Abstract
The most frequently recurring translocations in mucosa-associated lymphoid tissue (MALT) B-cell non-Hodgkin lymphoma, t(11;18)(q21;q21) and t(14;18)(q32; q21), lead to formation of an API2-MALT1 fusion or IgH-mediated MALT1 overexpression. Various approaches have implicated these proteins in nuclear factor κB (NF-κB) signaling, but this has not been shown experimentally in human B cells. Immunohistochemistry showed that MALT1 is predominantly expressed in normal and malignant germinal center B cells, corresponding to the differentiation stage of MALT lymphoma. We expressed MALT1 and apoptosis inhibitor-2 API2/MALT1 in human B-cell lymphoma BJAB cells and found both transgenes in membrane lipid rafts along with endogenous MALT1 and 2 binding partners involved in NF-κB signaling, B-cell lymphoma 10 (BCL10) and CARMA1 (caspase recruitment domain [CARD]-containing membrane-associated guanylate kinase [MAGUK] 1). API2-MALT1 and exogenous MALT1 increased constitutive NF-κB activity and enhanced IκB kinase (IKK) activation induced by CD40 stimulation. Both transgenes protected BJAB cells from FAS (CD95)-induced death, consistent with increases in NF-κB cytoprotective target gene expression, and increased their proliferation rate. Expression of a dominant-negative IκBα mutant showed that these survival and proliferative advantages are dependent on elevated constitutive NF-κB activity. Our findings support a model in which NF-κB signaling, once activated in a CD40-dependent immune response, is maintained and enhanced through deregulation of MALT1 or formation of an API2-MALT1 fusion.
Introduction
Four recurrent chromosomal translocations have been described in non-Hodgkin B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT) type. Two of them, t(14;18)(q32;q21) and t(11; 18)(q21;q21), are found in 30% to 50% of extranodal MALT lymphomas. The former juxtaposes the MALT1 (MLT1 or paracaspase 1) gene to the IgH promoter region, resulting in the deregulation of MALT1 expression, whereas the latter creates a fusion between MALT1 and the inhibitor of apoptosis gene API2 (cIAP2/HIAP1).1-6 Unlike the low-grade indolent MALT lymphomas that are dependent on the continuous presence of Helicobacter pylori, MALT lymphomas with translocations targeting the MALT1 gene can be found in cases without H pylori and are refractory to treatment against H pylori.7,8 The other 2 much rarer translocations, t(1;14)(p22;q32) and t(1;2)(p22;p12), target the BCL10 gene on the short arm of chromosome 1.9,10
MALT1 was independently identified as a member of the human paracaspase family and an interacting partner of B-cell lymphoma 10 (BCL10).11 In vitro, MALT1 synergizes with BCL10 to enhance nuclear factor κB activation,12 and the association of the 2 proteins has been shown to mediate IκB kinase (IKK) activation by facilitating the ubiquitinylation of the NF-κB essential modulator (NEMO) by the ubiquitin-conjugating enzyme UBC13.13 BCL10 also interacts with a group of proteins that contain an N-terminal caspase recruitment domain (CARD) domain and show overall structural homology to MAGUK (membrane-associated guanylate kinase) proteins.14-17 These proteins were subsequently termed CARMA (CARD-containing MAGUK) proteins, of which CARMA1 (CARD11) expression is predominantly lymphoid specific. It has been shown recently that MALT1 not only interacts with BCL10 directly but also associates with CARMA1, suggesting that a complex containing MALT1, CARMA1, and BCL10 plays an important role in lymphoid cell signaling.18 The critical function of MALT1 in lymphocyte signaling has been revealed by the analyses of MALT1-deficient mice,19,20 whose T cells fail to proliferate in response to CD3/CD28 costimulation or to phorbol myristate acetate (PMA) and ionomycin. Similarly, B cells of MALT1-/- mice fail to proliferate in response to immunoglobulin M (IgM), CD40, or lipopolysaccharide (LPS) stimulation. The lymphoid compartments of these mice showed severe reduction of marginal zone B cells and a lack of germinal center formation in spleens, as well as deregulated maturation of T-cell subsets in the thymus. The phenotype of MALT1-deficient mice closely resembles that of mice lacking Bcl10, in particular with respect to the lack of marginal zone B cells, and in the lack of response to T- and B-cell stimuli leading to NF-κB activation.21,22 Consistently, phenotypes of mice lacking CARMA1 resemble those of MALT1- and Bcl10-deficient mice.23-26 These observations confirm the in vitro data that MALT1 and CARMA1 both interact with BCL1012,13,27 and show that this ternary complex is critical in B- and T-cell stimulation leading to NF-κB activation and downstream immunologic responses.28
The above evidence strongly suggests that translocations involving BCL10 and MALT1 lead to elevated NF-κB activity in MALT B-cell lymphoma. In addition, it has been proposed that chronic antigenic stimulation caused by H pylori infection triggers T-cell-mediated B-cell expansion early in the disease process via activation of the CD40 and NF-κB pathway.29,30 We therefore investigated the involvement of MALT1 and its fusion gene product, apoptosis inhibitor-2 (API2)/MALT1, in NF-κB signaling in the presence and absence of CD40 stimulation. Genetic analyses of immunoglobulin gene hypermutation of MALT lymphoma cells suggest that the tumor has a germinal center/post-germinal center origin,31 and the development of MALT lymphoma may be caused by inhibition of germinal center B-cell apoptosis.32 Using a germinal center/post-germinal center-derived B-lymphoma cell line, we examined the effect of stably expressed MALT1 or API2-MALT1 on the NF-κB pathway and on apoptosis induced by FAS (CD95)/apolipoprotein 1 (APO1), which is a mediator of cell death in the germinal center counterbalanced by CD40 signaling.32,33 This study is the first report addressing the functional consequence of MALT1 and API2-MALT1 overexpression in human B-cell lymphoma cells. Our results support a model of MALT lymphomagenesis in which cytoprotection mediated by NF-κB, once activated in a CD40-dependent immune response (eg, against H pylori), is maintained and enhanced through deregulation of MALT1 or formation of an API2-MALT1 fusion.
Material and methods
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue blocks from 65 non-Hodgkin B-cell lymphomas and 12 normal lymphoid tissues were retrieved from the archives of the Department of Pathology at Geneva University Hospital. Lymphoid tumors were classified according to the Revised European-American classification of lymphoid neoplasms. After deparaffinization, sections were incubated for 10 minutes in methanol plus peroxide at room temperature, followed by washing with phosphate-buffered saline (PBS) solution. Slides were heated in citrate buffer using a pressure cooker for 3 minutes. Incubation with the primary antibody, monoclonal αMALT1 antibody directed to the C-terminal part of MALT1, was carried out for 1 hour at room temperature.5 Immunoreactivity detection was done by using the Envision system (DAKO, Carpinteria, CA).
Images were visualized under a Nikon Eclipse E400 microscope equipped with Plan Fluor 40 × (main panels) or 100 × (insets) objective lenses and aCF110X eyepiece (Nikon, Tokyo, Japan). Images were captured with a Nikon CoolPix E995 camera (Nikon) and processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Cell lines and retroviral constructs
Epstein-Barr virus (EBV)-negative BJAB human B-cell lymphoma cell lines were grown in RPMI-1640 with 10% fetal bovine serum. The human high-grade B-cell lymphoma cell lines SU-DHL-6, OCI-Ly3, and OCI-Ly7 were maintained as described previously.34 Full-length MALT1 or API2-MALT1 joining nucleotides 1 to 2048 of cIAP2 to nucleotides 814 to 2475 of MALT1 with c-terminal myc followed by 6 × His epitope tags in pCDNA3.1 vector (Invitrogen, Carlsbad, CA) were subcloned into pMSCV-IRES-EGFP retroviral vector.1,35 Following viral infection, enhanced green fluorescent protein (EGFP)-positive cells were selected by fluorescence-activated cell sorter (FACS)-assisted cell sorting. Selected single-cell clones were reinfected with empty retroviral vector vIRES-puromycin or vector carrying S32G/S36A mutant of IκB with N-terminal FLAG epitope tag.36 A similar retroviral vector (vIRES-hygromycin) carrying MALT1 or API2-MALT1 cDNA and hygromycin resistance was also generated. Infected cells were selected with 3 μg/mL puromycin (Sigma, St Louis, MO) or 500 μg/mL hygromycin (Invitrogen). The IκBα-luciferase reporter cDNA was assembled in pBluescript, excising from pIκB-EGFP (Clontech, Palo Alto, CA) its Kozak and IκBα sequence (which lacks a stop codon and is followed by a BamHI site) and inserting in-frame the luciferase coding region from pGL3 (Promega, Madison, WI), and transferred to vIRES-puromycin. The study was performed in accordance with ethical standards of the Helsinki Declaration of the World Medical Association and conformed with Geneva University Hospital ethical committee guidelines at the time of commencing the study.
Sucrose gradient raft fractionation
An established protocol for raft isolation was used.37 Briefly, 50 × 106 cells were washed once in cold buffer A (50 mM Tris-HCl, pH 7.4; 25 mM KCl; 5 mM MgCl2; and 1 mM EGTA [ethyleneglycotetraacetic acid]), then lysed in 680 μL buffer A supplemented with 1% Triton TX-100 and protease inhibitors. This lysate was incubated on ice for 1 hour before mixing with 820 μL buffer A containing 73% (wt/vol) sucrose to give a final volume of 1.5 mL in 40% sucrose. This is transferred to a SW41 centrifugation tube and overlaid with 6 mL buffer A containing 35% sucrose and then 3.5 mL buffer A containing 5% sucrose. The gradient is centrifuged at 250 000g for 16 hours at 4°C. Following centrifugation, 11 fractions, of 1 mL each, were collected from the top of the centrifugation tube. All fractions were immunoblotted for LCK/YES-related novel tyrosine kinase (LYN; Santa Cruz Biotechnology, Santa Cruz, CA) to confirm the presence of lipid rafts in fractions 3 to 5. The pooled fractions were diluted in TKM and further centrifuged at 250 000g for 2 hours. The pellet containing the Triton-resistant membrane proteins was boiled in denaturing sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
SDS-PAGE and immunoblotting
Thirty to 50 μg of protein from cell lysates was analyzed by SDS-PAGE and immunoblotted with the appropriate antibodies: rabbit polyclonal anti-CARMA1 antibody against the N-terminus of CARMA1 (AL-220; Alexis Biochemicals, Lausanne, Switzerland) and monoclonal anti-BCL10 antibody (A-6; Alexis Biochemicals); rabbit anti-human BclXL antibody (A-3535; DAKO); polyclonal antibodies against API2 and actin (H-85; Santa Cruz Biotechnology).
IKK activity and nuclear NF-κB measurements
BJAB cells stably infected with vIRES-puromycin vector containing the IκB-luciferase reporter alone or reinfected with vIRES-hygromycin vector carrying either MALT1 or API2-MALT1 cDNA were analyzed. Briefly, triplicates of 150 000 cells per well were either incubated in medium only (controls) or in the presence of 10 ng/mL tumor necrosis factor α (TNFα; R&D Systems, Minneapolis, MN), 40 ng/mL PMA with 2 μM ionomycin (Sigma), or 0.1 μ/mL CD40L (Alexis Biochemicals) cross-linked with 2.5 μg/mL M2 anti-FLAG antibody (Sigma) for 4 hours before analysis. Collected cells were lysed with 50 μL of Glo Lysis buffer (Promega) and mixed with 50 μL of Bright Glo stable substrate (Promega), and emission was measured in a luminometer. The values obtained for untreated controls were taken as 100%, and the stimulated samples were expressed as percentage of the corresponding controls.
Enzyme-linked immunosorbent assay (ELISA)-based measurement of nuclear NF-κB subunits was performed according to the manufacturer's protocol (Active Motif, Rixensart, Belgium). Briefly, 3 μg of BJAB nuclear extract was used per sample point in triplicates in the 96-well plate precoated with consensus oligonucleotides for all NF-κB subunits (p50, p65/RelA, RelB, p52, and c-Rel). After washing to remove nonspecific binding, specific antibodies to NF-κB subunits were added. After antibody binding, the plate was washed again before adding a horseradish peroxidase-conjugated secondary antibody. The peroxidase substrate was added and colorimetric change was measured at an optical density of 450 (OD450).
FACS analyses for FAS and CD83 expression
For FAS or CD83 expression, 5 × 105 to 10 × 105 BJAB cells were incubated in 100 μL FACS buffer (PBS/1% bovine serum albumin [BSA]/0.05% sodium azide) with 1 μg/mL 2R2 anti-FAS antibody (Alexis Biochemicals), the recommended concentration of anti-CD83 antibody (Pharmingen, San Diego, CA), or the corresponding isotype control IgG (Jackson ImmunoResearch, West Grove, PA). The cells were washed twice and incubated in 1:700 dilution of phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Jackson ImmunoResearch) for 30 minutes. The cells were washed again and resuspended in 300 μL of the same buffer for FACS analyses for the presence of FAS or CD83 using FacSCAN (Becton Dickinson, San Jose, CA).
Cytotoxic assays
The cytotoxic assays were performed using the tetrazolium compound MTS (3-(4,5-dimethythiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Promega) and phenazine methosulfate (PMS; Sigma-Aldrich, St Louis, MO), as described previously.38 Briefly, 50 000 cells in a volume of 100 μL were subjected to serial dilutions of soluble FAS-L (gift of Pascal Schneider, Lausanne, Switzerland). Where stated, CD40L-FLAG (Alexis Biochemicals) cross-linked with anti-FLAG antibody (Sigma) or anti-CD154 monoclonal antibody (Pharmingen) was added to the medium with various concentrations of FAS-L. After overnight incubation, cell viability was determined by incubation with MTS/PMS reagent for 2 hours, followed by measuring the absorbance at 490 nm. In experiments with BJAB cells expressing the IκBα superrepressor, FAS-L at indicated concentrations was used in triplicate wells under the same experimental conditions.
3[H]-thymidine incorporation
Exactly 5000 cells per well were plated in triplicates in a 96-well culture plate. The cells were incubated for 24 hours before pulsing with 0.5 μCi/well (0.0185 MBq/well) 3[H]-thymidine (Amersham, Arlington Heights, IL) for another 12 hours. Cells were harvested with a cell harvester (Inotech, Rockville, MD) and the counts per minute (cpm) counted with a Wallac Trilux scintillation counter (Perkin Elmer, Shelton, CT).
Quantitative PCR and RNA preparation
Total RNA was isolated from cells with Trizol reagent (Invitrogen) according to the manufacturer's instructions. To eliminate DNA contamination, the final preparations were treated with RNAse-free DNAse (Roche, Indianapolis, IN). Reverse transcription of 0.5 to 1 μg of total RNA was carried out using the cDNA first-strand synthesis kit (Roche), and 1 μLof a 100-fold dilution of this reaction was used to proceed real-time polymerase chain reaction (PCR) in iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Expression of mRNA was evaluated by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis using an icycler (Bio-Rad). For each target, PCR reactions were performed in triplicate in 2 independent experiments. The primers used are listed in Table 1. Selected primers for API2 detect only endogenous API2 but not the API-MALT1 fusion, whereas MALT1 primers detect all species (endogenous, overexpressed MALT1, and the API2-MALT1 fusion). Expression levels of target mRNAs were normalized to the endogenous mRNA levels of GAPDH gene (ΔCT method). The thermal cycling conditions for all reactions were 95°C for 10 minutes followed by 40 cycles of 95°C 15 seconds and 60°C 1 minute.
RT-PCR primers used for quantification of putative cytoprotective NF-κB target genes
Genes . | Forward primers . | Reverse primers . |
|---|---|---|
| hA20 | 5′–aga tca tcc aca aag ccc tca tc –3′ | 5′–aat tgc cgt cac cgt tcg t–3′ |
| hXAIP1 | 5′–ggt gtt ttc tca gta gtt ctt acc aga ca–3′ | 5′–atg cta aat ggt atc cag ggt gc–3′ |
| Survivin | 5′–acc agg tga gaa gtg agg ga–3′ | 5′–aac agt aga gga gcc agg ga–3′ |
| Bcl-XL | 5′–gaa cgg cgg ctg gga t–3′ | 5′–agc ggt tga agc gtt cct g–3′ |
| Bfl1/A1 | 5′–aca cag gag aat gga taa ggc aaa–3′ | 5′–agt cat cca gcc aga ttt agg ttc–3′ |
| cFLIP | 5′–gtg gag acc cac ctg ctc a–3′ | 5′–gga cac atc aga ttt atc caa atc c–3′ |
| API1 | 5′–gcc ttt ctc caa acc ctc tt–3′ | 5′–cat tcg agc tgc atg tgt ct–3′ |
| API2 | 5′–taa tac cgg gaa cat gaa g–3′ | 5′–gga cac tga tca aaa aag ac–3′ |
| Bcl2 | 5′–agg aag tga aca ttt cgg tga c–3′ | 5′–gct cag ttc cag gac cag g–3′ |
| Gadd45β | 5′–tgg aag agc tcg tgg cgt g–3′ | 5′–cct cgt caa tgg aga ggc–3′ |
| MALT1 | 5′–gga tct tac tga ata tga gat gc–3′ | 5′–gac aat ttt cag acc tat atg g–3′ |
Genes . | Forward primers . | Reverse primers . |
|---|---|---|
| hA20 | 5′–aga tca tcc aca aag ccc tca tc –3′ | 5′–aat tgc cgt cac cgt tcg t–3′ |
| hXAIP1 | 5′–ggt gtt ttc tca gta gtt ctt acc aga ca–3′ | 5′–atg cta aat ggt atc cag ggt gc–3′ |
| Survivin | 5′–acc agg tga gaa gtg agg ga–3′ | 5′–aac agt aga gga gcc agg ga–3′ |
| Bcl-XL | 5′–gaa cgg cgg ctg gga t–3′ | 5′–agc ggt tga agc gtt cct g–3′ |
| Bfl1/A1 | 5′–aca cag gag aat gga taa ggc aaa–3′ | 5′–agt cat cca gcc aga ttt agg ttc–3′ |
| cFLIP | 5′–gtg gag acc cac ctg ctc a–3′ | 5′–gga cac atc aga ttt atc caa atc c–3′ |
| API1 | 5′–gcc ttt ctc caa acc ctc tt–3′ | 5′–cat tcg agc tgc atg tgt ct–3′ |
| API2 | 5′–taa tac cgg gaa cat gaa g–3′ | 5′–gga cac tga tca aaa aag ac–3′ |
| Bcl2 | 5′–agg aag tga aca ttt cgg tga c–3′ | 5′–gct cag ttc cag gac cag g–3′ |
| Gadd45β | 5′–tgg aag agc tcg tgg cgt g–3′ | 5′–cct cgt caa tgg aga ggc–3′ |
| MALT1 | 5′–gga tct tac tga ata tga gat gc–3′ | 5′–gac aat ttt cag acc tat atg g–3′ |
Results
Expression of MALT1 in normal and malignant lymphoid tissues
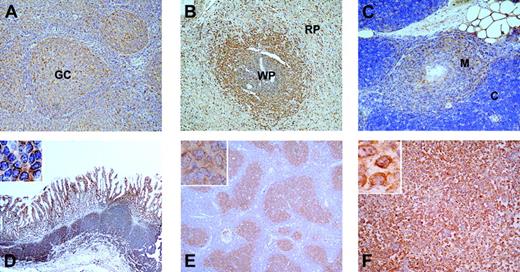
To investigate the localization of MALT1 in human normal and neoplastic lymphoid tissues, we performed immunohistochemical studies using a monoclonal antibody against the C-terminal part of MALT1.5 MALT1 is expressed predominantly in B cells in the germinal center (white pulp) and marginal zone of the spleen, the germinal center of tonsils, and the medulla of the thymus. In contrast, mantle zone B cells and interfollicular T cells of tonsils express relatively low amounts of MALT1 (Figure 1A-C). We also stained 6 cases of MALT lymphoma, which showed strong staining in infiltrating neoplastic lymphocytes in the gastric mucosa (Figure 1D). MALT1 was strongly expressed in large and small follicular cells in all 20 cases of follicular lymphomas examined (Figure 1E) and in large B-cell blasts in all 20 cases of diffuse large B-cell lymphoma (DLBCL; Figure 1F). Overall, these findings are consistent with a germinal center/marginal zone origin for MALT lymphoma and with deficiencies in marginal zone B cells and germinal centers in MALT1-deficient mice.20
Expression of MALT1 in normal human lymphoid tissues. Sections of normal reactive tonsil (panel A), spleen (panel B), and thymus (panel C) were stained with antibodies against MALT1. The germinal center (GC) of the tonsil, the white pulp (WP) and red pulp (RP) of the spleen, and the medulla (M) and cortex (C) of the thymus are indicated. The same staining was performed with sections of gastric MALT lymphoma (panel D), follicular lymphoma (panel E), and diffuse large B-cell lymphoma (DLBCL; panel F). Images were acquired as described in “Immunohistochemical staining.”
Expression of MALT1 in normal human lymphoid tissues. Sections of normal reactive tonsil (panel A), spleen (panel B), and thymus (panel C) were stained with antibodies against MALT1. The germinal center (GC) of the tonsil, the white pulp (WP) and red pulp (RP) of the spleen, and the medulla (M) and cortex (C) of the thymus are indicated. The same staining was performed with sections of gastric MALT lymphoma (panel D), follicular lymphoma (panel E), and diffuse large B-cell lymphoma (DLBCL; panel F). Images were acquired as described in “Immunohistochemical staining.”
MALT1 and API2-MALT1 are components of lipid rafts of malignant B cells
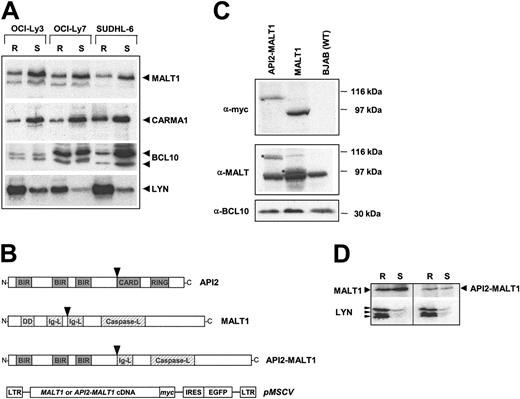
Detergent-resistant lipid-rich microdomains, or rafts, on the plasma membrane are believed to be signaling platforms harboring molecular complexes that are essential for signal transduction.39 The in vitro binding partner of MALT1, BCL10, is recruited to rafts by CARMA1 upon T-cell receptor (TCR) stimulation.27 We therefore examined several B-cell lymphoma cell lines established from DLBCL patients for MALT1 localization. We found that a pool of MALT1 is constitutively present in rafts, together with CARMA1 and BCL10 (Figure 2A). To study the biologic consequence of MALT1 and AP12/MALT1 expression in B cells, we generated BJAB cells stably expressing MALT1 or API2-MALT1 using retroviral vectors (Figure 2B). Expression of the transgenes was confirmed in the transfected cell populations and compared with that of the endogenous levels (Figure 2C). Similar expression levels of exogenous and endogenous MALT1 were observed (Figure 2C middle panel). The presence of its binding partner, BCL10, was detected in similar levels in transfected and parental BJAB cells (Figure 2C bottom panel). Individual cell clones were selected expressing either MALT1 or API2-MALT1 and used in some of the following experiments. Like endogenous MALT1 in DLBCL cells, exogenously expressed MALT1 and API2-MALT1 in BJAB cells were also found to be constitutively present in lipid rafts (Figure 2D).
Constitutive expression of MALT1 in lipid rafts of human B-cell lymphoma cells. (A) Western blotting shows MALT1, as well as CARMA1 and BCL10, to be located in both lipid raft (R) and soluble fractions (S) in DLBCL cell lines separated by sucrose gradient centrifugation. Arrowheads mark the band of the expected full-length size for each protein. The origin of the smaller band on the MALT1 blots, not observed in blots from BJAB cells, is uncertain. The multiple bands of BCL10 represent phosphorylation isoforms as they disappear after phosphatase treatment (data not shown). The partitioning of LYN, a B-cell-specific protein of the Src protein kinase family known to be constitutively present in rafts, confirms effective separation of raft and nonraft protein fractions. (B) Structure of C-terminal myc-tagged MALT1 and API2-MALT1 transgenes and pMSCV retroviral vectors used in the study. Arrowheads show positions of the most common breakpoints found in t(11;18) that give rise to the API2-MALT1 fusion gene, which is used for all of the following experiments. (C) Lysates from equivalent cell numbers of pools of BJAB cells transfected with API2-MALT1 or MALT1 were immunoblotted for myc, MALT1, and BCL10. The exogenous API2-MALT1 and MALT1 are detected by anti-myc and with the MALT1 antibody (*). (D) Lipid raft and soluble fractions of lysates from pools of MALT1- and API2-MALT1-expressing BJAB cells were immunoblotted for myc and LYN. LTR indicates long-term repeat; BIR, baculovirus inhibitor of apoptosis repeat; DD, death domain; RING, RING domain; and IRES, internal ribosomal entry site.
Constitutive expression of MALT1 in lipid rafts of human B-cell lymphoma cells. (A) Western blotting shows MALT1, as well as CARMA1 and BCL10, to be located in both lipid raft (R) and soluble fractions (S) in DLBCL cell lines separated by sucrose gradient centrifugation. Arrowheads mark the band of the expected full-length size for each protein. The origin of the smaller band on the MALT1 blots, not observed in blots from BJAB cells, is uncertain. The multiple bands of BCL10 represent phosphorylation isoforms as they disappear after phosphatase treatment (data not shown). The partitioning of LYN, a B-cell-specific protein of the Src protein kinase family known to be constitutively present in rafts, confirms effective separation of raft and nonraft protein fractions. (B) Structure of C-terminal myc-tagged MALT1 and API2-MALT1 transgenes and pMSCV retroviral vectors used in the study. Arrowheads show positions of the most common breakpoints found in t(11;18) that give rise to the API2-MALT1 fusion gene, which is used for all of the following experiments. (C) Lysates from equivalent cell numbers of pools of BJAB cells transfected with API2-MALT1 or MALT1 were immunoblotted for myc, MALT1, and BCL10. The exogenous API2-MALT1 and MALT1 are detected by anti-myc and with the MALT1 antibody (*). (D) Lipid raft and soluble fractions of lysates from pools of MALT1- and API2-MALT1-expressing BJAB cells were immunoblotted for myc and LYN. LTR indicates long-term repeat; BIR, baculovirus inhibitor of apoptosis repeat; DD, death domain; RING, RING domain; and IRES, internal ribosomal entry site.
Effects of exogenous MALT1 and API2-MALT1 on constitutive and CD40-mediated NF-κB activity
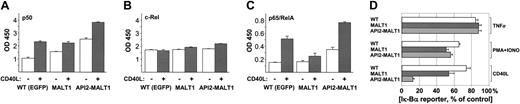
We examined the effect on NF-κB activity of API2-MALT1 or MALT1 overexpression in BJAB cells using an ELISA-based assay to quantify NF-κB subunits (p50, p65/RelA, RelB, p52, and c-Rel) in nuclear extracts. In unmodified BJAB cells there were relatively high amounts of p50 and c-Rel compared with p65 (Figure 3) and other subunits (data not shown). After stimulation of CD40, which is known to activate the IKK complex, nuclear c-Rel was relatively unchanged but there were increases in p50 and p65/RelA (Figure 3A-C). Analysis of unstimulated cells showed that basal NF-κB activity was increased by API2-MALT1 (p50 and p65/RelA) or exogenous MALT1 (p50 only). The response to CD40 stimulation was also increased by API2-MALT1 (p50, c-Rel, and p65/RelA), and MALT1 produced a modest but significant rise in nuclear c-Rel after CD40 stimulation.
Constitutive and CD40-stimulated NF-κB activation in BJAB cells expressing MALT1 or API2-MALT1. BJAB cells transfected with pMSCV-IRES-EGFP vector alone or single clones transfected with vector carrying MALT1 or API2-MALT1 were untreated (□) or treated with 0.1 μg/mL cross-linked CD40L for 4 hours (▦). Two micrograms of nuclear protein extracts from each cell line was used in an ELISA assay measuring (A) p50, (B) c-Rel, and (C) p65/RelA subunits of NF-κB. The results represent triplicates of 1 of 3 representative experiments. (D) Enhanced NF-κB activation by API2-MALT1 and MALT1 upon CD40L but not PMA/ionomycin (PMA + IONO) or TNFα stimulation. BJAB cells expressing IκBα reporter only (WT; □) or reporter with MALT1 (▦) or API2-MALT1 (▧) were untreated or treated for 4 hours with TNFα, PMA/ionomycin, or cross-linked CD40L. Lysates were used in a luciferase assay to measure the amount of exogenous IκBα-luciferase, inversely related to the activity of IKK. Each bar represents the reporter level as a percentage normalized to the corresponding untreated cells. Values are obtained from triplicates of one of several representative experiments. Error bars indicate the standard deviations obtained from triplicate samples. The experiment is representative of several independent experiments.
Constitutive and CD40-stimulated NF-κB activation in BJAB cells expressing MALT1 or API2-MALT1. BJAB cells transfected with pMSCV-IRES-EGFP vector alone or single clones transfected with vector carrying MALT1 or API2-MALT1 were untreated (□) or treated with 0.1 μg/mL cross-linked CD40L for 4 hours (▦). Two micrograms of nuclear protein extracts from each cell line was used in an ELISA assay measuring (A) p50, (B) c-Rel, and (C) p65/RelA subunits of NF-κB. The results represent triplicates of 1 of 3 representative experiments. (D) Enhanced NF-κB activation by API2-MALT1 and MALT1 upon CD40L but not PMA/ionomycin (PMA + IONO) or TNFα stimulation. BJAB cells expressing IκBα reporter only (WT; □) or reporter with MALT1 (▦) or API2-MALT1 (▧) were untreated or treated for 4 hours with TNFα, PMA/ionomycin, or cross-linked CD40L. Lysates were used in a luciferase assay to measure the amount of exogenous IκBα-luciferase, inversely related to the activity of IKK. Each bar represents the reporter level as a percentage normalized to the corresponding untreated cells. Values are obtained from triplicates of one of several representative experiments. Error bars indicate the standard deviations obtained from triplicate samples. The experiment is representative of several independent experiments.
MALT1 and API2-MALT1 act in the CD40-inducing NF-κB pathway upstream of IKK complex activation
To address at which point the transgenes affect the CD40 pathway to NF-κB activation, we created a cell-based assay for IKK activity in BJAB cells by stably expressing an IκBα-luciferase fusion reporter gene. The assay derives from observations that the exogenous IκBα-reporter protein (like endogenous IκBα) is degraded as a result of IKK activity, but its transcription (unlike that of endogenous IκB) is relatively independent of NF-κB activity (ie, its production can be regarded as constitutive). Therefore, the level of the reporter protein (easily measured as luciferase activity) can serve as an indicator of IKK activity, falling as IKK activity increases and vice versa. As expected, BJAB cells stably expressing the reporter alone showed a substantial decline in the reporter level after stimulation by PMA and ionomycin, indicating an acute increase in IKK activity with less of a response to TNFα (Figure 3D). BJAB cells also expressing MALT1 or API2-MALT1 showed a similar pattern and degree of response to these agents, indicating that these transgenes had no effect on the respective pathways by which these agents increase IKK activity. In contrast, it was clear that these transgenes further augmented the increase in IKK activity caused by CD40 signaling in BJAB cells. The fact that API2-MALT1 was the more effective transgene, despite similar levels of expression shown by Western blots, is in agreement with NF-κB activation abilities observed in 293T cells (data not shown).
API2-MALT1 and MALT1 expression protect BJAB cells from FAS-mediated apoptosis
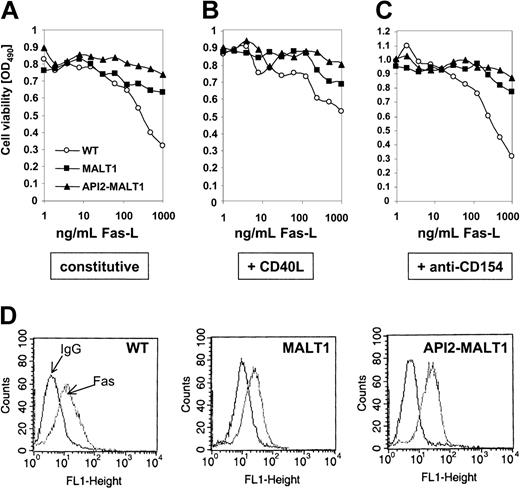
Our results so far indicate that MALT and API2-MALT1 can function in the CD40 signaling pathway, which blocks apoptosis induced by FAS (APO1) in B-cell lines and primary cells from the germinal center stage.33,40 We found that BJAB cells expressing MALT1 or API2-MALT1 were more resistant to FAS-induced apoptosis than were parental mock-infected cells (Figure 4A). This difference in FAS sensitivity was not due to differences in expression of FAS, which was essentially the same between the 3 cell lines (Figure 4D). Wild-type (WT) cells were more resistant to FAS in the presence of CD40 ligand (CD154; Figure 4B), approaching the resistance displayed by unstimulated cells expressing either transgene. The specificity of protection caused by CD40L (CD154) on WT cells was further confirmed when the sensitivity to FAS-L shifted back to that of untreated cells in the presence of an anti-CD154 antibody (Figure 4C).
Protection from FAS-induced apoptosis by MALT1 and API2-MALT1. (A) Parental BJAB cells with vIRES-puromycin vector alone (WT) or single clones of cells expressing MALT1 or API2-MALT1 were challenged for 16 hours with serial dilutions of soluble FAS-L alone, with (B) 0.1 μg/mL cross-linked CD40L, or with (C) a CD40L neutralizing antibody (anti-CD154). Results shown are representative of 4 different experiments. (D) FACS analyses of FAS expression in the 3 cell lines.
Protection from FAS-induced apoptosis by MALT1 and API2-MALT1. (A) Parental BJAB cells with vIRES-puromycin vector alone (WT) or single clones of cells expressing MALT1 or API2-MALT1 were challenged for 16 hours with serial dilutions of soluble FAS-L alone, with (B) 0.1 μg/mL cross-linked CD40L, or with (C) a CD40L neutralizing antibody (anti-CD154). Results shown are representative of 4 different experiments. (D) FACS analyses of FAS expression in the 3 cell lines.
Constitutive NF-κB activation is critical for MALT1- and API2-MALT1-mediated FAS resistance and proliferation
It is perhaps surprising that CD154 did not further increase the FAS resistance of cells expressing MALT1 or API2-MALT1 (Figure 4B), since these cells respond to CD40 stimulation in several other measures (eg, in the ELISA assay for nuclear subunits) and are in fact even more responsive than WT cells. However, this finding suggests that FAS resistance imparted by the transgenes is due to their impact on constitutive NF-κB activity, an effect shown by the ELISA assay in the absence of stimulation. To examine this, BJAB cells expressing no transgene, MALT1, or API2-MALT1 were secondarily infected with a “superrepressor” (IκBα-SR) form of IκBα, bearing mutations in the 2 serine phosphorylation sites (S32G, S36A). This mutant cannot be phosphorylated by IKK and subsequently degraded and acts thus as a dominant negative in the NF-κB pathway.36 MALT1- and API2-MALT1-expressing cells were resistant to FAS-mediated apoptosis even at 2 μg/mL FAS-L. In contrast, the corresponding lines coexpressing SR were significantly less resistant, similar to their parental cells (Figure 5A). In the parental cells, the superrepressor had no effect on sensitivity to FAS-L. Given that all the SR+ lines express similar levels of IκBα-SR (Figure 5D), these results indicate that MALT1- or API2-MALT1-mediated resistance to FAS-induced apoptosis is dependent on their induction of constitutive NF-κB activity.
NF-κB activation by MALT1 and API2-MALT1 is necessary for resistance to FAS-mediated apoptosis and proliferation. (A) BJAB cells expressing no primary transgene (WT) or expressing MALT1 or API2-MALT1 were secondarily infected with empty vector or IκBα superrepressor (SR) and incubated in triplicates with soluble FAS-L. Cell viability was assayed after 16 hours. The results are representative of 2 independent experiments. (B) Cell proliferation was measured by incorporation of 3[H]-thymidine measured in single clones or pools of BJAB cells expressing no transgene (WT), MALT1, or API2-MALT1. Results are shown as cpm, with error bars representing standard deviation obtained from triplicate samples. (C) The same BJAB cells as in panel A with empty second vector (□) or IκBα superrepressor (▪) were assayed for 3[H]-thymidine incorporation as described above. The data shown are 1 of 3 independent experiments. (D) Lysates of equal numbers of the above BJAB cells were immunoblotted against the N-terminal FLAG epitope tag of SR-IκBα to show specific and equal expression.
NF-κB activation by MALT1 and API2-MALT1 is necessary for resistance to FAS-mediated apoptosis and proliferation. (A) BJAB cells expressing no primary transgene (WT) or expressing MALT1 or API2-MALT1 were secondarily infected with empty vector or IκBα superrepressor (SR) and incubated in triplicates with soluble FAS-L. Cell viability was assayed after 16 hours. The results are representative of 2 independent experiments. (B) Cell proliferation was measured by incorporation of 3[H]-thymidine measured in single clones or pools of BJAB cells expressing no transgene (WT), MALT1, or API2-MALT1. Results are shown as cpm, with error bars representing standard deviation obtained from triplicate samples. (C) The same BJAB cells as in panel A with empty second vector (□) or IκBα superrepressor (▪) were assayed for 3[H]-thymidine incorporation as described above. The data shown are 1 of 3 independent experiments. (D) Lysates of equal numbers of the above BJAB cells were immunoblotted against the N-terminal FLAG epitope tag of SR-IκBα to show specific and equal expression.
Because NF-κB activation in malignant lymphomas has been shown to increase proliferation as well as survival,41 we examined the effect of MALT1 and API2-MALT1 on proliferation as measured by incorporation of 3[H] thymidine into DNA. Both transgenes conferred a modest but significant growth advantage to pools and single clones expressing the transgenes (Figure 5B). Because this advantage was largely eliminated by IκBα-SR (Figure 5C), it can be attributed to constitutive NF-κB activation. In contrast, expression of IκBα-SR did not affect proliferation of the parental BJAB cells.
MALT1 and API2-MALT1 specifically enhance CD40-stimulated NF-κB activation
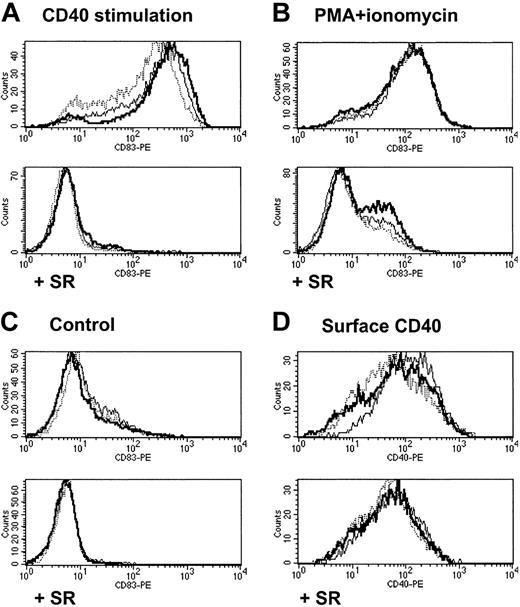
The effect of the superrepressor (SR) as an effective inhibitor of NF-κB activation in these lines was further demonstrated by measuring surface expression of CD83, a known direct target of NF-κB.42,43 Among cell lines infected in parallel with the empty second vector, and in keeping with the results so far, the CD83 increase after 4 hours of CD40 stimulation was augmented by the transgenes (Figure 6A), although they did not affect the response to PMA/ionomycin (Figure 6B). A similar pattern of enhancement by transgenes was seen over a spectrum of lower doses and shorter duration of CD40 stimulation and in experiments using transient transfection of an NF-κB-driven luciferase reporter plasmid (data not shown). In sharp contrast, all SR lines were completely unresponsive to CD40 stimulation (Figure 6A), ie, CD83 remained virtually undetectable and lower than in the non-SR lines without stimulation (Figure 6C). The effect of the transgenes on CD40 signaling was not explained by baseline CD83 levels or levels of surface CD40 itself, which were little affected by the transgenes and only modestly reduced by IκBα-SR (Figure 6D). Furthermore, reduced CD40 was unlikely to be the only effect of the IκBα-SR on NF-κB activation by the CD40 pathway, since the CD83 response to PMA/ionomycin stimulation was also markedly reduced in SR lines.
Effect of MALT1 and API2-MALT1 on NF-κB activation as measured by CD83 expression. FACS measurement of surface markers in BJAB lines with no transgene (dotted line), MALT1 (thin line), or API2-MALT1 (bold line) secondarily infected with empty vector (top panels) or IκBα superrepressor (bottom panels). Histograms show CD83 expression after 4 hour incubation with cross-linked CD40L (A), PMA+ionomycin (B), or medium alone (C). Histogram (D) shows resting CD40 expression in the 6 lines.
Effect of MALT1 and API2-MALT1 on NF-κB activation as measured by CD83 expression. FACS measurement of surface markers in BJAB lines with no transgene (dotted line), MALT1 (thin line), or API2-MALT1 (bold line) secondarily infected with empty vector (top panels) or IκBα superrepressor (bottom panels). Histograms show CD83 expression after 4 hour incubation with cross-linked CD40L (A), PMA+ionomycin (B), or medium alone (C). Histogram (D) shows resting CD40 expression in the 6 lines.
MALT1 and API2-MALT1 enhance expression of distinct cytoprotective NF-κB target genes induced by CD40 signaling
Many NF-κB target genes protect against apoptotic stimuli in B cells and other cell types44 ; in particular, CD40 signaling increases B-cell survival through NF-κB-mediated up-regulation of BclXL and BfL1/A1.40 We therefore compared expression of a series of known antiapoptotic NF-κB target genes (API1, API2, BclXL, hA20, Bfl1/A1, and Bcl2) in BJAB cells expressing MALT1, API2-MALT1, or vector only (Figure 7A). While resting mRNA levels of API2 and hA20 were significantly higher in MALT1-expressing cells than in others, that of Bcl2 was significantly higher in API2-MALT1-expressing cells, suggesting that there may be differences in how MALT1 and API2-MALT1 produce constitutive NF-κB activation. CD40 stimulation of these lines produced significant elevations in mRNA levels of most targets (Figure 7B), but BclXL, rose significantly only in the presence of API2-MALT1 or overexpressed MALT1, confirming the previous suggestion that both transgenes are involved in CD40-mediated NF-κB activation. The protein levels of BCLXL and API2 rose significantly following 24 hours of CD40 stimulation, consistent with the rise in their mRNA levels (Figure 7C). In the same experiment, no change due to transgenes and/or CD40 stimulation was found in the mRNA levels of several other known cytoprotective NF-κB target genes (cFLIP, hXIAP, SURVIVIN, and Gadd45β; data not shown), showing that these do not mediate the observed changes in FAS resistance in BJAB cells and that target gene expression is influenced by cell type.
Constitutive and CD40-enhanced transcription of cytoprotective genes. (A) Quantitative PCR of cDNAs from unstimulated BJAB cells with no transgene (▪), API2-MALT1 (□), or MALT1 (▨). Relative expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. Standard deviation bars were obtained from 2 independent experiments, each performed in triplicates. (B) In parallel, the same cells were stimulated with cross-linked CD40L for 4 hours and total RNA was extracted for quantitative RT-PCR analyses. (C) BJAB cells with and without the transgenes were stimulated with CD40 ligand for 0, 4, or 24 hours, and 30 μg of lysate was charged per lane and immunoblotted for BclXL and API2.
Constitutive and CD40-enhanced transcription of cytoprotective genes. (A) Quantitative PCR of cDNAs from unstimulated BJAB cells with no transgene (▪), API2-MALT1 (□), or MALT1 (▨). Relative expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. Standard deviation bars were obtained from 2 independent experiments, each performed in triplicates. (B) In parallel, the same cells were stimulated with cross-linked CD40L for 4 hours and total RNA was extracted for quantitative RT-PCR analyses. (C) BJAB cells with and without the transgenes were stimulated with CD40 ligand for 0, 4, or 24 hours, and 30 μg of lysate was charged per lane and immunoblotted for BclXL and API2.
Discussion
It has been known for some time that a small set of recurring chromosomal translocations is associated with MALT B-cell lymphomas, but the functional consequences of these genetic alterations have only recently come to light. Chromosomal translocations t(11;18)(q21;q21) and t(14;18)(q32;q21), found in up to 50% of MALT lymphomas, lead to overexpression of MALT1 or formation of an API2-MALT1 fusion protein.7,8 Although there was reason to believe that these would lead to NF-κB activation in MALT B-cell lymphoma, and immunostains of primary MALT lymphomas have shown evidence of NF-κB activation,45 this has not been shown experimentally in human B cells. Here we demonstrate that both MALT1 and API2-MALT1 confer constitutive NF-κB activity in a human B-cell line, resulting in increased proliferation and resistance to FAS-induced apoptosis. Furthermore, both transgenes act upstream of the IKK complex to enhance NF-κB activation induced by CD40. Many lymphoid malignancies have been associated with constitutive NF-κB activation and poor prognosis, and elevated NF-κB activity in MALT B-cell lymphoma would favor malignant B-cell proliferation and survival.41,46-48 Our results are consistent with recent findings that NF-κB activation by API2-MALT transforms NIH 3T3 cells and inhibits p53-induced cell death.49
Based on previous studies and our results presented here, we propose the following model for the role of MALT1 and API2-MALT1 in MALT lymphomagenesis (Figure 8). In early stages, the immune response triggered by H pylori infection induces NF-κB activation in B cells via CD40/CD40L interaction with T cells, increasing the expression of specific cytoprotective genes. The acquisition of recurring chromosomal translocations results in the expression of API2-MALT1 or overexpression of MALT1, producing constitutive NF-κB activation and enhancing the response to CD40 stimulation. Both of these are potentially advantageous to lymphoma cells, but constitutive NF-κB activity would confer independence from the immune response to H pylori, and we have shown that it alone may be sufficient for enhanced proliferation and resistance to FAS-induced apoptosis. Finally, because the API2-MALT1 fusion is itself regulated by NF-κB, a positive feedback loop may be maintained that further supports bacterium- and CD40-independent NF-κB activation, leading to progression of MALT lymphoma.7,8 It should be noted that at least parts of this model may not be restricted to gastric MALT lymphoma or H pylori infection, although those have generated much of the research implicating CD40 and antigen drive in MALT lymphoma. MALT1 translocations are found in tumors from other sites, and recently similar causal associations have been found between Chlamydia psittaci and MALT lymphoma of the orbit and between Campylobacter jejuni and a MALT lymphoma-like immunoproliferative lesion of the small intestine.50,51
Model of the role of MALT1 and API2-MALT1 in the NF-κB activation pathway leading to MALT lymphoma.H pylori infection induces a chronic T-cell-dependent immune reaction leading to CD40/CD40L interaction. Stimulation of B cells through CD40 activates IKK and NF-κB via a complex containing MALT1, BCL10, and CARMA. Overexpression of MALT1 or expression of API2-MALT1 as a consequence of chromosomal translocations [t(11;18)(q21;q21) or t(14;18)(q32; q21)] results in elevated constitutive and CD40-stimulated IKK activity, increasing transcription of NF-κB target genes and favoring survival and proliferation. In cases with t(11;18)(q21;q21), API2-MALT feeds back positively to sustain high constitutive NF-κB activity and reduce dependence on H pylori infection.
Model of the role of MALT1 and API2-MALT1 in the NF-κB activation pathway leading to MALT lymphoma.H pylori infection induces a chronic T-cell-dependent immune reaction leading to CD40/CD40L interaction. Stimulation of B cells through CD40 activates IKK and NF-κB via a complex containing MALT1, BCL10, and CARMA. Overexpression of MALT1 or expression of API2-MALT1 as a consequence of chromosomal translocations [t(11;18)(q21;q21) or t(14;18)(q32; q21)] results in elevated constitutive and CD40-stimulated IKK activity, increasing transcription of NF-κB target genes and favoring survival and proliferation. In cases with t(11;18)(q21;q21), API2-MALT feeds back positively to sustain high constitutive NF-κB activity and reduce dependence on H pylori infection.
Constitutive NF-κB activation by API2-MALT1 or overexpressed MALT1 is a primary feature of the proposed model. Normal mature B cells require some degree of constitutive NF-κB activity for survival, as shown by their disappearance from mice after conditional deletion of NEMO or IKKβ.52 Deficiencies in germinal centers and splenic marginal zone B cells are features of mice with germ line inactivation of members of the NF-κB family,53 as they are of mice with germ line inactivation of MALT1, BCL10, or CARMA1. Since there is in vitro evidence that these proteins can produce IKK activation, it is plausible that they normally function in a common pathway contributing to constitutive NF-κB activity in B cells. Using a human B-cell line corresponding to the germinal center stage of B-cell differentiation, we have shown that API2-MALT1 or overexpressed MALT1 produces increased constitutive NF-κB activity, resulting in increased proliferation and resistance to FAS-induced apoptosis. These benefits are plausible given that many NF-κB target genes have antiapoptotic functions and some (eg, cyclin D2) are associated with cell-cycle progression. However, this may not fully model the consequences of increased constitutive NF-κB activity in MALT lymphoma or other types of lymphoma in which it is found. In particular, while IκBα-SR merely abrogates the effects attributable to API2-MALT or overexpressed MALT1 in BJAB cells, returning them to a state comparable to that of wild-type cells, it is lethal to cell lines from lymphoma types characterized by increased constitutive NF-κB activity.34 In other words, increased constitutive NF-κB activity is not merely advantageous but an essential feature of the biology of these types of lymphoma; whether this is the case for MALT lymphoma remains unknown.
The mechanisms of constitutive IKK activation by API2-MALT and overexpressed MALT1 may be multifaceted and somewhat different, related to our findings that the 2 transgenes had both similar and different effects by several measures. In vitro studies showed that IKK activation by MALT1 or BCL10 requires both proteins and this was supported by studies using embryonic fibroblasts from MALT1 knockout mice.12 Using purified proteins, Sun et al54 successfully reconstituted the pathway from BCL10 to IKK activation, in which oligomerization of MALT1 by a BCL10/CARMA1/MALT1 complex leads to the recruitment of a ubiquitin ligase (TNF receptor-associated factor 6 [TRAF6]), followed by activation of a kinase (transforming growth factor-β [TGFβ]-activated kinase 1 [TAK1]), which activates the IKK complex and NF-κB. In contrast, BCL10 is not required for IKK activation by API2-MALT, and BCL10 was not among several proteins found to bind to API2-MALT in one study.12,55 API2-MALT is more stable than MALT1 or API2 because it lacks domains of those proteins that promote proteasomal degradation.56 Subcellular localization is also likely to be a major factor affecting the function of API2-MALT and MALT1. We found that endogenous MALT1 in several DLBCL cell lines and exogenously expressed MALT1 and API2-MALT1 in BJAB cells are constitutively present in lipid raft fractions together with BCL10 and CARMA1. This is contrary to previous studies using primary T cells that showed that BCL10 is only recruited into rafts upon TCR stimulation.27 Studies using cells from DLBCL patients have shown that preassembled signaling complexes in rafts, named signalosomes, contain molecules of the CD40 signaling pathway and may be responsible for elevated constitutive NF-κB activity.57 These observations suggest that MALT1 and API2-MALT1 may be preassembled into so-called signalosomes, contributing to their ability to activate IKK constitutively and/or enhance CD40 signaling. However, it is likely that there are additional regulating factors since DLBCL cell lines with similar findings on lipid raft analysis for MALT1, CARMA, and BCL10 (Figure 2A) have substantially different levels of constitutive NF-κB activity and a starkly different response to IκBα-SR expression.34
A second key feature of our proposed model is that both API2-MALT and overexpressed MALT1 enhance the effect of CD40 stimulation on IKK activation. This is consistent with one study of germ line inactivation of MALT1, which found that MALT1 was required for the proliferative response of mouse B cells to CD40 stimulation.21 CD40 signaling has previously been implicated to play an essential role in MALT lymphomagenesis29,30 and is more significant given our finding that API2-MALT or MALT1 overexpression can potentiate CD40 signaling to NF-κB. CD40 signaling affects multiple points in the normal B-cell response to antigen, including B-T-cell interaction and germinal center formation, entry, and clonal selection.33,58 Immunoglobulin gene hypermutation indicates that cells of MALT lymphoma traverse this same process, at least initially, so there are many opportunities for enhanced CD40 signaling to promote survival, although questions remain about B-cell physiology. For example, CD40 signaling can block spontaneous in vitro apoptosis of normal human germinal center B cells, which originates from complexes centered around FAS (highly expressed at this differentiation stage).59 However, lpr mice with inactivating mutations in FAS do not show a simple reduction in apoptosis of germinal center B cells but rather more subtle changes in number and autoreactivity of memory cells, implying a more modest role for FAS in germinal center physiology.60 Nonetheless, resistance to FAS-induced apoptosis has been implicated as a factor in MALT lymphomagenesis, in that FAS mutations have been found in some primary tumors,61 and we have shown that FAS killing of BJAB cells is opposed by the constitutive effects of API2-MALT or MALT1 overexpression.
A third key aspect of our model is that since API2 is regulated by NF-κB,62 API2-MALT is still subject to regulation by NF-κB at the API2 locus and has the potential to establish a positive feedback loop, as has previously been speculated.12 This could be critical in enabling gastric MALT lymphoma with t(11;18)(q21;q21) not to require ongoing stimulation by H pylori infection and would be consistent with the observation that cases with this translocation do not regress with eradication of infection. In contrast, endogenous MALT1 mRNA levels in BJAB cells were not changed by CD40 stimulation or IκBα-SR expression (data not shown), confirming that endogenous MALT1 is not an NF-κB target gene in BJAB cells and cannot be part of a positive feedback loop.
In summary, we have used a B-cell line model to provide evidence that MALT1 and its fusion protein API2-MALT1 contribute to MALT lymphomagenesis through constitutive and CD40-stimulated increases in NF-κB activity. To study the roles of MALT1 and its fusion protein API2-MALT1 in lymphomagenesis in vivo, transgenic mouse models overexpressing these proteins would be informative. Due to the role of NF-κB as a common downstream mediator of a large number of signal transduction pathways, its targeted therapeutic inhibition may be difficult to achieve without untoward side effects.63 Identification and functional characterization of the upstream activators of NF-κB in particular types of lymphoma could provide more specific therapeutic targets. Since germ line inactivation of the MALT1 gene in mice seems almost exclusively to affect the lymphoid system,20,21 blocking MALT1 function may be associated with controllable side effects.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-06-2297.
Supported by grants (J.S.) from the Swiss National Science Foundation (SNF-3100-056984.99), the Swiss Cancer League (KFS-01077-09-2000), and the Cancer and Solidarité Foundation (Geneva, Switzerland).
L.H. and R.E.D. contributed equally to this study.
Presented partially in poster and abstract forms at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to P. Schneider for his gift of FAS-L and for technical advice, to V. Dixit for kindly providing MALT1 antibodies, and M. Baens and P. Marynen for the MALT1 and API2-MALT1 cDNAs. We also thank J. Zaric for initial participation on the project, M. Thome for helpful discussions, D. Hoessli for technical advice on raft preparation, and P. Gindre for performing the immunohistochemical stainings.

![Figure 5. NF-κB activation by MALT1 and API2-MALT1 is necessary for resistance to FAS-mediated apoptosis and proliferation. (A) BJAB cells expressing no primary transgene (WT) or expressing MALT1 or API2-MALT1 were secondarily infected with empty vector or IκBα superrepressor (SR) and incubated in triplicates with soluble FAS-L. Cell viability was assayed after 16 hours. The results are representative of 2 independent experiments. (B) Cell proliferation was measured by incorporation of 3[H]-thymidine measured in single clones or pools of BJAB cells expressing no transgene (WT), MALT1, or API2-MALT1. Results are shown as cpm, with error bars representing standard deviation obtained from triplicate samples. (C) The same BJAB cells as in panel A with empty second vector (□) or IκBα superrepressor (▪) were assayed for 3[H]-thymidine incorporation as described above. The data shown are 1 of 3 independent experiments. (D) Lysates of equal numbers of the above BJAB cells were immunoblotted against the N-terminal FLAG epitope tag of SR-IκBα to show specific and equal expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-06-2297/6/m_zh80070576470005.jpeg?Expires=1767787825&Signature=3YM276O7kKa3NpJm9VUek5GdaDb20s14IFl1w4v-aSz2io9wy7YX1q6LJfL7D8LPtCppf8iEHifPwfOXWaCVz~JFzCaCc833bE~17LwYHaCPsFRy5igTT8XsaIPuMGiqe~pX1g7-b1QrvcQupL1EFwO003qgRe6vAsGqyJh3GGQEOUM0uWz58X~OEDVcZ2n11KR8j26Yy-2xRjtlx2qpw9pM3aMYQVDuEvUgzRA6ZYN2ptTznpTUIpJzNyJFCXAUZQ8IGqwgGuQaHZKO5djEuoWhpk01f8xBysTdTgtzHk1l3mnr~tCwIdB7S4bwOjv93tsLLYFYMtP1S7Yx3jbSSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 8. Model of the role of MALT1 and API2-MALT1 in the NF-κB activation pathway leading to MALT lymphoma. H pylori infection induces a chronic T-cell-dependent immune reaction leading to CD40/CD40L interaction. Stimulation of B cells through CD40 activates IKK and NF-κB via a complex containing MALT1, BCL10, and CARMA. Overexpression of MALT1 or expression of API2-MALT1 as a consequence of chromosomal translocations [t(11;18)(q21;q21) or t(14;18)(q32; q21)] results in elevated constitutive and CD40-stimulated IKK activity, increasing transcription of NF-κB target genes and favoring survival and proliferation. In cases with t(11;18)(q21;q21), API2-MALT feeds back positively to sustain high constitutive NF-κB activity and reduce dependence on H pylori infection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-06-2297/6/m_zh80070576470008.jpeg?Expires=1767787825&Signature=boE39WTB~ck1Ins8fCuVD1oMeHDlFGm~YGSqEGzcJtWnHkHf~qyVyDsRu6TcFPMcgMDrC5B5gqvJmoHx-8a-2HPn4NM5XlhwlKlfIJOTVVPFCsIZXPOyi108-qM2vWOhhF68wwN9MmZpWNYb8alei6exigNMfCKm0qdpk46tVMCL7QkBhSyoAX3tuvOOhaZhY4cisMNGe1jNRTMmwfCgnHf~aRJF3sKC-fw83K73B4rkbiO4i2fUEmxU7Fp6nfeIs-9eEvpogQ0joZU7BuZSvN4ZoKhz2ppRVhKi1ETou2e9Mk1vC0dESYOeCujITzp2di7gl1ob5bvv55UYYXOVFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal