Abstract
Protein Z is a vitamin K–dependent glycoprotein that plays a role in the regulation of coagulation. A nucleotide substitution of G by C in exon II of the protein Z gene, resulting in the replacement of Glu-30 with Gln (E30Q), and a G to A transition at the 79th nucleotide in intron F (IntF79G/A) were heterozygously identified in a patient with a severe thrombotic tendency, whose plasma protein Z level was about 15% of normal. Other vitamin K–dependent coagulation factors were within normal ranges. Glu-30 is one of 13 γ-carboxylation sites in protein Z and is well conserved among vitamin K–dependent proteins. Expression studies revealed that the E30Q mutant was not released from synthesizing cells, although wild-type protein Z was readily secreted in a vitamin K–dependent fashion. The E30Q mutant was N-glycosylated, γ-carboxylated, and translocated from the endoplasmic reticulum (ER) to the Golgi in the presence of vitamin K, as was the wild type. Coexpression of E30Q with wild-type protein Z interfered with the secretion of the wild type, while only a minor or no effect was observed on the secretion of factor X and plasminogen. The IntF79A allele has been reported to be also associated with lowered protein Z levels.
Introduction
Protein Z (PZ) is a vitamin K–dependent plasma glycoprotein homologous to coagulation factors VII, IX, and X and protein C. Mature human PZ consists of 360 amino acids containing 13 γ-carboxyglutamic acid (Gla) residues, and 1 Gla, 2 epidermal growth factor (EGF), and 1 serine protease domain(s).1,2 PZ is not the zymogen of a serine protease, because it lacks the His and Ser residues of the catalytic triad. The gene for human protein Z is localized to chromosome 13q34 where the genes for factors VII and X exist side by side, and it spans approximately 14 kilobase (kb) consisting of 9 exons, including 1 alternative exon.3
PZ is synthesized in the liver and secreted into the circulation. Plasma concentrations of PZ vary widely among individuals (0.6-5.7 mg/L) with an average of 2.9 mg/L,4 and concentrations are low in newborn infants5 and patients with chronic liver disease.6 Warfarin treatment reduces the PZ antigen in plasma to levels much lower than those of other warfarin-treated vitamin K–dependent proteins.4
PZ forms a calcium ion–dependent complex with factor Xa on the phospholipid surface and thereby serves as a cofactor for the inhibition of factor Xa by a PZ-dependent protease inhibitor.7,8 PZ-null mice have an apparently normal phenotype, whereas PZ deficiency dramatically enhances the thrombotic phenotype in mice carrying the factor VLeiden genotype.9 This situation is also reported in humans.10 These findings indicate an important role of PZ in the regulation of coagulation.
Recently, many disease states have been reported in relation to PZ deficiency: ischemic stroke,11 antiphospholipid syndrome,12 unexplained early fetal loss,13 acute coronary syndrome,14 ischemic colitis,15 and so on. However, the mechanism of PZ deficiency has not been elucidated thus far. At least some of these cases are of hereditary origin.13 In the present study, we analyzed the PZ gene in a patient with a very low concentration of PZ in plasma and identified a nucleotide substitution in the coding region leading to the replacement of a Glu-30 amino acid residue in the Gla domain. We also performed a cDNA expression study to examine whether the amino acid substitution was causative of PZ deficiency.
Patient, materials, and methods
Patient
A German female born in 1966 had had at least 3 episodes of deep vein thrombosis and 1 of spontaneous miscarriage as described elsewhere (B.K.-M. et al30 ). Although she was a heterozygote of the factor VLeiden mutation with activated protein C resistance, all other parameters of coagulation, fibrinolysis, and platelets, including vitamin K–dependent coagulation factors, were within normal ranges except her plasma PZ concentration, which was substantially low, 0.32 and 0.4 mg/L on 2 separate occasions, despite the absence of warfarin treatment or liver dysfunction. No blood samples were available from members of the patient's family.
Approval was obtained from the institutional review board of Yamagata University School of Medicine (Yamagata, Japan) for these studies. Informed consent was provided according to the Declaration of Helsinki.
Sequencing
Nine exons and their boundaries of the PZ gene, including 1 alternative splicing exon, were amplified from genomic DNA of the patient by a polymerase chain reaction (PCR) using primers as described previously.3 The PCR products were directly sequenced by using a Big-Dye Terminator Cycle Sequencing Kit and a 310 Genetic Analyzer (Applied Biosystems, Foster City, CA). The PCR products of several exons were also cloned into a pBluescript II vector (Stratagene, La Jolla, CA), and resultant subclones were sequenced.
PCR-restriction fragment length polymorphism (RFLP) analysis
An antisense primer Q30-R (5′-CAGTGACTACTTCATTTTCAAACAATT-3′), containing 1 base mismatch (bold italic face), was designed to introduce a restriction site for the MfeI endonuclease into a PCR product of the mutant allele (Figure 1A). PCR was performed using Q30-R and a sense primer (5′-GGCTGAGAGCCTGTGGAG-3′), and genomic DNA from individuals as a template, for 35 cycles of 96°C for 30 seconds, 52°C for 30 seconds, and 72°C for 30 seconds. PCR product (5 μL) was reacted with 2 U MfeI in a 20-μL reaction mixture at 37°C for 4 hours. The samples were electrophoresed using a 2% agarose gel. PCR-RFLP for R255H was also performed using 2 oligonucleotides: forward primer (VIII-S), 5′-GAA GGATCCAAGACCCGCTGATGATCAAGA-3′, and reverse primer (PZ-RH), 5′-CGCTGAGGAGGCCCCTGGCG-3′. The latter was a mutagenesis primer designed to create a new restriction site for HhaI by substituting the T nucleotide with C (bold italic). PCR products were digested with the HhaI endonuclease at 37°C for 2 hours, and the reaction mixture was applied to a 2% agarose gel.
E30Q mutation identified in patient's PZ gene. (A) The normal nucleotide sequence of the PZ gene is shown at the top and a nucleotide substitution in exon II is indicated by a bold letter. A primer for PCR-RFLP analysis, the Q30R primer, was designed with 1 base mismatch (in italics) to generate an MfeI endonuclease restriction site in a PCR product from the mutant allele (bottom). (B) PCR products obtained from the patient (P) and healthy controls (1-8) were applied to agarose gel electrophoresis after MfeI digestion. Only the mutant Q30 allele was cleaved into 262–base pair (bp) and 25-bp fragments, although the latter is too small to detect. (C) Alignment of the amino acid sequences of Gla domains among vitamin K–dependent proteins. The position of E30 in PZ is shown in bold. GAS6 indicates growth arrest specific 6; BGP, bone Gla protein; MGP, matrix Gla protein.
E30Q mutation identified in patient's PZ gene. (A) The normal nucleotide sequence of the PZ gene is shown at the top and a nucleotide substitution in exon II is indicated by a bold letter. A primer for PCR-RFLP analysis, the Q30R primer, was designed with 1 base mismatch (in italics) to generate an MfeI endonuclease restriction site in a PCR product from the mutant allele (bottom). (B) PCR products obtained from the patient (P) and healthy controls (1-8) were applied to agarose gel electrophoresis after MfeI digestion. Only the mutant Q30 allele was cleaved into 262–base pair (bp) and 25-bp fragments, although the latter is too small to detect. (C) Alignment of the amino acid sequences of Gla domains among vitamin K–dependent proteins. The position of E30 in PZ is shown in bold. GAS6 indicates growth arrest specific 6; BGP, bone Gla protein; MGP, matrix Gla protein.
Expression vector construction
PZ cDNA was prepared by a reverse-transcriptase (RT)–PCR from human liver RNA by using the primers BF, 5′-TCCGGATCCGAATGGCAGGCTGCGTCCCAC-3′ (sense), and BR, 5′-GCTGGGATCCAGTTAGTTCATGATCTGTTTAAA-3′ (antisense) containing a BamHI restriction site (underlined). Mutant cDNA was prepared by PCR site-directed mutagenesis by using the primers QF, 5′-TCTATGAAGAAGCAAGACAAGTGTTTGAAAATGAAG-3′ (sense), and QR, 5′-CTTCATTTTCAAACACTTGTCTTGCTTCTTCATAGA-3′ (antisense), for E30Q. The amplified cDNA was digested with BamHI and inserted into a BamHI site of a mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA). Finally, the plasmid was purified using a plasmid MAXI kit (Qiagen, Valencia, CA).
Human factor X cDNA was also prepared by a RT-PCR from HepG2 RNA by using the primers FX-BF, 5′-CTCGGATCCCACCATGGGGCGCCCACTGCAC-3′ (sense), and FX-BR, 5′-GTGGGATCCTCACTTTAATGGAGAGGACGTTAT-3′ (antisense), containing a BamHI restriction site (underlined). The amplified cDNA was digested with BamHI and inserted into a BamHI site of an expression vector pcDNA3. Human plasminogen cDNA in a vector named ZEM219b/Glu-PLG (Asako Ooe and A.I., unpublished data, March 1998) was cleaved out by restriction enzymes BamHI and XbaI and inserted into BamHI-XbaI sites of an expression vector pcDNA3.
Transfection
Baby hamster kidney (BHK) cells were cultured in Dulbecco modified Eagle medium (D-MEM) containing 10% (vol/vol) fetal bovine serum (FBS), 1 × nonessential amino acid, and 1 × antibiotics-antimycotics. A 20-μg plasmid DNA was transfected to approximately 2 × 105 BHK cells by the calcium phosphate precipitation method. Mock-transfected cells were prepared by using a pcDNA3 vector without an inserted cDNA. For coexpression studies, medium was changed to D-MEM containing 2% FBS with 5 μg/mL vitamin K (Sigma, St Louis, MO), and cells were further cultured for 24 hours. To establish cells stably expressing PZ, 1/100 of cells 48 hours after transfection were seeded and further cultured in the presence of 2.5 mg/mL G418 (GIBCO, Grand Island, NY). Cell clones highly expressing PZ were selected by Northern blot screening using a digoxygenin (DIG)–labeled antisense PZ RNA as a probe and a DIG-nucleotide detection kit (Roche, Indianapolis, IN). All the subsequent expression studies were performed in the presence of 0.1 mg/mL G418.
Western blotting
Culture medium from transformed cells was mixed with an equal volume of a sodium dodecyl sulfate (SDS)–sample buffer consisting of 125 mM Tris (tris(hydroxymethyl)aminomethane) (pH 6.8), 2% SDS, 15% glycerol, 0.05% bromphenol blue. For barium-binding and cotransfection studies, 1 mL culture medium collected from a culture dish was mixed with 50 μL 0.25 M sodium citrate, then added to 50 μL 1 M barium chloride and rotated at 4°C for 1 hour. After centrifugation, the pellet was rinsed twice with 0.5 mL 20 mM Tris (pH 7.5), 150 mM sodium chloride, 10 mM barium chloride, and 10 mM benzamidine, dissolved with 50 μL 20 mM Tris (pH 7.5), 150 mM sodium chloride, 0.1 M ethylenediaminetetraacetate (EDTA), and 10 mM benzamidine, and boiled in 50 μL SDS-sample buffer with β-mercaptoethanol (β-ME). Cells were washed twice with phosphate-buffered saline and harvested in a solution containing 1% Triton X-100, 20 mM Tris (pH 7.5), 150 mM sodium chloride, 10 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The cell suspension was sonicated and boiled in an equal volume of the SDS-sample buffer, and 5% β-ME. Samples were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane (Advantec, Tokyo, Japan). After blocking, the membrane was incubated with 1:10 000-dilution of an anti–human PZ antibody (BioPur AG, Budendorf, Switzerland), followed by reaction with 1:2000-dilution of a horseradish peroxidase–conjugated anti–rabbit immunoglobulin G (IgG) antibody (Amersham, Piscat-away, NJ). Detection was achieved using an enhanced-chemiluminescent substrate (Amersham).
Pulse-chase experiments
Stably transformed cells (2 × 105 cells) grown in a standard medium for 24 hours were preincubated in methionine-free D-MEM containing 2% dialyzed FBS for 60 minutes. [35S]-methionine (50 μCi [1.85 MBq]) was then added to the cells and incubated at 37°C for 60 minutes. For chase, the medium was replaced by the standard medium containing 5 μg/mL vitamin K after labeling, and the cells were further incubated for an appropriate time before immunoprecipitation with the anti-PZ antibody. The radioactivity of the protein Z bands was quantified by using a FLA-2000 Fluoroimage Analyzer (Fuji Film, Tokyo, Japan).
Immunoprecipitation
Cells were harvested in 0.1% SDS, 0.1% Triton X-100, 10 mM Tris (pH 7.5), 150 mM sodium chloride, 1 mM benzamidine, and 1 mM PMSF. Preimmunized rabbit serum (5 μL) and 20% PANSORBIN (20 μL; Calbiochem, La Jolla, CA) were added to cell lysates and rotated for 1 hour to remove nonspecific materials. After centrifugation, 5 μL anti–human PZ antibody was added to the supernatant and incubated at 4°C overnight. The reaction mixture was mixed with 20 μL 20% PANSORBIN, rotated for 1 hour, and then collected by centrifugation. The precipitate was rinsed 3 times with 0.1% SDS, 0.1% Triton X-100, 10 mM Tris (pH 7.5), 150 mM sodium chloride, boiled in the SDS sample buffer, and subjected to SDS-PAGE.
Immunofluorescent microscopy
Stably transformed cells (2 × 105 cells) were seeded and cultured overnight without vitamin K. The medium was changed to a fresh one with or without 5 μg/mL vitamin K, and the cells were incubated for 24 hours, then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. An anti–protein disulfide isomerase (PDI) monoclonal antibody (StressGen, Victoria, Canada; 1:1000 dilution) was reacted with the permeabilized cells, followed by reaction with a rhodamine-conjugated anti–mouse IgG antibody (ICN, Aurora, OH). The cells were further incubated with a 1:1000 dilution of a rabbit anti–human PZ antibody coupled with a fluorescein isothiocyanate (FITC)–conjugated anti–rabbit IgG antibody (Organon Teknika, Durham, NC). Fluorescence was detected using an Olympus IX70/FLA microscope system and imaging was achieved by a DP70 imaging system (Olympus, Tokyo, Japan).
Results
Identification of a novel G/C nucleotide substitution (E30Q)
All 9 exons, including 1 alternative exon3 and their exon-intron boundaries of the PZ gene, in the subject with a reduced PZ level were amplified by PCR, and the products were directly sequenced. There were several nucleotide substitutions in the patient's PZ gene: A heterozygous G to C transversion at the 138th nucleotide position in exon II, a heterozygous A to G transition at the 42nd nucleotide position in intron C, a heterozygous G to A transition at the 79th nucleotide in intron F (IntF79G/A), and a heterozygous A to G transition at the 193rd position in exon VIII. The IntF79A allele has been reported to be associated with lower PZ levels.16
The nucleotide substitutions in exons II and VIII located in the coding region and resulted in the amino acid replacements of Glu-30 with Gln (E30Q; Figure 1A) and of Arg-255 with His (R255H), respectively. PCR-RFLP analyses for E30Q and R255H were carried out to search for these same nucleotide substitutions in healthy German individuals. E30Q was detected in the patient heterozygously but not in 127 healthy control subjects (Figure 1B). However, R255H was found in 14 of 222 control alleles (gene frequency = 0.063), and no correlation between plasma concentrations and the R255H allele was observed among individuals (data not shown). R255H as well as the remaining substitutions described in the first paragraph of this subsection must be genetic polymorphisms since the same nucleotide changes have also been identified homozygously or heterozygously in many other individuals.
Impaired secretion of Glu-30 mutant PZ
Glu-30 is 1 of 13 γ-carboxylation sites of PZ,1 and it is highly conserved among vitamin K–dependent proteins (Figure 1C), indicating the importance of this residue for the synthesis and structure or function of PZ. Accordingly, cDNA expression studies of wild-type (WT) and E30Q mutant PZs were carried out using BHK cells. The mRNAs for both PZs appeared to be produced equally well as shown in Figure 2A.
Expression of wild-type and E30Q mutant PZs in BHK cells. (A) Northern blotting of PZ mRNA obtained from BHK cells transfected with either WT or E30Q PZ vectors. The amount of mRNA for the E30Q mutant was comparable to that of the WT, as shown by RNA bands at the bottom. (B) Stably transformed BHK cells were incubated for 24 hours in the absence or the presence of 5 μg/mL vitamin K. Culture medium was directly subjected to SDS-PAGE under nonreducing conditions, followed by Western blotting using an anti–human PZ antibody. Protein (10 μg) of cell lysates was also analyzed by SDS-PAGE under reducing conditions and Western blotting. Mutant PZ was never detected in the culture medium, while WT PZ was readily found only when cultured in the presence of vitamin K. Note that the upper band (Gla) appeared inside cells only in the presence of vitamin K.
Expression of wild-type and E30Q mutant PZs in BHK cells. (A) Northern blotting of PZ mRNA obtained from BHK cells transfected with either WT or E30Q PZ vectors. The amount of mRNA for the E30Q mutant was comparable to that of the WT, as shown by RNA bands at the bottom. (B) Stably transformed BHK cells were incubated for 24 hours in the absence or the presence of 5 μg/mL vitamin K. Culture medium was directly subjected to SDS-PAGE under nonreducing conditions, followed by Western blotting using an anti–human PZ antibody. Protein (10 μg) of cell lysates was also analyzed by SDS-PAGE under reducing conditions and Western blotting. Mutant PZ was never detected in the culture medium, while WT PZ was readily found only when cultured in the presence of vitamin K. Note that the upper band (Gla) appeared inside cells only in the presence of vitamin K.
WT and E30Q PZs of about 60 kDa under reducing conditions were detected inside transfected cells, but they were not observed in the culture medium in the absence of vitamin K, a cofactor of γ-carboxylase (Figure 2B). In the presence of vitamin K, WT and E30Q PZs showed upper bands of about 68 kDa (Gla form) under reducing conditions inside cells, and about 64 kDa WT PZ under nonreducing conditions could be detected in the medium, indicating vitamin K–dependent modification (γ-carboxylation) and secretion of WT PZ. E30Q PZ was also synthesized and modified inside cells but could not be secreted outside even in the presence of vitamin K. These findings were again confirmed by pulse-chase experiments in which carboxylation was initiated by the addition of vitamin K. A newly synthesized nascent WT PZ was modified to a larger form (about 30%), and then the same amount was secreted into the medium, while a similar amount of E30Q PZ also became larger but never did secrete into the medium (Figure 3A).
Vitamin K–dependent intracellular modification and secretion of PZ. (A, top) Pulse-chase experiment. Cells grown under the vitamin K–free medium were labeled with [35S]-Met for 60 minutes and incubated with vitamin K in a standard medium. Radio-labeled cell lysate and culture medium, harvested at various time intervals after labeling, were immunoprecipitated with an anti-PZ antibody, followed by SDS-PAGE. Only the modified form (Gla) of WT PZ was secreted into the medium. Nas indicates nascent protein Z. (Bottom) Time course of the radioactive bands for protein Z (○, wild type; •, E30Q mutant). (B) Barium binding. Ten micrograms WT PZ or the E30Q expression vector was transfected to BHK cells. Forty-eight hours after transfection, cells were treated with vitamin K for 24 hours. PZs in the medium were collected by barium-citrate absorption. (C) Effect of warfarin. BHK cells stably expressing PZs were treated with vitamin K, 10 μg/mL warfarin, or both. The cell lysate and culture medium were analyzed by SDS-PAGE/Western blotting as described as in Figure 2B. Warfarin treatment completely reversed the vitamin K–dependent intracellular modification of WT and E30Q PZs.
Vitamin K–dependent intracellular modification and secretion of PZ. (A, top) Pulse-chase experiment. Cells grown under the vitamin K–free medium were labeled with [35S]-Met for 60 minutes and incubated with vitamin K in a standard medium. Radio-labeled cell lysate and culture medium, harvested at various time intervals after labeling, were immunoprecipitated with an anti-PZ antibody, followed by SDS-PAGE. Only the modified form (Gla) of WT PZ was secreted into the medium. Nas indicates nascent protein Z. (Bottom) Time course of the radioactive bands for protein Z (○, wild type; •, E30Q mutant). (B) Barium binding. Ten micrograms WT PZ or the E30Q expression vector was transfected to BHK cells. Forty-eight hours after transfection, cells were treated with vitamin K for 24 hours. PZs in the medium were collected by barium-citrate absorption. (C) Effect of warfarin. BHK cells stably expressing PZs were treated with vitamin K, 10 μg/mL warfarin, or both. The cell lysate and culture medium were analyzed by SDS-PAGE/Western blotting as described as in Figure 2B. Warfarin treatment completely reversed the vitamin K–dependent intracellular modification of WT and E30Q PZs.
Vitamin K–dependent modification of PZ
γ-Carboxylation of WT PZ was further demonstrated as shown in Figure 3B, whereby Gla-containing PZs were collected by barium-citrate absorption. Only Gla forms of the WT secreted into the culture medium was observed by Western blotting, indicating that its Gla domain was functionally intact in terms of the binding capability to barium-citrate.
The vitamin K–dependent modification and secretion of WT PZ was completely suppressed by the addition of warfarin, a potent inhibitor of γ-carboxylation, into the culture medium (Figure 3C). The fact that the upper bands of both the WT and E30Q PZs inside cells disappeared because of the warfarin treatment confirmed that the intracellular modification of nascent PZs was through the γ-carboxylation reaction.
N-glycosylation of PZ during its biosynthesis
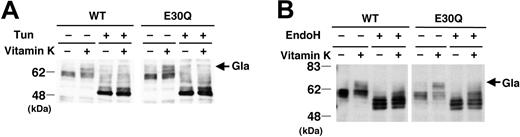
Human PZ is a glycoprotein and contains 5 potential N-linked carbohydrate attachment sites.1,2 Tunicamycin, a potent inhibitor of N-glycosylation, completely blocked the appearance of 60- to 64-kDa bands of WT and E30Q PZs inside cells (Figure 4A), indicating that both PZs undergo normal posttranslational processing in the endoplasmic reticulum (ER). In addition, both the WT and E30Q PZs were sensitive to endoH digestion (Figure 4B), indicating that most PZs are in a compartment(s) before medial Golgi.
N-Glycosylation of PZ. (A) Stably transformed cells were treated with 5μg/mL tunicamycin (Tun) for 24 hours in the presence or the absence of vitamin K. (B) Treatment of PZ with an endoglycosidase H (EndoH). Protein (10 μg) of cell lysate was treated with 2 mU EndoH for 24 hours and subjected to SDS-PAGE/Western blotting. N-glycosylation of PZs was completely suppressed by Tun treatment, and the carbohydrate moiety of PZs was removed by EndoH digestion.
N-Glycosylation of PZ. (A) Stably transformed cells were treated with 5μg/mL tunicamycin (Tun) for 24 hours in the presence or the absence of vitamin K. (B) Treatment of PZ with an endoglycosidase H (EndoH). Protein (10 μg) of cell lysate was treated with 2 mU EndoH for 24 hours and subjected to SDS-PAGE/Western blotting. N-glycosylation of PZs was completely suppressed by Tun treatment, and the carbohydrate moiety of PZs was removed by EndoH digestion.
Intracellular transportation of PZ in the presence of vitamin K
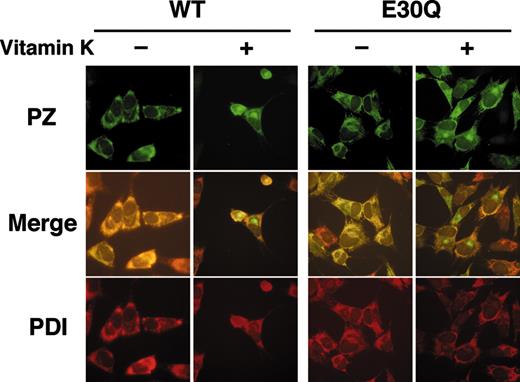
Stably transfected cells of WT and E30Q PZs showed a typical staining pattern of secretory proteins in the presence of vitamin K near the nuclei, which likely corresponds to the Golgi, and in a reticular pattern throughout the cytoplasm (Figure 5, top). On the contrary, cells of both PZs were stained only for the cytoplasm in the absence of vitamin K. These signals were colocalized with those for an ER marker protein, PDI (Figure 5, bottom). It is noteworthy that the intense signals near the nuclei observed only in the presence of vitamin K were not colocalized with those for PDI (Figure 5, middle), confirming that this structure is the Golgi apparatus.
Immunofluorescent microscopy of cells stably expressing PZ. Cells were first cultured for 24 hours in the presence or the absence of vitamin K. After fixation and permeabilization, cells were incubated with a rabbit anti–human PZ antibody, followed by reaction with a FITC-labeled anti–rabbit IgG (top). The same cells were also stained with an anti-PDI monoclonal antibody coupled with a rhodamine-labeled anti–mouse IgG (bottom). Merged images are shown in the middle. Note that both the WT and Q30E PZs were detected in the Golgi apparatus only in the presence of vitamin K. Fluorescence was detected as described in the methods section (magnification × 40).
Immunofluorescent microscopy of cells stably expressing PZ. Cells were first cultured for 24 hours in the presence or the absence of vitamin K. After fixation and permeabilization, cells were incubated with a rabbit anti–human PZ antibody, followed by reaction with a FITC-labeled anti–rabbit IgG (top). The same cells were also stained with an anti-PDI monoclonal antibody coupled with a rhodamine-labeled anti–mouse IgG (bottom). Merged images are shown in the middle. Note that both the WT and Q30E PZs were detected in the Golgi apparatus only in the presence of vitamin K. Fluorescence was detected as described in the methods section (magnification × 40).
These results clearly indicate that intracellular transportation of PZs to the Golgi is also dependent on vitamin K and the γ-carboxylation reaction of the PZ molecules.
Suppression of WT PZ secretion by E30Q mutant
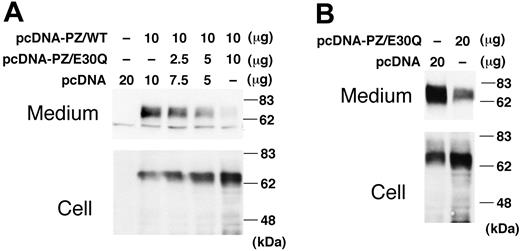
Since the E30Q mutation was identified only heterozygously in the patient's gene despite our extensive search, the WT PZ was coexpressed with the E30Q mutant in a transient expression system. The amount of the intracellular PZ increased with increasing amounts of the E30Q expression vector (Figure 6A). On the contrary, the more that the E30Q vector was cotransfected with WT PZ, the less the amount of PZ was secreted into the medium.
Coexpression of E30Q mutant with WT PZ. (A) WT PZ expression vector (10 μg) was cotransfected to BHK cells with varying amounts of the E30Q expression vector. Forty-eight hours after transfection, cells were treated with vitamin K for 24 hours. PZ in the medium was collected by barium-citrate absorption because of the low levels of PZ expressed by transient transfection. (B) The E30Q expression plasmid was transfected to the BHK cells stably expressing WT PZ.
Coexpression of E30Q mutant with WT PZ. (A) WT PZ expression vector (10 μg) was cotransfected to BHK cells with varying amounts of the E30Q expression vector. Forty-eight hours after transfection, cells were treated with vitamin K for 24 hours. PZ in the medium was collected by barium-citrate absorption because of the low levels of PZ expressed by transient transfection. (B) The E30Q expression plasmid was transfected to the BHK cells stably expressing WT PZ.
These results were confirmed when the E30Q expression vector was transfected into the cells stably expressing WT PZ. The amount of secreted PZ dramatically decreased with the addition of the mutant vector when compared with that of a mock vector (Figure 6B), whereas the amount of intracellular PZ increased. Accordingly, coexpression of the E30Q mutant clearly interferes with the secretion of the WT molecule, but not with its biosynthesis.
When the E30Q mutant was coexpressed with another vitamin K–dependent protein, factor X, or a non–vitamin K–dependent protein, plasminogen, only a slight decrease of the secretion of factor X was observed, while no effect was seen on plasminogen (data not shown). These results were consistent with the fact that coagulation and fibrinolytic factors were within normal ranges in the patient in vivo, as described in “Patient.”
Discussion
In this study we present the first case of naturally occurring genetic defects in the human PZ gene that result in its deficiency both in vivo and in vitro. The G/C (E30Q) substitution must be a mutation, not a mere polymorphism, because it was not detected in 127 individuals of the same ethnic population as the patient. Unfortunately, we could not conclude whether this nucleotide replacement had been inherited or had occurred de novo, since no samples were available from the patient's family members.
The pathologic implications of the E30Q mutation seem to be 2-fold as shown by the present in vitro studies: The E30Q molecule itself could not be secreted outside the synthesizing cells at all. Accordingly, a homozygote of this mutation would have a severe case of PZ deficiency, if he or she were born. Second, the E30Q mutant interferes with the secretion of WT PZ when coexpressed in the same cells. It is very likely, therefore, that a heterozygote of this mutation would not possess half of the normal amount of PZ in his or her plasma; rather, his or her plasma PZ level would be much less than half because of the suppressing effect of the mutant allele on the normal allele. This is consistent with the fact that the subject heterozygous for E30Q has about 15% of normal PZ level in her plasma. Additional studies on patients, transgenic animals, or both with this mutation, however, are needed to confirm this hypothesis in vivo.
In addition, the very low plasma PZ level in the patient may be also explained at least in part by low-level expression of the opposite E30 (non-E30Q) allele, since the patient possessed the IntF79A polymorphism as well. Its linkage to reduced PZ levels was suggested by Lithy et al16 of about 25% decrease in heterozygotes and 50% decrease in homozygotes. The promoter/enhancer sequences of human PZ, however, have not been reported thus far. The possibility of lowered expression of the opposite E30 allele could be also clarified, if samples from the patient's family were available.
At the moment, the mechanism(s) by which the E30Q mutation impairs the secretion of both the WT and the mutant itself is unclear. The E30Q PZ molecule was synthesized and N-glycosylated and γ-carboxylated in the ER, and then transported to the Golgi, as was the WT PZ; however, it was not secreted. Accordingly, transportation of the mutant from the Golgi to the plasma membrane may be impaired, it may be trapped by some quality control system outside the ER. γ-Carboxylase binds and releases vitamin K–dependent proteins. It is kept tightly bound to its substrates via their propeptides until the end of the full reaction of all target Glu residues in the Gla domains.17 The processivity of γ-carboxylase for the mutant seemed to be lower than that for the WT PZ, since the Gla form of E30Q appeared in an amount similar to the WT, but remained longer, as shown by pulse-chase experiments. Accordingly, a larger amount of the undercarboxylated mutant molecule may be retained by γ-carboxylase18,19 and cannot be secreted.
Alternatively, if the amount of γ-carboxylase in the synthesizing BHK cells in vitro (and hepatocytes in vivo) was too small and rate limiting, the coexpression of mutant PZ would result in the reduction of γ-carboxylation and secretion of the WT PZ as well as other vitamin K–dependent proteins. However, the coexpression studies demonstrated that both wild-type and mutant PZs did not drastically suppress the secretion of factor X, indicating that the amount of γ-carboxylase was apparently sufficient for processing both PZs and factor X.
Several amino acid substitutions of the Gla residues in other vitamin K–dependent proteins have been reported previously: E14K, E19A, and E32Q in factor X20-23 ; E7D, E20A, and E26K in protein C24-26 ; and so on. Among these studies, expression levels of both WT factor X and E7G mutant were similar in a mammalian cell system, indicating that this mutation has no affect on protein folding or secretion.27 Factor X antigen levels in the medium were also identical for the E25K mutant and WT expression constructs.28 Expression studies of E19A revealed that factor X antigen levels in the culture medium, the secretion efficiency, and the stability of WT and E19A were virtually identical.23 Thus, no mutations of the Glu residues in the Gla domain of factor X had any significant effect on the secretion of mutant molecules, unlike the E30Q mutation of PZ.
Finally, there is a wide range of variation in plasma PZ levels among individuals,4 and its acquired and hereditary deficiencies are not uncommon.13 Malnutrition such as vitamin K deficiency, liver dysfunction, or both must be responsible for most PZ deficiency but not for all. Thus, it is very likely that there are a number of mutations and polymorphisms in the human PZ gene, which may affect its biosynthesis.16,29
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-06-2250.
Supported in part by research grants from The Japanese Society for the Promotion of Sciences, and The Naito Foundation, Tokyo, Japan.
Presented in part at the 44th meeting of the Gesellschaft fur Thrombose und Hamostaseforschung (GTH), Freiburg, Germany, February 2000, and at the 26th annual meeting of the Japanese Society of Thrombosis and Hemostasis, Tokyo, Japan, November 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr F. Tokunaga for his invaluable discussion, and Drs T. Okumura and L. Gao for their help in sequencing the PZ gene of many individuals, Dr S. Alaoui for providing anti-PZ antibodies during preliminary experiments, and Mrs L. B. Joshi for her assistance in preparation of the manuscript.



![Figure 3. Vitamin K–dependent intracellular modification and secretion of PZ. (A, top) Pulse-chase experiment. Cells grown under the vitamin K–free medium were labeled with [35S]-Met for 60 minutes and incubated with vitamin K in a standard medium. Radio-labeled cell lysate and culture medium, harvested at various time intervals after labeling, were immunoprecipitated with an anti-PZ antibody, followed by SDS-PAGE. Only the modified form (Gla) of WT PZ was secreted into the medium. Nas indicates nascent protein Z. (Bottom) Time course of the radioactive bands for protein Z (○, wild type; •, E30Q mutant). (B) Barium binding. Ten micrograms WT PZ or the E30Q expression vector was transfected to BHK cells. Forty-eight hours after transfection, cells were treated with vitamin K for 24 hours. PZs in the medium were collected by barium-citrate absorption. (C) Effect of warfarin. BHK cells stably expressing PZs were treated with vitamin K, 10 μg/mL warfarin, or both. The cell lysate and culture medium were analyzed by SDS-PAGE/Western blotting as described as in Figure 2B. Warfarin treatment completely reversed the vitamin K–dependent intracellular modification of WT and E30Q PZs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-06-2250/6/m_zh80080576930003.jpeg?Expires=1765949477&Signature=HH3qRjgVlCu~xSCzn6-xCmjBCX~0iBSfiUnF03iELGHyhkTjsouqLnHNe5EXUH-jUCfihXm3lgK1qAJ~Ks1im-pSTnX-iufv4OEaYNHp~nV14tpDgqT23q40tL4nJRqcn~y6OfdqAK0i4VcQB4oNUXlbpNwnls76VR8JyzOkfy3b6r5bVcn75sdJdL2kUdF243rmPcoE7HK4kakJRBMCi3q3RDEJfGmzyenoOq-xruTm3m6i5ZVbuqBaAoGcple6FeYTnLIXE~qdumdFZcHdFcGGQDuGd353SxwetPbhJtVFxwWGzQm5rBIV80-Myz1ohe4c84AiVghUrke-cCfHpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal