Abstract
Erythropoiesis in the adult mammal depends critically on erythropoietin, an inducible cytokine with pluripotent effects. Erythropoietin gene expression increases under conditions associated with lowered oxygen content such as anemia and hypoxia. HIF-1α, the founding member of the hypoxia-inducible factor (HIF) alpha class, was identified by its ability to bind and activate the hypoxia-responsive enhancer in the erythropoietin regulatory region in vitro. The existence of multiple HIF alpha members raises the question of which HIF alpha member or members regulates erythropoietin expression in vivo. We previously reported that mice lacking wild-type HIF-2α, encoded by the EPAS1 gene, exhibit pancytopenia. In this study, we have characterized the etiology of this hematopoietic phenotype. Molecular studies of EPAS1-null kidneys reveal dramatically decreased erythropoietin gene expression. EPAS1-null as well as heterozygous mice have impaired renal erythropoietin induction in response to hypoxia. Treatment of EPAS1-null mice with exogenous erythropoietin reverses the hematopoietic and other defects. We propose that HIF-2α is an essential regulator of murine erythropoietin production. Impairments in HIF signaling, involving either HIF-1α or HIF-2α, may play a prominent role in conditions involving altered hematopoietic or erythropoietin homeostasis.
Introduction
Hematopoiesis involves complex interactions of specialized cell types and molecular signaling events that vary according to development.1 The major sites of murine hematopoietic development depend on the particular developmental or postnatal stage.2,3 Erythropoiesis, that aspect of hematopoiesis concerned with generation of erythrocytes, occurs in 2 distinct developmental phases characterized by primitive erythropoiesis in the yolk sac blood islands and definitive erythropoiesis in the fetal liver or bone marrow later in development.1,4,5 Besides location, primitive and definitive erythropoiesis also differ in their growth factor or cytokine requirements.
Erythropoietin was first described as an endocrine regulator of erythropoiesis produced in the kidneys; later studies revealed a paracrine role of this cytokine in global hematopoiesis and other aspects of mammalian physiology.6-9 Primitive erythropoiesis in the yolk sac is erythropoietin-independent, but requires other cytokines such as vascular endothelial growth factor (VEGF) and c-Kit.10-12 In contrast, definitive erythropoiesis in the fetal liver and in the adult bone marrow is erythropoietin-dependent as evident by gene disruption studies of the erythropoietin13 or erythropoietin receptor14 gene. The temporal distinction of primitive and definitive erythropoiesis is in part due to developmental timing of erythropoietin and erythropoietin receptor gene expression.15
The sites of definitive erythropoiesis are also sites of erythropoietin production.16 These sites include resident macrophages in the adult bone marrow as well as in the fetal liver blood islands,17 the predominant sites of definitive erythropoiesis in the adult and neonate, respectively. Erythropoietin produced in these locations may function as a paracrine growth factor for global hematopoiesis. However, despite its production in and impact on the bone marrow compartment, the kidney is the major production site of circulating erythropoietin in the adult mammal.18
Although the kidney has long been recognized as the source of endocrine erythropoietin,18 the exact site of renal erythropoietin production has been a topic of debate.6 Molecular and transgenic mouse studies provide evidence for erythropoietin production in at least 2 different candidate cell types, the proximal tubular cells19,20 and the peritubular or interstitial cells.21-24 Transgenic mice generated using a human erythropoietin transgene demonstrated erythropoietin production in the renal peritubular interstitial cell population,25 whereas transgenic mice using a mouse erythropoietin promoter-lacZ transgene revealed lacZ expression in the proximal tubular cells.26 Another transgenic mouse strain resulting from homologous recombination of a simian virus 40 (SV40) epitope-tagged erythropoietin transgene localized erythropoietin production to the fibroblast interstitial population.27 Hence, several cell populations are candidate sites for erythropoietin production in the kidney.
Renal erythropoietin production is regulated primarily at the transcriptional level and is markedly induced by anemia or global hypoxia.28 Identifying the molecular mechanisms controlling erythropoietin gene expression was enabled by the identification of a hepatic cell line that expressed erythropoietin in a hypoxia-dependent manner.29,30 Investigators defined the enhancer element in the erythropoietin gene responsible for hypoxia induction31-33 and used DNA affinity chromatography to identify31 and purify34 a heterodimer protein complex, hypoxia inducible factor 1 (HIF-1). HIF-1 consists of 2 members of the basic helix-loop-helix (bHLH): Per-Arnt-Sim (PAS) domain containing transcription factor family, HIF-1 alpha (HIF-1α) and HIF-1 beta (HIF-1β), with the latter previously identified as the aryl hydrocarbon nuclear translocator (ARNT).35
Biologic specificity of bHLH-PAS domain transcription factors is dictated by the alpha component whereas biologic activity is conferred by the beta component. With the use of bioinformatics, additional members of the HIF alpha family were identified. Endothelial PAS domain protein 1 (EPAS1),36 also known as HIF-2α, is closely related to HIF-1α in composition. Although early studies of HIF-2α primarily evaluated whether biologic actions of HIF-2α were similar to those of HIF-1α, more recent investigations have highlighted unique aspects for HIF-2α or HIF-1α.37-40 Evidence for dual requirements of HIF-1α and HIF-2α in the in vivo regulation of HIF target genes in the adult mouse has not been presented to date.
Targeted disruption of HIF members provided insights into their roles in hematopoiesis. Embryos lacking ARNT have defects in yolk sac vascularization, suggesting a defect in primitive hematopoiesis.41 Differentiation assays of ARNT–/– embryonic stem (ES) cells suggest ARNT is essential for global hematopoiesis.42 Further characterization with ARNT–/– ES cell assays suggested that the absence of VEGF might be responsible for the global hematopoietic defect. Since VEGF is required for primitive erythropoiesis and since VEGF is a target gene for HIF-1, the ARNT–/– ES cell experiments suggest a role for HIF members in primitive erythropoiesis. Moreover, the ineffectiveness of exogenous erythropoietin upon ARNT–/– ES cell differentiation constitute further evidence that the ARNT–/– hematopoietic phenotype may involve abnormal primitive erythropoiesis.42
Chimeric and conditional knockout mice have provided some insights into the role of HIF-1 in B-cell development43 and myeloid cell function,44 respectively. Examination of HIF-1α+/– mice revealed a minor impairment in chronic continuous hypoxia-stimulated erythrocytosis,45 whereas a brief period of intermittent or continuous hypoxia treatment revealed a lack of induction of renal erythropoietin gene expression.9 These results suggest a role for HIF-1 in erythropoietin gene expression in the adult mouse and hence in definitive erythropoiesis. However, a specific role for HIF-1 in global hematopoiesis has been hampered by the unavailability of HIF-1α–/– mice.
The generation of adult mice globally lacking HIF-2α (EPAS1–/– mice) allowed biologic roles for HIF-2α to be defined in the context of the intact organism.46,47 One aspect of the EPAS1–/– phenotype is hematopoietic deficiency.47 Transplantation experiments using irradiated EPAS1–/– mice as recipients of syngeneic EPAS1+/+ hematopoietic stem cells revealed that the hematopoietic defect in EPAS1–/– mice is due to altered bone marrow microenvironment function and/or to effects of the EPAS1–/– state on expression of systemic mediators.47 Gene expression of VEGF members and VEGF receptors in the heterogeneous bone marrow cell population is not significantly different between EPAS1–/– and EPAS1+/+ mice, implying that abnormalities in VEGF signaling may not be responsible for the hematopoietic defect in EPAS1–/– mice.47 We reasoned that a systemic mediator of hematopoietic development might be impaired in EPAS1–/– mice. Given the potential role of erythropoietin in global hematopoiesis and the likely role of HIF members in erythropoietin regulation, we were particularly interested in determining whether abnormalities in erythropoietin signaling might be responsible for the hematopoietic defect observed in EPAS1–/– mice.
Materials and methods
Animal studies
We generated F1 hybrid mice by crossing 129+/– female mice with C57+/– male mice carrying a single mutant EPAS1 allele as described.47 Mice housed in a standard 12:12 light/dark cycle were fed ad lib with 4% fat-containing chow (mating pairs) or 11% fat-containing chow (pregnant females and newborn mice up to one month old). Polymerase chain reaction (PCR) genotyping was performed as described.47
We performed hypoxia experiments using either short-term, medium-term, or long-term treatment protocols. For short-term hypoxia treatments, mice were housed in a 13-L plexiglass container and exposed to air mixtures using flow-through air delivery by pressurized tanks at a rate of 2 L per minute. For room air (RA) treatments, age- and gender-matched mice were exposed to 6 RA/RA cycles with each cycle consisting of 5 minutes of RA (21% oxygen/79% nitrogen) followed by 5 minutes of RA (21% oxygen/79% nitrogen). For short-term intermittent hypoxia (STIH), age- and gender-matched mice were exposed to 6 RA/hypoxia cycles with each cycle consisting of 5 minutes of RA (21% oxygen/79% nitrogen) followed by 5 minutes of hypoxia air mixture (6% oxygen/94% nitrogen). Mice were harvested for tissues 1 hour after completion of the STZH treatment protocol (2 hours after the initiation of the first hypoxia cycle) for RNA or harvested immediately for protein. For short-term continuous hypoxia (STCH) or medium-term continuous hypoxia (MTCH), age- and gender-matched mice were exposed to hypoxia air mixture (6% oxygen/94% nitrogen) for 2 hours or 4 hours, respectively. Mice were harvested for tissues immediately after completion of the STCH or MTCH treatment protocol.
For long-term continuous hypoxia (LTCH), age- and gender-matched mice were housed individually in cages contained within a large plexiglass chamber. Mice were exposed for 12 days to hypoxia air mixture (10% oxygen/94% nitrogen) using flow-through air delivery by pressurized tanks at a rate of 2 L per minute. Carbon dioxide scrubbers were present to keep levels below 0.5% as assessed by daily checks. Cage changes were made halfway through the hypoxia treatment protocol and mice were fed ad lib with standard chow.
We performed rescue experiments with intraperitoneal injections on Mondays, Wednesdays, and Fridays of low-dose48 (500 U/kg body weight) or high-dose49 (5000 U/kg body weight) recombinant human erythropoietin (Epogen; Amgen, Thousand Oaks, CA) prepared in phosphate-buffered saline (PBS). Erythropoietin injections with 500 U/kg began after postnatal day 30 and increased to 5000 U/kg after postnatal day 50. Age- and gender-matched EPAS1–/– and EPAS1+/+ mice were used in the rescue experiments. We obtained approval for all experimental protocols from the UT Southwestern Medical Center Institutional Animal Care and Use Committee.
Gross pathologic and histologic studies
For whole mouse and kidney weights, we collected information from age- and gender-matched EPAS1–/– and EPAS1+/+ mice. Organ–to–body-weight ratios were calculated from wet organ weights measured from exsanguinated mice normalized to the respective body weight obtained prior to organ harvest.
For β-galactosidase staining, we used EPAS1+/+, EPAS1+/– or EPAS1–/– mice perfused with 4% parafomaldehyde/PBS by cardiac puncture. We removed and postfixed select organs for 1 additional hour prior to subsequent manipulations. We embedded samples in paraffin for further sectioning when indicated. For β-galactosidase staining, we stained kidney vibratome sections, embedded the stained sections in paraffin for sectioning, and imaged the resultant sections (×40 fields). We performed hematoxylin and eosin (H&E) staining on paraformaldehyde-fixed, paraffinembedded samples (×20 fields, kidney), oil red O staining on frozen sections (×40, liver), and Giemsa staining on decalcified bone marrow left in situ (×40).
For in situ analyses, kidneys were removed from paraformaldehyde-perfused EPAS1+/+ mice maintained under STCH conditions as described. Sagittal-oriented paraffin sections (5 μm) were placed on slides for 35S-labeled RNA radioactive in situ hybridization. The antisense or sense complementary RNA (cRNA) probes used were generated from pGEM T-easy vectors (Promega Corporation, Madison, WI) containing complementary DNA (cDNA) inserts from murine erythroprotein (mEpo) (nucleotide [nt] 194-579, RefSeq NM_007942) or murine EPAS1 (mEPAS1) (nt 1378-1890, RefSeq NM_010137) using a SP6/T7 Maxiscript Kit (Ambion, Woodland Hills, TX) and 35S-uridine 5′-triphosphate (Amersham Biosciences, Piscataway, NJ).
Microscopy and image acquisition
We reviewed all histological preparations on a Leica Laborlux S photomicroscope (Leica Microsystems, Wetzlar, Germany) equipped with bright-field and incidental dark-field illumination. We imaged paraffin-embedded or frozen tissue sections without oil or water immersion using 20 × or 40 × Leica objectives with plan-achromatic optics of 0.45 and 1.00 numerical aperture, respectively. We photographed images using this same Leica photomicroscope and an Optronics DEI-750 analog CCD color camera (Optronics, Goleta, CA) interfaced with a Scion CG-7 frame-digitizer-equipped (Scion Corporation, Frederick, MD) Macintosh G4 desktop computer (Apple Computer, Cupertino, CA). We captured images using Image J v1.23 acquisition and analysis software (Scion Corporation, Frederick, MD & NIH, Bethesda, MD). We processed and adjusted the images with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Samples were imaged under identical light conditions. Posthoc image adjustments of brightness/contrast or magnification, if performed, were made with identical modification of parameters for EPAS1–/– and EPAS1+/+ samples.
Hematopoietic and serum studies
For spun hematocrits, we collected blood using tail-vein sampling with heparinized capillary tubes. For complete blood cell counts, we collected blood samples from anesthetized mice via retro-orbital punctures just prior to euthanasia and performed automated cell counts (Dallas Children's Hospital Pathology Laboratory, Dallas, TX). Serum was prepared from blood and frozen at –80°C until use. We processed samples using automated analyses for routine metabolites or electrolytes (Dallas Children's Hospital).
Molecular studies
We obtained kidney samples from freshly euthanized mice matched for genotype, gender, age, litter, and treatment group. For each sample, we generated total RNA followed by first-strand cDNA preparations. Quantitative real-time reverse transcription–polymerase chain reaction (rtRT-PCR) reactions identified changes in gene expression of candidate genes with SYBR-green based rtRT-PCR measured in an ABI Prism 7700 sequence detection system instrument and software (Applied Biosystems, Foster City, CA). The genes examined included erythropoietin (Epo) as well as a housekeeping gene for the internal standard (β-actin) with primers designed to span introns and validated prior to use as described earlier.9,46 Gene expression for EPAS1–/– or EPAS1+/– samples were compared with results obtained from EPAS1+/+ samples using the threshold cycle method normalized to β-actin (User Bulletin Number 2; Applied Biosystems). The data for each genotype are the overall mean of 4 individual subject means with each subject mean generated from duplicate rtRT-PCR data points.
Western blot analyses
For total kidney protein extracts, freshly harvested whole kidney was minced in ice-cold harvesting buffer (50 mM Tris, pH 7.4; 500 mM NaCl; 2 mM EDTA [ethylenediaminetetraacetic acid], pH 7.9; 0.5% Nonidet-P40; 100 μM CoCl2; 1 mM dithiothreitol [DTT]) supplemented with protease inhibitor cocktail (Complete Mini; Roche, Indianapolis, IN; cat no. 1 836 153). The tissues then were extracted with 10:1 volume/weight ice-cold homogenization buffer (25 mM Tris, pH 7.4; 1 mM EDTA, pH 7.9; 10% glycerol; 100 μM CoCl2; 1 mM DTT) with protease inhibitor cocktail. Protein concentrations were determined by bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL; cat no. 1 856 210) with bovine serum albumin as standard.
Nuclear extracts were prepared by centrifugation of the whole kidney extracts at 800g for 5 minutes at 4°C. The nuclear pellets were gently resuspended with ice-cold X-wash buffer (0.25 M sucrose; 20 mM Tris, pH 7.8; 1.5 mM MgCl2; 8.5% glycerol; 5% Triton X-100; 100 μM CoCl2; 1 mM DTT) in presence of protease cocktail. After centrifugation at 1500g for 10 minutes at 4°C, the pellets were washed with ice-cold X-wash buffer minus Triton X-100 and then centrifuged. The pellets were homogenized in ice-cold lysis buffer (20 mM Tris, pH 7.8; 450 mM NaCl; 5 mM EDTA, pH 7.9; 10 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid]; 25% glycerol; 100 μM CoCl2; 1 mM DTT) in presence of protease inhibitor cocktail and incubated for 1 hour at 4°C. Nuclear debris was pelleted by centrifugation at 13 000g at 4°C for 30 minutes and the supernatants collected as nuclear extract.
For Western blot analyses of HIF proteins, nuclear extract protein preparations (HIF-1α, 50 μg per lane; HIF-2α, actin: 15 μg per lane) were separated by 6% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences, Buckinghamshire, United Kingdom). The blots were blocked in Blotto-Tween solution (5% nonfat dry milk, 0.1% Tween in PBS) for 1 hour and then incubated overnight in Blotto-Tween with a mouse monoclonal antibody for HIF-1α (1:500; Novus, Littleton, CO), HIF-2α (1:1000; Novus), or β-actin (1:2000; Sigma, St Louis, MO). Peroxidase-coupled AffiniPure rabbit anti–mouse immunoglobulin G (1:10 000; Jackson Immunoresearch Laboratories, West Grove, PA) was used in secondary incubations for 1 hour followed by the detection of reactive bands with a chemiluminescence detection system (ECL) plus kit (Amersham Biosciences, Piscataway, NJ).
For serum erythropoietin protein evaluation, serum was prepared from blood collected from the inferior vena cava of anesthetized mice. Total serum protein (150 μg per lane) was separated by a 10% SDS–polyacrylamide gel electrophoresis (PAGE) gel and transferred to PVDF membranes. The blots were blocked in Blotto-Tween solution (5% nonfat dry milk, 0.05% Tween in PBS) for 1 hour and then incubated in Blotto-Tween for 2 hours with a rabbit anti–human erythropoietin (H-162) polyclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The blots were then incubated with horseradish peroxidase (HRP)–conjugated goat anti–rabbit immunoglobulin (Ig) G (1:5000; Santa Cruz Biotechnology) for 30 minutes. Labeled protein was visualized by using an ECL (Amersham Biosciences, Piscataway, NJ) and quantified by densitometry. For control of loading, the blots were first incubated in stripping buffer (62.5 mM Tris-HCl, 100 mM β-mercaptoethanol, 2% SDS) at 50°C for 30 minutes, and then incubated with an HRP-conjugated sheep anti–mouse IgG (1:1000; Amersham Biosciences) for 1 hour prior to detection by chemiluminescence. The bands corresponding to the light chain of normal mouse IgG were used as an internal reference to serum erythropoietin.
Statistics
We reported data as the mean with standard error of the mean (SEM). Statistical analyses were performed using Excel (Microsoft, Redmond, WA) or JMP Statistics Software (SAS, Cary, NC). Comparisons were by Student t tests for paired data, analysis of variance for 3-way comparisons, and chi-squared or the exact binomial test for proportions.
Results
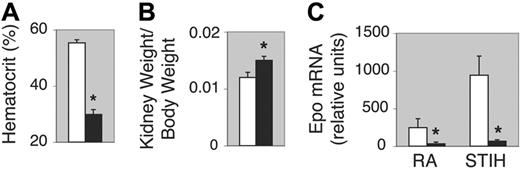
Mice normally exhibit developmental aspects during the first month of life, including anemia of prematurity and a lack of response to exogenous erythropoietin administration.1 For this reason, we evaluated mice older than 1 month of age to assess for abnormalities in global hematopoiesis attributable to defects in erythropoietin synthesis rather than to altered developmental sequelae. Spun hematocrit measurements of 2-month-old EPAS1–/– mice confirmed a reduced red blood cell mass (Figure 1A) as also seen with 1-month-old EPAS1–/– mice.47 We noted increased kidney–to–body-weight ratios in 2-month-old EPAS1–/– mice, indicating that reduced renal mass was not a cause of the anemia (Figure 1B). Serum creatinine levels were not significantly different between EPAS1–/– and EPAS1+/+ mice, indicating preservation of gross renal function (data not shown).
Anemia of EPAS1–/– mice accompanied by depressed erythropoietin mRNA levels. (A) Hematocrit levels for EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 11 per genotype). Statistically meaningful difference in means between the hematocrits of EPAS1+/+ and EPAS1–/– mice is indicated with an asterisk (1-tailed t test with P < .001). (B) Kidney weight–to–body weight ratios (KW/BW) for EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 7 per genotype). Statistically meaningful difference between the KW/BW of EPAS1+/+ and EPAS1–/– mice is indicated with an asterisk (1-tailed t test with P < .005). (C) Real-time RT-PCR quantification of erythropoietin (Epo) mRNA levels in EPAS1+/+ (□) or EPAS1–/– (▪) kidney for mice maintained at room air (RA) or after exposure to short-term intermittent hypoxia (STIH) (n = 4 per genotype per treatment group). Statistically meaningful differences in means between the erythropoietin mRNA levels of EPAS1+/+ and EPAS1–/– mice maintained at RA (1-tailed t test with P < .001) or after STIH exposure (1-tailed t test with P < .001) are indicated with an asterisk. Values are expressed as the mean plus or minus standard error of the mean.
Anemia of EPAS1–/– mice accompanied by depressed erythropoietin mRNA levels. (A) Hematocrit levels for EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 11 per genotype). Statistically meaningful difference in means between the hematocrits of EPAS1+/+ and EPAS1–/– mice is indicated with an asterisk (1-tailed t test with P < .001). (B) Kidney weight–to–body weight ratios (KW/BW) for EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 7 per genotype). Statistically meaningful difference between the KW/BW of EPAS1+/+ and EPAS1–/– mice is indicated with an asterisk (1-tailed t test with P < .005). (C) Real-time RT-PCR quantification of erythropoietin (Epo) mRNA levels in EPAS1+/+ (□) or EPAS1–/– (▪) kidney for mice maintained at room air (RA) or after exposure to short-term intermittent hypoxia (STIH) (n = 4 per genotype per treatment group). Statistically meaningful differences in means between the erythropoietin mRNA levels of EPAS1+/+ and EPAS1–/– mice maintained at RA (1-tailed t test with P < .001) or after STIH exposure (1-tailed t test with P < .001) are indicated with an asterisk. Values are expressed as the mean plus or minus standard error of the mean.
Given its key role in definitive erythropoiesis, we were interested in assessing erythropoietin mRNA levels in EPAS1–/– mice. Despite the anemia, we found markedly reduced renal erythropoietin mRNA levels in 2-month-old EPAS1–/– mice in comparison to EPAS1+/+ mice maintained at room air (RA) (Figure 1C). Treatment of EPAS1–/– mice with hypoxia (short-term intermittent hypoxia; STIH) resulted in a relative increase in erythropoietin mRNA levels compared with RA, but remained markedly depressed in the absolute level of erythropoietin mRNA expression compared with even EPAS1+/+ mice maintained at RA (Figure 1C).
We next performed histologic and in situ hybridization studies to evaluate for any pathologic damage and to localize sites of EPAS1 expression. There were no overt histologic abnormalities in kidneys from 2-month-old EPAS1–/– mice (Figure 2A) versus EPAS1+/+ mice (Figure 2B). Activity staining for nuclear-localized β-galactosidase, a surrogate marker for EPAS1 in this knockout,50 revealed strong expression predominantly in vascular endothelial cells, but also in the renal interstitial cell compartment; β-galactosidase staining was not evident in renal tubular cells of 2-month-old EPAS1–/– (Figure 2C) or EPAS1+/– mice (data not shown). The in situ hybridization analyses revealed prominent expression of EPAS1 mRNA in vascular endothelial cells (Figure 2D) as well as in a subset of the renal interstitial cell population (Figure 2E) of EPAS1+/+ mice. Expression of Epo mRNA was characterized by strong expression in a subset of the renal interstitial cells (Figure 2F).
EPAS1 is expressed in renal interstitial cells. Representative kidney sections from hematoxylin and eosin–stained (A) EPAS1–/– (–/–) or (B) EPAS1+/+ (+/+) kidney (×40 fields) and (C) β-galactosidase–stained EPAS1–/– (–/–) kidney (×40 field). Dark-field (top) and bright-field images (bottom) of in situ studies performed on kidney samples from EPAS1+/+ mice after exposure to short-term continuous hypoxia (STCH) with probes specific for (D) and (E) EPAS1 or (F) Epo mRNA. The arrows indicate areas of prominent signal in vascular endothelial cells (VECs), juxtaglomerular complex cells (JGCs), or interstitial cells (ICs) within the kidney. The black bars in panel B and the white bar in panel C correspond to 100 μm; the black bar in panel F corresponds to 200 μm.
EPAS1 is expressed in renal interstitial cells. Representative kidney sections from hematoxylin and eosin–stained (A) EPAS1–/– (–/–) or (B) EPAS1+/+ (+/+) kidney (×40 fields) and (C) β-galactosidase–stained EPAS1–/– (–/–) kidney (×40 field). Dark-field (top) and bright-field images (bottom) of in situ studies performed on kidney samples from EPAS1+/+ mice after exposure to short-term continuous hypoxia (STCH) with probes specific for (D) and (E) EPAS1 or (F) Epo mRNA. The arrows indicate areas of prominent signal in vascular endothelial cells (VECs), juxtaglomerular complex cells (JGCs), or interstitial cells (ICs) within the kidney. The black bars in panel B and the white bar in panel C correspond to 100 μm; the black bar in panel F corresponds to 200 μm.
We next addressed whether a haploinsufficiency state of EPAS1 would alter renal erythropoietin gene expression. Renal erythropoietin expression was not significantly different between EPAS1+/– and EPAS1+/+ mice under normoxic (ie, RA) conditions (Figure 3A). However, after exposure to short-term intermittent hypoxia (STIH) or short-term continuous hypoxia (STCH) treatment, induction of Epo gene expression in EPAS1+/– mice was impaired relative to EPAS1+/+ mice (Figure 3A). Exposure of EPAS1+/+ or EPAS1+/– mice to STIH or STCH conditions induced activation of the HIF pathway to a comparable degree as defined by stabilization of HIF-1α protein (Figure 3B). With respect to HIF-2α, EPAS1+/– mice had reduced HIF-2α protein levels relative to EPAS1+/+ mice under RA, STIH, or STCH conditions (Figure 3B) although there were no significant differences within each genotype for the 3 conditions.
Erythropoietin levels in EPAS1+/– mice are not induced by hypoxia. (A) Renal erythropoietin mRNA levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice exposed to room air (RA), short-term intermittent hypoxia (STIH), or short-term continuous hypoxia (STCH) (n = 4 per genotype per treatment group). Statistically meaningful differences in means between erythropoietin mRNA levels from EPAS1+/+ or EPAS1+/– mice after STIH exposure (one-tailed t test with P = .07) or STCH exposure (one-tailed t test with P = .06) are indicated with an asterisk. (B) HIF-1α, HIF-2α, or actin protein levels as assessed by Western blot analyses of nuclear extracts from representative EPAS1+/+ (+/+) or EPAS1+/– (+/–) mice maintained under RA, STIH, or STCH conditions. A nonspecific (NS) band seen with use of the HIF-2α monoclonal antibody is indicated for comparison between samples. (C) Hematocrit levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice maintained at RA (n = 21 per genotype), STIH (n = 22 per genotype), or STCH (n = 13 per genotype). Statistically meaningful difference in means between the hematocrit levels of EPAS1+/+ or EPAS1+/– mice maintained at RA is indicated with an asterisk (one-tailed t test with P = .01). (D) Hematocrit levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice maintained at RA or after long-term continuous hypoxia (LTCH) exposure (n = 6 per genotype per treatment group). Statistically meaningful difference in means between the hematocrit levels of EPAS1+/+ or EPAS1+/– mice maintained at LTCH is indicated with an asterisk (one-tailed t test with P = .003). Values are expressed as the mean plus or minus standard error of the mean. (E) Erythropoietin protein levels as assessed by Western blot analyses of serum from representative EPAS1+/+ (+/+) or EPAS1+/– (+/–) mice maintained under room air (RA) or medium-term continuous hypoxia (MTCH) conditions. The same membrane was reprobed for mouse serum IgG as a comparison between samples.
Erythropoietin levels in EPAS1+/– mice are not induced by hypoxia. (A) Renal erythropoietin mRNA levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice exposed to room air (RA), short-term intermittent hypoxia (STIH), or short-term continuous hypoxia (STCH) (n = 4 per genotype per treatment group). Statistically meaningful differences in means between erythropoietin mRNA levels from EPAS1+/+ or EPAS1+/– mice after STIH exposure (one-tailed t test with P = .07) or STCH exposure (one-tailed t test with P = .06) are indicated with an asterisk. (B) HIF-1α, HIF-2α, or actin protein levels as assessed by Western blot analyses of nuclear extracts from representative EPAS1+/+ (+/+) or EPAS1+/– (+/–) mice maintained under RA, STIH, or STCH conditions. A nonspecific (NS) band seen with use of the HIF-2α monoclonal antibody is indicated for comparison between samples. (C) Hematocrit levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice maintained at RA (n = 21 per genotype), STIH (n = 22 per genotype), or STCH (n = 13 per genotype). Statistically meaningful difference in means between the hematocrit levels of EPAS1+/+ or EPAS1+/– mice maintained at RA is indicated with an asterisk (one-tailed t test with P = .01). (D) Hematocrit levels for EPAS1+/+ (□) or EPAS1+/– (▦) mice maintained at RA or after long-term continuous hypoxia (LTCH) exposure (n = 6 per genotype per treatment group). Statistically meaningful difference in means between the hematocrit levels of EPAS1+/+ or EPAS1+/– mice maintained at LTCH is indicated with an asterisk (one-tailed t test with P = .003). Values are expressed as the mean plus or minus standard error of the mean. (E) Erythropoietin protein levels as assessed by Western blot analyses of serum from representative EPAS1+/+ (+/+) or EPAS1+/– (+/–) mice maintained under room air (RA) or medium-term continuous hypoxia (MTCH) conditions. The same membrane was reprobed for mouse serum IgG as a comparison between samples.
The differences in renal erythropoietin mRNA levels between EPAS1+/– and EPAS1+/+ mice translated into functional deficits as well (Figure 3C). Baseline (RA) hematocrit values were mildly decreased in EPAS1+/– as compared with EPAS1+/+ mice. Hematocrit determinations performed shortly after initiation of STIH or STCH trended toward lower values for EPAS1+/– as compared with EPAS1+/+ mice (Figure 3C). Following prolonged or long-term continuous hypoxia exposure (LTCH), there remained a small, but significant, difference in the hematocrits of EPAS1+/– versus EPAS1+/+ mice (Figure 3D).
The molecular characterization and hematocrit results suggest that a haploinsufficiency state for EPAS1 has measurable effects on short-term (ie, Epo gene expression) as well as long-term (ie, erythropoiesis) responses to hypoxic exposure. Since there is a temporal delay between changes in de novo erythropoietin gene expression and protein levels, we examined serum erythropoietin protein levels for an intermediate-length continuous hypoxia protocol, medium-term continuous hypoxia (MTCH). The results obtained revealed reduced serum erythropoietin protein levels in EPAS1+/– versus EPAS1+/+ mice (Figure 3E), consistent with the observed changes in renal erythropoietin gene expression found at earlier time points in the continuous hypoxia protocol (ie, STCH).
To determine if the hematopoietic defect in EPAS1–/– mice would respond to erythropoietin replacement therapy, we administered exogenous erythropoietin to EPAS1–/– mice. Erythropoietin treatment using low-dose (500 U/kg body weight) erythropoietin dosing of EPAS1–/– mice from 30 to 50 days of age resulted in a time-dependent increase in hematocrit levels close to that of normal (ie, untreated EPAS1+/+) mice (Figure 4A). All EPAS1–/– mice universally responded to 20 days of low-dose erythropoietin treatment, although EPAS1–/– mice that began treatment with the most depressed hematocrit levels were slightly below average wild-type levels after 20 days of erythropoietin treatment.
Pancytopenia of EPAS1–/– mice responds to erythropoietin treatment. (A) Hematocrit levels for EPAS1+/+ (□) compared with EPAS1–/– (▪) littermate mice treated with erythropoietin (n = 4 per genotype per time point). The erythropoietin dosage was either low-dose (500 U/kg) for days 0 to 20 or high-dose (5000 U/kg) for days 21 to 30 of treatment. Statistically meaningful differences in means between the hematocrits of EPAS1+/+ or EPAS1–/– mice prior to initiation of erythropoietin treatment (one-tailed t test with P = .002) or after 10 days of low-dose erythropoietin treatment (one-tailed t test with P = .02) are indicated by an asterisk. No further differences between groups of mice were noted for all other time points (one-tailed t test with P > .1). (B) Complete blood cell count analyses for untreated EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 6 per genotype). Statistically meaningful differences in means between values from EPAS1+/+ or EPAS1–/– mice for white blood cells (WBCs; one-tailed t test with P = .0001), red blood cells (RBCs; one-tailed t test with P = .003), platelets (PLTs; one-tailed t test with P = .0018), or reticulocytes (RETs; one-tailed t test with P = .049) are indicated by an asterisk. (C) Complete blood cell count analyses for EPAS1+/+ (□) or EPAS1–/– (▪) littermate mice after erythropoietin treatment (n = 4 per genotype). Statistically meaningful differences in means between values of EPAS1+/+ or EPAS1–/– mice for platelets (one-tailed t test with P = .0001) or reticulocytes (one-tailed t test with P = .0005) are indicated by an asterisk. The units correspond to 103/μL for WBCs, 106/μL for RBCs, 105/μL for PLTs, and 105/μL for RETs. Values are expressed as the mean plus or minus standard error of the mean.
Pancytopenia of EPAS1–/– mice responds to erythropoietin treatment. (A) Hematocrit levels for EPAS1+/+ (□) compared with EPAS1–/– (▪) littermate mice treated with erythropoietin (n = 4 per genotype per time point). The erythropoietin dosage was either low-dose (500 U/kg) for days 0 to 20 or high-dose (5000 U/kg) for days 21 to 30 of treatment. Statistically meaningful differences in means between the hematocrits of EPAS1+/+ or EPAS1–/– mice prior to initiation of erythropoietin treatment (one-tailed t test with P = .002) or after 10 days of low-dose erythropoietin treatment (one-tailed t test with P = .02) are indicated by an asterisk. No further differences between groups of mice were noted for all other time points (one-tailed t test with P > .1). (B) Complete blood cell count analyses for untreated EPAS1+/+ (□) or EPAS1–/– (▪) mice (n = 6 per genotype). Statistically meaningful differences in means between values from EPAS1+/+ or EPAS1–/– mice for white blood cells (WBCs; one-tailed t test with P = .0001), red blood cells (RBCs; one-tailed t test with P = .003), platelets (PLTs; one-tailed t test with P = .0018), or reticulocytes (RETs; one-tailed t test with P = .049) are indicated by an asterisk. (C) Complete blood cell count analyses for EPAS1+/+ (□) or EPAS1–/– (▪) littermate mice after erythropoietin treatment (n = 4 per genotype). Statistically meaningful differences in means between values of EPAS1+/+ or EPAS1–/– mice for platelets (one-tailed t test with P = .0001) or reticulocytes (one-tailed t test with P = .0005) are indicated by an asterisk. The units correspond to 103/μL for WBCs, 106/μL for RBCs, 105/μL for PLTs, and 105/μL for RETs. Values are expressed as the mean plus or minus standard error of the mean.
To assess if supra-physiologic levels (5000 U/kg) of erythropoietin would further stimulate red blood cell production, we increased the erythropoietin dosage administered to EPAS1–/– mice from low- to high-dose levels from 50 to 60 days of age. After 10 days of high-dose erythropoietin treatment, hematocrit levels of erythropoietin-treated EPAS1–/– mice were slightly higher on average than untreated EPAS1+/+ mice (Figure 4A). Continued treatment of EPAS1–/– mice with high-dose erythropoietin for an additional 10 days resulted in erythrocytosis, similar to that observed in wild-type or heterozygous control mice treated in an identical manner (data not shown), indicating that erythropoietin resistance or a decreased erythroid progenitor reserve is not a cause of the hematopoietic defect observed in untreated EPAS1–/– mice. EPAS1–/– mice at baseline exhibit marked abnormalities in all the major hematopoietic lineages (Figure 4B). After 30 days of erythropoietin treatment, we observed correction of all major blood lineages in the peripheral blood of 2-month-old EPAS1–/– mice, revealing an effect of erythropoietin on global hematopoiesis (Figure 4C).
Erythropoietin treatment of EPAS1–/– mice alleviated other aspects of the EPAS1-null state. The hypocellular bone marrow of EPAS1–/– mice (Figure 5A) was corrected in erythropoietin-treated EPAS1–/– mice (Figure 5B), resulting in bone marrow similar in appearance to that of EPAS1+/+ mice (Figure 5C). The liver–body weight ratios of EPAS1–/– mice improved after erythropoietin treatment compared with untreated EPAS1–/– mice (data not shown). The lipid deposits found in EPAS1–/– livers (Figure 5D) reversed after erythropoietin treatment (Figure 5E) so as to be indistinguishable from EPAS1+/+ mice (Figure 5F).
Erythropoietin treatment alters other aspects of the EPAS1–/– phenotype. Giemsa staining of bone marrow from representative (A) untreated (-Epo) EPAS1–/– (–/–), (B) erythropoietin-treated (+Epo) EPAS1–/– (–/–), or (C) EPAS1+/+ (+/+) mice (all images are ×20 fields, equivalent size). The black bar in panel C corresponds to 100 μm. Representative oil red O (ORO) staining of liver from (D) untreated (-Epo) EPAS1–/– (–/–), (E) erythropoietin-treated (+Epo) EPAS1–/– (–/–), or (F) EPAS1+/+ (+/+) mice (all images are ×20 fields, equivalent size). The black bar in panel F corresponds to 100 μm.
Erythropoietin treatment alters other aspects of the EPAS1–/– phenotype. Giemsa staining of bone marrow from representative (A) untreated (-Epo) EPAS1–/– (–/–), (B) erythropoietin-treated (+Epo) EPAS1–/– (–/–), or (C) EPAS1+/+ (+/+) mice (all images are ×20 fields, equivalent size). The black bar in panel C corresponds to 100 μm. Representative oil red O (ORO) staining of liver from (D) untreated (-Epo) EPAS1–/– (–/–), (E) erythropoietin-treated (+Epo) EPAS1–/– (–/–), or (F) EPAS1+/+ (+/+) mice (all images are ×20 fields, equivalent size). The black bar in panel F corresponds to 100 μm.
The biochemical abnormalities normally seen in EPAS1–/– mice also were affected by erythropoietin treatment. We noted improvements in serum glucose (no significant differences between groups) and lactate levels for erythropoietin-treated EPAS1–/– mice, although lactate levels were still mildly elevated relative to EPAS1+/+ mice (2.0 ± 0.5 versus 6.4 ± 0.3, EPAS1+/+ versus erythropoietin-treated EPAS1–/– mice). The treatment protocol as performed did not alleviate all aspects of the EPAS1–/– phenotype; the retinopathy and cardiac hypertrophy observed in EPAS1–/– mice did not differ significantly after erythropoietin treatment (data not shown). Hence, other aspects of the EPAS1–/– phenotype likely have additional molecular etiologies besides erythropoietin deficiency or are multifactorial in nature.
Discussion
We have determined that HIF-2α is a major regulator of renal erythropoietin gene expression in vivo. A reduction in EPAS1 gene dosage has profound effects for EPAS1–/– mice and intermediate effects for EPAS1+/– mice on erythropoiesis. EPAS1–/– mice exhibit a moderate-to-severe anemia under ambient oxygen conditions whereas EPAS1+/– mice have a mild anemia under these same conditions. Molecular characterization revealed a marked depression of renal erythropoietin gene expression for EPAS1–/– mice maintained at room air and no significant differences in renal erythropoietin gene expression for EPAS1+/– mice under these same conditions.
Although renal erythropoietin mRNA levels in EPAS1+/– mice were not significantly different from those of EPAS1+/+ mice under ambient oxygen conditions, we observed a slight reduction in resting hematocrits in EPAS1+/– mice compared with EPAS1+/+ mice. Since erythropoietin mRNA levels are near the limits of detection for RA samples even by real-time RT-PCR, we cannot exclude a minor reduction in erythropoietin levels as being responsible for this difference in EPAS1+/– mice compared with EPAS1+/+ mice. We also cannot exclude an effect of EPAS1 haploinsufficiency on paracrine erythropoietin production in the bone marrow and resultant effects on definitive hematopoiesis as a result.
The hypoxia treatment protocols revealed impaired induction of erythropoietin gene expression in EPAS1–/– as well as EPAS1+/– mice. The absolute degree of erythropoietin induction by hypoxia is dramatically blunted in EPAS1–/– mice relative to EPAS1+/+ mice and mildly decreased in EPAS1+/– mice. Blunting of erythropoietin gene expression in EPAS1+/– mice occurs despite HIF-1α activation as suggested by HIF-1α stabilization. In the data presented, no significant increases in HIF-2α protein were observed with nuclear extracts prepared from hypoxia-treated mice; however, we have noted stabilization of HIF-2α protein in some hypoxia-treated samples similar to previously reported results with hypoxia-exposed rodents.51 HIF-2α protein stabilization may still occur in localized regions of the kidney and would not be evident in these experiments using whole kidney extracts. It is also possible that HIF-2α signaling in these experiments involves mechanisms other than protein stabilization.
Long-term continuous hypoxia treatment reveals a blunted erythropoietic response of EPAS1+/– mice compared with EPAS1+/+ mice. Thus, haploinsufficiency for EPAS1, as with HIF-1α,45 has long-term effects on erythrocyte steady-state levels under these conditions. In the kidney, as opposed to most other tissues of the body, expression of HIF-1α and HIF-2α protein is mutually exclusive.52,53 Based on in situ and immunohistochemical studies, HIF-2α is expressed in renal interstitial and endothelial cells51,54 whereas HIF-1α is expressed predominantly in the tubular cells.54,55 Using a sensitive surrogate marker for EPAS1 gene expression, we found EPAS1 gene expression in EPAS1–/– mice is coincident with the interstitial cells and vascular endothelial cells, cell types previously implicated in erythropoietin production.21,23,25 The in situ analyses presented in this study support earlier findings indicating prominent expression of Epo in a subset of renal interstitial cells. Since the in situ analyses also reveal expression of EPAS1 in a subset of the renal interstitial cells, it is likely that HIF-2α directly participates in Epo gene expression regulation.
The inability to establish primary erythropoietin-producing renal interstitial fibroblast or other renal cell lines is indirect evidence of an in vivo multicellular requirement for renal erythropoietin production. In conjunction with earlier studies examining the regulation of erythropoietin gene expression by HIF-1α,9 these results reveal that in vivo renal erythropoietin production requires multiple HIF members. It remains to be determined why both HIF-1α and HIF-2α are essential for optimal renal erythropoietin regulation and whether HIF-1α and HIF-2α alter erythropoietin gene expression directly or indirectly.
We attribute the depressed erythropoietin gene expression in EPAS1–/– mice to the absence of functional HIF-2α. It is unlikely that HIF-1α signaling is impaired due to metabolic abnormalities in EPAS1–/– mice since renal Epo mRNA levels remain depressed in EPAS1–/– mice treated with exogenous erythropoietin (data not shown). HIF-independent mechanisms may contribute to the reduced basal level of erythropoietin expression seen in EPAS1–/– mice under ambient oxygen conditions; however, these mechanisms cannot completely compensate in the complete absence of HIF-2α, either under ambient oxygen conditions or after exposure to hypoxia. HIF-1α–dependent pathways are likely activated in EPAS1–/– mice exposed to hypoxia, but activation of HIF-1α–dependent pathways does not result in efficient induction of Epo gene expression.
Exogenous erythropoietin has the expected impact on erythropoiesis in EPAS1–/– mice, including increased reticulocytosis, and may also be responsible for increased thrombocytosis.56,57 Surprisingly, erythropoietin treatment also corrected the nonerythroid deficiencies in EPAS1–/– mice. The restoration of all hematopoietic lineages of EPAS1–/– mice after erythropoietin treatment supports the contention that global hematopoietic maturation is dependent on erythropoietin. Since both erythropoietin58 as well as the erythropoietin receptor are expressed in early hematopoietic precursor/progenitor cells,59 erythropoietin may play an important role in the early maturation process. Erythropoietin is also produced locally in several tissues, including the bone marrow where it may have paracrine or autocrine effects.60 The hematopoietic response of EPAS1–/– mice to exogenous erythropoietin may reflect efficient replenishment of an otherwise absent paracrine source of erythropoietin.61 We cannot exclude an indirect effect of erythropoietin on global hematopoiesis via erythropoietin-dependent induction of other cytokines, synergistic interactions of erythropoietin with other cytokines, or induction by erythropoietin of a downstream signaling pathway that is affected in EPAS1–/– mice but not normally regulated by erythropoietin.62
Several cell types within the bone marrow express HIF-2α, including vascular endothelial cells, bone-lining cells, and resident macrophages.47 Erythropoietin may originate from some of these cell types. Consistent with the existence of a cytokine-producing cell type that may originate from within the blood marrow compartment, aged irradiated mice (2 years after transplantation) that are recipients of bone marrow stem cells from EPAS1–/– mice exhibit lower hematocrit levels in comparison to irradiated recipient mice that received bone marrow stem cells from EPAS1+/+ mice (data not shown). Since these 2 groups of mice do not exhibit differences in their hematocrit levels at younger ages, the long-term differences in their erythrocyte masses may be due to time-dependent turnover of long-lived erythropoietin-producing EPAS1+/+ stromal cell types within the bone marrow. In this scenario, endogenous EPAS1+/+ stromal cells in transplant-recipient mice that are a paracrine source of erythropoietin in the bone marrow are eventually replaced by EPAS1–/– stromal cells, originating from the transplanted EPAS1–/– stem cells, in which erythropoietin production is impaired. However, other causes may also contribute to this age-related phenotype.
The systemic lipid abnormalities otherwise seen in untreated EPAS1–/– mice, including the hepatosteatosis, are also reversed with erythropoietin treatment of EPAS1–/– mice, similar to that observed after treatment with an antioxidant mimetic.46 The liver is an extra-hepatic site of erythropoietin production in neonatal mice and in anemic adult mice. Since HIF members have been implicated in control of erythropoietin expression in extra-renal sites,49,63 it is possible that the pathologic findings observed in the livers of EPAS1–/– mice are in part due to absence of paracrine erythropoietin production. Moreover, other environmental factors such as oxidative stress modulate erythropoietin gene expression.64-66 Therefore, molecular defects in oxidative stress responses, another aspect of the EPAS1–/–-null phenotype,46 also may negatively impact erythropoietin gene expression.
Erythropoietin, major antioxidant enzymes, and other HIF target genes may constitute a battery of cellular factors that alleviate oxidative stress associated with anemia, ischemia/reperfusion, and hypoxia/reoxygenation. The importance of erythropoietin signaling in pathologic states is an active source of investigation. Therapeutic modulators that specifically augment HIF-2α activity may thus provide adjunct or alternative therapies to exogenous erythropoietin treatments. Future studies of EPAS1-deficient mice will have important translational implications for disease states involving erythropoietin deficiencies.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-05-1695.
Supported by grants to J.G. from the National Institutes of Health, National Heart, Lung, Blood Institute (NIH/NHLBI); NIH/National Institute of Mental Health (NIMH) Conte Center, the American Heart Association (AHA)/Texas Affiliate, the American Cancer Society, and the Donald W. Reynolds Foundation.
M.S. and K.D. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Das for excellent technical assistance, J. Repa for assistance with real-time RT-PCR, D. Foster and S. McKnight for generous support, and R. Bruick for critical review of this manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal