. Lane R indicates resting cells; lane D, dibucaine-treated cells; and lane DC, calpeptin- and dibucaine-treated cells. (D-F) In vitro cleavage of WAVEs. WAVE1 or WAVE 2 was precipitated from platelets either by GST–profilin I (for WAVE1) or GST-IRSp53 (for WAVE2) as indicated. FLAG-tagged WAVE3 was expressed in Cos7 cells and immunoprecipitated by anti-FLAG monoclonal antibody. The precipitates were untreated or incubated with purified μ-calpain in the presence or absence of calcium ion as indicated. After denaturing in SDS, WAVE1 (D), WAVE2 (E), and WAVE3 (F) were detected by immunoblotting. (G) Cleavage of WAVEs during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After the addition of EGTA (5 mM) and ethylenediaminetetraacetic acid (5 mM), platelets were lysed by boiling in SDS sample buffer. WAVEs were detected by immunoblotting as described in panels A to C. Lane 1 indicates resting platelets; lane 2, thrombin stimulation of platelets for 30 minutes with stirring; and lane 3, thrombin stimulation for 30 minutes without stirring.
WAVE1 and WAVE2 were precipitated by immobilized GST–profilin I and GST-IRSp53, respectively. The precipitates were incubated with μ-calpain in the presence or the absence of ionized calcium. The data of the blot shown in Figure 4D and 4E indicate that, following incubation with calpain, the immunoreactivity of both WAVE1 and WAVE2 was abolished in the presence of ionized calcium, indicating that μ-calpain cleaves WAVE1 and WAVE2 in vitro. We performed similar experiments using WAVE3 expressed in Cos7 cells (Figure 4F) and found that the same is true for WAVE3. Calpain activation is also an event downstream of platelet aggregation. When platelets were activated with thrombin and simultaneously stirred to induce platelet aggregation, a significant loss of WAVE immunoreactivity was observed (Figure 4G, middle column). Omitting stirring to minimize aggregation or prevention of aggregation inhibited the loss of immunoreactivity (Figure 4G, third column). Because activation of calpain could occur during lysis of platelets, these experiments were performed by the addition of EGTA and EDTA immediately before the lysis of platelets to prevent artificial cleavage.36
Localization of WAVEs in spreading platelets
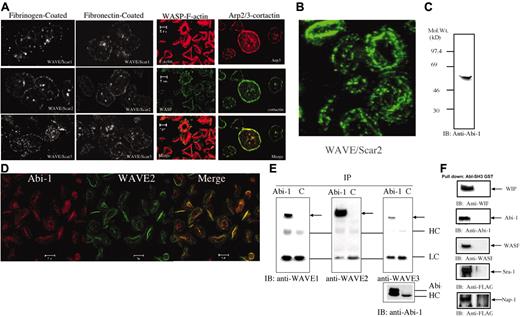
Cortactin, a recognized Arp2/3 complex regulator, is known to localize at the rim of lamellipodia of platelet, where the Arp2/3 complex is also localized.37,38 In glass-activated spreading human platelets, portions of WAVE1 to WAVE3 localized to the edge of the lamellipodia, as did Arp3, polymerized actin (F-actin), WASP, and cortactin (Figure 5A), indicating that the accumulation in the highly dynamic actin-cytoskeleton is a common feature of all potential regulators of the Arp2/3 complex, including WASP and WAVEs, suggesting that they potentially regulate actin polymerization during spreading. However, on close examination, among the 3 isoforms, WAVE2 appears to be most consistently concentrated in the edges of lamellipodia. Further, a significant portion of WAVE1 to WAVE3 appears to be distributed in other parts of lamellipodia. To determine the exact location of WAVE proteins, we simultaneously fixed and permeabilized spread platelets as described previously.14 WAVE2 was detected at linear structures throughout the lamellipodia, including the rim (Figure 5B). However, WAVE1 and WAVE3 staining showed poorly defined dotlike structures, which are not concentrated in the rim (data not shown). Given this information, among the 3 isoforms, WAVE2 likely plays the most critical role in reorganization of the lamellipodia.
Fluorescence micrographs showing the localization of WAVEs, WASP, cortactin, Arp3, and F-actin in human platelets. (A) Platelets were allowed to attach and spread on fibrinogen-coated (0.1 mg/mL) or fibronectin-coated (20 μg/mL) or glass coverslips and were fixed and stained for WAVEs with specific affinity-purified antibodies. Platelets spread on uncoated glass coverslips were stained for F-actin, WASP, Arp3, and cortactin as indicated. Before use, coated coverslips were blocked with bovine serum albumin (1% in phosphate buffered saline) for 1 hour at 37°C. (B) WAVE2 is observed in the rim of the lamellipodia following permeabilization and fixation of spread platelets and stained with anti-WAVE2. The presence of WAVE2 was examined as in panel A, except that spread platelets were fixed in 4% paraformaldehyde in PHEM buffer (described in “Materials and methods”) containing a 1/50 dilution of phalloidin with 0.25% Triton for 20 minutes at room temperature. (C) The presence of Abi-1. Platelet whole-cell lysate was subjected to SDS-PAGE, followed by immunoblotting using an anti–Abi-1 monoclonal antibody (4E2). (D) Colocalization of WAVE2 and Abi-1. Fixation of spread platelets was done as in panel B, except that the sample was stained with both anti-WAVE2 and anti–Abi-1 (4E2). (E) Association of WAVE/Scars and Abi-1. Whole-cell lysates from resting platelets were subjected to immunoprecipitation using either anti–Abi-1 (1G9) or control IgG (C). The precipitates were equally divided into 4 aliquots; SDS-PAGE and immunoblotting were performed by using anti-WAVE1 (left), anti-WAVE2 (middle), anti-WAVE3 (right), and anti–Abi-1(1G9; bottom right). The blotting was performed on the same membrane. HC and LC indicate heavy and light chains of IgG. The relative positions of Wave/Scars are indicated by arrows. (F) WIP, Abi-1, WASP, Sra-1, and WASP are precipitated by GST-SH3 of abl but not GST. For WIP, Abi-1, and WASP, platelet proteins were precipitated by GST-SH3 of abl. The precipitates were subjected to SDS-PAGE, followed by immunoblotting by using the antibodies indicated. For Sra-1 and Nap-1, the FLAG-tagged proteins were expressed in Cos7 cells. Equal amount of the cell lysates were incubated with GST-SH3 of abl or GST alone. The precipitates were subjected to SDS-PAGE, followed by immunoblotting by using an anti-FLAG monoclonal antibody.
Fluorescence micrographs showing the localization of WAVEs, WASP, cortactin, Arp3, and F-actin in human platelets. (A) Platelets were allowed to attach and spread on fibrinogen-coated (0.1 mg/mL) or fibronectin-coated (20 μg/mL) or glass coverslips and were fixed and stained for WAVEs with specific affinity-purified antibodies. Platelets spread on uncoated glass coverslips were stained for F-actin, WASP, Arp3, and cortactin as indicated. Before use, coated coverslips were blocked with bovine serum albumin (1% in phosphate buffered saline) for 1 hour at 37°C. (B) WAVE2 is observed in the rim of the lamellipodia following permeabilization and fixation of spread platelets and stained with anti-WAVE2. The presence of WAVE2 was examined as in panel A, except that spread platelets were fixed in 4% paraformaldehyde in PHEM buffer (described in “Materials and methods”) containing a 1/50 dilution of phalloidin with 0.25% Triton for 20 minutes at room temperature. (C) The presence of Abi-1. Platelet whole-cell lysate was subjected to SDS-PAGE, followed by immunoblotting using an anti–Abi-1 monoclonal antibody (4E2). (D) Colocalization of WAVE2 and Abi-1. Fixation of spread platelets was done as in panel B, except that the sample was stained with both anti-WAVE2 and anti–Abi-1 (4E2). (E) Association of WAVE/Scars and Abi-1. Whole-cell lysates from resting platelets were subjected to immunoprecipitation using either anti–Abi-1 (1G9) or control IgG (C). The precipitates were equally divided into 4 aliquots; SDS-PAGE and immunoblotting were performed by using anti-WAVE1 (left), anti-WAVE2 (middle), anti-WAVE3 (right), and anti–Abi-1(1G9; bottom right). The blotting was performed on the same membrane. HC and LC indicate heavy and light chains of IgG. The relative positions of Wave/Scars are indicated by arrows. (F) WIP, Abi-1, WASP, Sra-1, and WASP are precipitated by GST-SH3 of abl but not GST. For WIP, Abi-1, and WASP, platelet proteins were precipitated by GST-SH3 of abl. The precipitates were subjected to SDS-PAGE, followed by immunoblotting by using the antibodies indicated. For Sra-1 and Nap-1, the FLAG-tagged proteins were expressed in Cos7 cells. Equal amount of the cell lysates were incubated with GST-SH3 of abl or GST alone. The precipitates were subjected to SDS-PAGE, followed by immunoblotting by using an anti-FLAG monoclonal antibody.
We recently applied mass spectrometry to identify a ligand for the SH3 domain of CrkL.39 To gain more insight into the mechanisms of the regulation of WAVE/Scars in platelets, we tried to identify the ligands of abl-SH3 in a similar fashion, because abl-SH3 binds to WAVE/Scar1 to WAVE/Scar332 (Figure 1), and it may be directly or indirectly associated with the interacting proteins of WAVE/Scar1 to WAVE/Scar3. Platelet proteins were precipitated by the SH3 domain of abl, expressed as a GST-fusion protein, and subjected to SDS-PAGE electrophoresis followed by CBB staining. We observed 140, 120, 110, 80, 55, and 45 kDa bands, which were consistently precipitated by GST–abl-SH3 but not GST alone. To identify the proteins present in these bands, the gels were sliced and, following digestion by trypsin, analyzed by MALDI-TOF/MS using a Voyager-DE/STR. The proteins were identified by comparing the molecular weights determined by MALDI-TOF/MS, and the theoretical peptide masses of the proteins were registered in the NCBInr database as described.39 The identified proteins are shown in Table 1. Although we only examined proteins visualized by CBB, we were able to identify WAVE2, the most abundant WAVE isoform in platelets. Another protein is VASP, which was reported to associate with the SH3 domain of abl.40 Interestingly, among them, Nap1 has been shown to form a complex with WAVE1.41 Moreover, evidence has been provided that in Drosophila cells the counterparts of mammalian cytoplasmic FMRP interacting protein 1/p140Sra-1 (Sra-1), Nap1, and spectrin SH3 domain binding protein1/Abi-1 may be essential for the stabilization of WAVE.42 More recently, it was reported that Sra-1, Nap1, and Abi-1 make a complex with WAVE2 and are essential mediators of Rac-dependent WAVE2 signaling in the lamellipodia of mammalian cells.43-45 These observations suggest that human platelets not only express WAVE1 to WAVE3 but also their potential interacting proteins. In agreement with this possibility, we were able to demonstrate that platelets express Abi-1 (Figure 5C) and that it extensively colocalizes with WAVE2 in spreading platelets (Figure 5D). We also found that anti–Abi-1 but not control IgG1 precipitate WAVE1 to WAVE3 from the lysates of resting platelets (Figure 5E). We also confirmed that WIP, Abi-1, and WASP are precipitated by GST-SH3 domain of abl but not GST alone (Figure 5F). Since antibodies against NAP-1 and Sra-1 have not been available to us, we expressed these proteins with a FLAG-tag in Cos7 cells. GST-SH3 domain of abl but not GST alone precipitated these proteins from Cos7 cells (Figure 5F).
List of proteins, which are precipitated with abl-SH3 and identified by mass spectrometry
Apparent molecular weight, kDa . | Protein name . | GenBank accession no. . |
|---|---|---|
| 140 | Cytoplasmic FMRP interacting protein 1/p 140Sra-1 | BC005097 |
| 120 | Nck-associated protein 1/HEM-2 | BAA77295 |
| 110 | Membrane-associated protein HEM-1 | M58285 |
| 80 | WAVE2 | AB026542 |
| 65 | WASP | U12707 |
| WASP interacting protein (WIP) | AF031588 | |
| 55 | Spectrin SH3 domain binding protein 1/Abl-interactor protein 1 long | U87166 |
| 45 | Vasodilator-stimulated phosphoprotein (VASP) | Z46389 |
Apparent molecular weight, kDa . | Protein name . | GenBank accession no. . |
|---|---|---|
| 140 | Cytoplasmic FMRP interacting protein 1/p 140Sra-1 | BC005097 |
| 120 | Nck-associated protein 1/HEM-2 | BAA77295 |
| 110 | Membrane-associated protein HEM-1 | M58285 |
| 80 | WAVE2 | AB026542 |
| 65 | WASP | U12707 |
| WASP interacting protein (WIP) | AF031588 | |
| 55 | Spectrin SH3 domain binding protein 1/Abl-interactor protein 1 long | U87166 |
| 45 | Vasodilator-stimulated phosphoprotein (VASP) | Z46389 |
Discussion
WAVE proteins are encoded by 3 separate genes in mammalians. Relatively little is known of their tissue distribution.1,2 On the basis of Northern blot analysis, it was reported that WAVE1 and WAVE3 are predominantly expressed in neuronal tissues of mice, while WAVE2 is more ubiquitously distributed.29 Surprisingly, we found that all 3 isoforms of the WAVE proteins are expressed in human platelets, suggesting that a more rigorous determination of the tissue distribution of WAVEs at the protein levels is warranted. After original submission of this manuscript, the presence of WAVE2 in platelets was reported, although its localization in platelets and other information concerning WAVE2 were not described.46
Our finding that all 3 WAVE isoforms localize at the edge of the developing lamellipodia in spreading platelets (Figure 5) suggests a role of WAVE proteins in the development of platelet lamellipodia, as has been described for other cell systems.43-45,47,48 However, we noticed that WAVE2 is more consistently concentrated in the lamellipodia than the other 2 isoforms, which appear to distribute throughout the cells as dots, suggesting the possibility that WAVE2 plays a predominant role in the regulation of lamellipodia in platelets. Consistent with the idea, only WAVE2 was found at linear structures (including the rim area) in lamellipodia following permeabilization and fixation of spread platelets (Figure 5B and data not shown). On the basis of reports that platelets do not express N-WASP or at best very small amounts,19,20 and that platelets from patients with WAS lacking WASP appear to spread normally on the surface,17,18,45 we postulate that WAVEs (especially WAVE2) and cortactin rather than WASP or N-WASP play the predominant role in the regulation of actin polymerization in the lamellipodia of platelets. Recently, it was reported that WAVE2 requires phosphatidylinositol 3,4,5-trisphosphate for localization and promotion of lamellipodia in cell lines.49 Since the development of lamellipodia requires D3-polyphosphoinositides,50,51 it is possible that platelet-associated WAVE2 contributes to the development of lamellipodia downstream of rac and D3-polyphosphoinositides. The presence of Sra-1, Nap1, and Abi-1 in platelets (Table 1; Figure 5C), the colocalization of WAVE2 and Abi-1 in the spreading cells (Figure 5D), and the coprecipitation of WAVE1 to WAVE3 with Abi-1 from the platelet lysates (Figure 5E) raise the possibility that, in platelets, Sra-1, Nap1, and Abi-1 are important interacting proteins for WAVE/Scar proteins, as has been reported for fibroblasts.43,44 Although we found that a significant portion of WASP is localized at the edges of lamellipodia (Figure 5A), the significance of the finding remains to be determined in view of previous reports.17,18,45 We observed that the incorporation of all 3 WAVE isoforms to the actin-rich Triton X-100–insoluble pellets following stimulation of the platelets by TRAP is dependent on integrin-mediated platelet aggregation (Figure 3), suggesting that, in platelets, all 3 WAVE isoforms are involved in the dynamic reorganization of the actin cytoskeleton following integrin-mediated aggregation. Similar data were reported for cortactin.38 Another common feature of WAVEs is their cleavage following aggregation or dibucaine treatment of platelets, likely mediated by calpain. Indeed, we have confirmed that μ-calpain cleaves WAVE1 to WAVE3, dependent on calcium ion in vitro (Figure 4). We have previously reported that WASP was incorporated into the actin cytoskeleton following integrin-mediated aggregation and that it was also a substrate for calpain.16 Thus, the involvement of integrin-dependent actin organization and cleavage by calpain is a common feature of all WASP family proteins expressed in platelets except for N-WASP. The physiologic significance of calpain-mediated cleavage of WASP family proteins remains to be determined. Our study also revealed distinct features of WAVE proteins. In vitro binding studies, using platelet lysates and immobilized GST-fusion proteins, indicated that WAVE1 and WAVE2 prefer different ligands. The SH3 domain of IRSp53 precipitated WAVE1 less efficiently than GST–profilin I and vice versa (Figure 2). The finding that the SH3 domain of IRSp53 binds strongly to WAVE2, weakly to WAVE1, and not at all to WAVE3 is in complete agreement with the results of the previous report that used cell lines.20
Unlike WASP, neither WAVE1 nor WAVE2 binds to the amino terminal SH3 domain of CrkL, and WAVE3 does not appear to bind to any of these GST-fusion proteins. It has previously been reported that only WAVE1 among the WAVE proteins binds to protein kinase A.32 Although these results do not establish a direct functional interaction between WAVEs and their ligands, WAVEs may bind to different signaling molecules directly or indirectly, and the different signaling pathways may use a distinct WAVE protein to regulate the actin cytoskeleton. Nozumi et al52 have demonstrated that in the neuroblastoma cell line NG108, N-WASP and WAVE isoforms play distinct roles in this process. A more recent study also pointed out that WAVE1 and WAVE2 have distinct functions in the regulation of actin cytoskeleton.23 These findings and our results suggest that WAVE isoforms have distinct features. Consistent with this view are the recent reports that targeted disruption of the WAVE1 gene in mice leads to developments of neural defects, suggesting that WAVE isoforms have nonredundant and distinct functions in vivo.53,54 Our results also suggest that WAVE proteins are likely involved in integrin-dependent actin cytoskeletal reorganization, probably through the Arp2/3 complex, which is known to play a critical role in the regulation of the cytoskeleton in platelets.11,14,15 On the basis of our experimental approach, we cannot rule out the involvement of WAVEs in the actin polymerization of suspended platelets in the absence of aggregation. Furthermore, the relationship between WAVEs and other regulators of actin polymerization such as VASP, kaptin/2E4, gelsolin, or cofilin remain to be determined.1-4,10,11,15,55
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2003-04-1319.
Supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Technology of Japan (I.W., H.F., K.M., A.O.), Core Research for Evolutional Science and Technology (CREST) (T.T., I.W.), the National Institutes of Health (HD17427; H.D.O.), the March of Dimes Birth Defects Foundation (6-F496-0330; H.D.O.), the Jeffrey Modell Foundation, the DeJoria Wiskott-Aldrich Research Fund (H.D.O.), Human Frontier Science Program (A.O., L.M.M.), Iijima Foundation for Food Science (A.O.), Asahi Breweries Foundation (A.O.), Akiyama Foundation (A.O.), Fuji Foundation For Protein Research (A.O.), Smoking Research Foundation (A.O.), and the Clark Foundation (A.O.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs. Brian J. Druker, Ann Marie Pendergast, and Takashi Nagase for generously providing reagents and Ms. Tomoko Kawasaki for technical assistance. We thank Dr. Elaine L. Bearer for helpful advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal