Abstract
CD97, a membrane protein expressed at high levels on inflammatory cells and some carcinomas, is a member of the adhesion G protein–coupled receptor family, whose members have bipartite structures consisting of an extracellular peptide containing adhesion motifs noncovalently coupled to a class B 7-transmembrane domain. CD97α, the extracellular domain of CD97, contains 3 to 5 fibrillin class 1 epidermal growth factor (EGF)–like repeats, an Arg-Gly-Asp (RGD) tripeptide, and a mucin stalk. We show here that CD97α promotes angiogenesis in vivo as demonstrated with purified protein in a directed in vivo angiogenesis assay (DIVAA) and by enhanced vascularization of developing tumors expressing CD97. These data suggest that CD97 can contribute to angiogenesis associated with inflammation and tumor progression. Strong integrin α5β1 interactions with CD97 have been identified, but αvβ3 also contributes to cell attachment. Furthermore, soluble CD97 acts as a potent chemoattractant for migration and invasion of human umbilical vein endothelial cells (HUVECs), and this function is integrin dependent. CD97 EGF-like repeat 4 is known to bind chondroitin sulfate. It was found that coengagement of α5β1 and chondroitotin sulfate proteoglycan by CD97 synergistically initiates endothelial cell invasion. Integrin α5β1 is the first high-affinity cellular counterreceptor that has been identified for a member within this family of adhesion receptors.
Introduction
The adhesion G protein–coupled receptors are membrane-bound proteins with long N-termini containing multiple and distinct combinations of adhesion motifs.1 These proteins contain domains such as epidermal growth factor (EGF)–like, cadherin, lectin, laminin, olfactomedin, immunoglobulin, or thrombospondin modules. The extracellular domains are bound to a conserved family of 7 transmembrane (TM) receptors that are distantly related to the class B, or secretin family, G protein–coupled receptors. A characteristic feature of the receptors within this family is their posttranslational proteolysis prior to expression at the cell membrane from a single peptide to 2 noncovalently associated subunits.2-4 The α subunit comprises the majority of the extracellular domain and contains the adhesion motifs, whereas the β subunit comprises the 7 TM receptor with a short extracellular extension. A lack of knowledge concerning high-affinity ligand or counterreceptor interactions has impeded progress in defining signaling pathways and physiologic actions of this family of receptors.
CD97 belongs to the EGF-TM7 subgroup of adhesion G protein–coupled receptors.5 The proteins in this group contain varying numbers of extracellular fibrillin class 1 type EGF-like repeats and include CD97, EMR1, EMR2, EMR3, and EMR4. CD97 is found in myeloid cells, lymphoid cells, and muscle cells, whereas the other members of this family are predominantly in myeloid lineage cells.3,6-8 In addition, CD97 is often expressed in advanced-stage thyroid, colorectal, gastric, esophageal, and pancreatic carcinomas and has been proposed to play a role in the progression of such cancers.8-10
CD97 is produced in alternatively spliced forms that contain between 3 and 5 EGF-like repeats.3 To date, low-affinity interactions specific for various splice variants of the EGF-like repeats have been identified. The variant containing EGF-like repeats 1, 2, and 5 interacts with CD55, a ubiquitously expressed, cell surface complement regulatory protein.11-13 The fourth EGF-like repeat, which is contained in only the largest variant, interacts in multimerized form with chondroitin sulfate (CS).14 CD97 binding to either CD55 or CS is solely dependent on interaction through the EGF-like repeats. No morphologic or physiologic response has been observed in CD97-expressing cells upon binding to either CD55 or CS, and thus, the physiologic role of these interactions is unknown.
CD97 contains an Arg-Gly-Asp (RGD) sequence 110 amino acids COOH-terminal to the end of the last EGF-like repeat.3 An RGD tripeptide can serve as a ligand recognition sequence for one or more integrins.15-17 The context of the RGD sequence, including flanking residues and 3-dimensional presentation, in addition to individual features of the integrin-binding pockets, determines whether productive interactions occur. Integrins are heterodimeric TM receptors that mediate cell adhesion to diverse components of the extracellular matrix (ECM), cell surface proteins, as well as plasma proteins.18,19 Integrin-mediated cellular interactions initiate signaling pathways that regulate a plethora of responses including cell morphology, survival, proliferation, migration, and invasion, which ultimately control processes such as development, tissue remodeling, and immune function.
A subset of integrins contributes to the regulation of angiogenesis.20,21 Angiogenesis is the process whereby new blood vessels develop from pre-existing vessels and it occurs normally during wound healing and the female reproductive cycle, or during pathophysiologic events such as tumorigenesis, diabetic retinopathy, or rheumatoid arthritis. Studies in experimental angiogenesis models and in mutant mice have provided consistent data demonstrating that engagement of α5β1, α1β1, and α2β1 integrins promote angigogenesis.22,23 The roles of αvβ3 and αvβ5 integrins are more open to interpretation. Antibodies and small molecule inhibitors of these integrins inhibit angiogenesis, whereas β3- or β5-deficient mice show enhanced tumor growth and angiogenesis, leading to the suggestion that αvβ3 and αvβ5 integrins act as integrators of positive and negative angiogenic signals by mediating distinct signals dependent on specific ligand interactions.24
We show here that cell adhesion to CD97 appears to be mediated mainly by integrin α5β1, although αvβ3 receptors bind CD97 as well. CD97 binding to endothelial cells stimulates their motility and invasion in an isoform-specific manner. Consistent with this, CD97 acts as a proangiogenic factor in vivo. These data are the first to suggest that CD97, which is highly expressed on various inflammatory cells and some carcinomas, contributes to inflammation-mediated angiogenesis and possibly tumor progression.
Materials and methods
Reagents
Cell culture media and supplements were purchased from Invitrogen (Carlsbad, CA) unless otherwise specified. Growth factors and fibronectin were obtained from Chemicon (Temecula, CA). Function-blocking anti-integrin monoclonal antibodies from Chemicon were anti-α2 clone P1E6, anti-αvβ3 clone LM609, and anti-αvβ5 clone P1F6. Blocking (IIA1) and nonblocking (VC5) antibodies directed to α5 were from BD Biosciences (San Jose, CA). RGD inhibitor peptide was from Bachem (King of Prussia, PA). Antifibronectin rat monoclonal antibodies (mAb 16G3)25 and anti-integrin β1 function-blocking rat monoclonal antibodies (mAb 13)26 were kindly provided by Kenneth Yamada (National Institute of Dental and Craniofacial Research, Bethesda, MD). Monoclonal antibodies were without azide. HT29 cell lines were a gift from Judith Varner (University of California at San Diego, La Jolla, CA), and the MEKDD/LC7ΔSX construct was a gift from Nadeem Mogul (Harvard University, Boston, MA).
Cell-culture conditions
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (Walkersville, MD) and maintained in endothelial cell basal medium (EBM2) with EGM2 additives as specified. NIH3T3 fibroblasts and HT29 integrin α5β1-positive and integrin α5-negative colon carcinoma cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (FCS) and gentamicin.
Expression constructs
Preparation of soluble CD97 protein
Soluble CD97-mFc fusion proteins were generated by transfecting COS-7L with CD97/pSec-mFc-Bio expression constructs using LipoFectamine 2000 (Invitrogen) according to the manufacturer's procedure. At 2 and 3 days after transfection, the serum-free conditioned medium was collected, centrifuged, and passed through a 0.22-μm filter. The mFc fusion proteins were purified by protein A agarose (Invitrogen) column chromatography as directed by the manufacturer and concentrated using Centricon centrifugal filter devices (Millipore, Bedford, MA). Coomassie stain following polyacrylamide gel electrophoresis showed a single protein band, and protein concentration was determined by the Coomassie Plus Protein Assay Reagent (Pierce, Rockford, IL).
HUVEC and HT29 adhesion
A quantity of 8 μL soluble protein (between 1 μg/mL and 40 μg/mL), diluted in phosphate-buffered saline (PBS), was spotted on the surface of a 35-mm Petri plate. Plates were incubated overnight at 4°C. Protein spots were aspirated, 2 mL blocking PBS/1% bovine serum albumin (BSA) was added to each plate, and left at room temperature for 1 hour. Blocking reagent was removed and 1.5 mL EBM2/0.1% BSA was added for HUVECs and DMEM/0.1% BSA for HT29 cells. A 70% confluent culture of cells was trypsinized, quenched with trypsin inhibitor, centrifuged, and resuspended in complete growth medium at 106 cells/mL. Cells were allowed to recover at room temperature with gentle rocking for 2 hours. Following recovery, HUVECs were resuspended in EBM2/0.1% BSA, and HT29 cells were resuspended in DMEM/0.1% BSA to a final concentration of 106 cells/mL. Cells were left untreated or treated as indicated and then 800 μL cell suspension was added to each Petri plate. Plates were incubated at 37°C for 30 minutes. Unbound cells were removed by gentle washing with PBS. Cells adhered to protein were stained with Hema3 stain set (Fischer Scientific, Pittsburgh, PA) and counted on a Nikon TMS microscope using a 20× objective. Photographs of adhered HT29 cells were taken using a Nikon DXM 1200 digital camera on a Zeiss Axiovert 100M microscope equipped with a 32×/0.40 Achrostigmat objective (Zeiss, Thornwood, NY).
HUVEC invasion
Invasion chambers were prepared by coating 8-μM pore, 24-well format membranes (BD Biosciences) with 100 μL (0.5 mg/mL) Matrigel (BD Biosciences) per well and incubating them overnight at room temperature. Cells were prepared as described for adhesion but 250 μL cells was placed into the upper chamber while 500 μL chemoattractant (100 ng/mL) was placed into the bottom. Cells were allowed to migrate for 15 hours at 37°C/5% CO2. Invasion was assayed as previously described.29
HUVEC migration
Chemotaxis was determined using 8-μM pore membranes in a Boyden chamber. Briefly, membrane filters (Osmonics, Livermore, CA) were coated overnight at room temperature with 10 μg/mL gelatin in 0.1% acetic acid. Cells were prepared as outlined for adhesion, and 56 μL cells was placed into the upper chamber and 28 μL chemoattractant (100 ng/mL) was placed into the bottom portion of the Boyden chamber. Cells were allowed to migrate for 15 hours at 37°C in an environment of 5% CO2. Migration was evaluated as described for invasion.
Fluorescence and confocal microscopy
To compare the morphology of HUVECs binding to CD97 protein or fibronectin in the absence and presence of RGD inhibitor peptide, cells were allowed to adhere to protein-coated coverslips (Daigger, Vernon Hills, IL). Bound cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Fixed cells were stained with Texas Red phalloidin (Molecular Probes, Eugene, OR) and antivinculin monoclonal antibody (Sigma, Saint Louis, MO) followed by goat antimouse fluorescein isothiocyanate (FITC)–conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Confocal scans were generated on a Zeiss LSM510 using a 100×/1.3 oil Plan NeoFluar objective (Zeiss).
Directed in vivo angiogenesis assay (DIVAA)
Angiogenic responses were compared and quantitated as described.30
In vivo angiogenesis model
MEK(218D,222D)–expressing NIH3T3 cells were produced by retroviral infection using a previously described protocol.31 Forty clones were selected and screened for surface CD7 by direct immunofluorescence using anti–CD7-FITC monoclonal antibody (BD Biosciences). Clones with moderate to high expression were tested for ability to generate poorly vascularized subcutaneous tumors in athymic mice. A clone possessing these characteristics was subsequently infected with CD97(3EGF)/pLRT or CD97(5EGF)/pLRT and selected with 5 μg/mL blastocidin (Invitrogen) to acquire permanently transfected, polyclonal populations of cells. The selected cells were sorted twice to enrich for CD97 expression. Clone 5-CD97(3EGF)/pLRT and clone 5-CD97(5EGF)/pLRT were chosen because they had the highest levels of CD97 expression. Expression levels of the 2 isoforms were the same as determined by mean fluorescence. Prior to injection, cells were trypsinized, washed with PBS, and resuspended in 0.9% saline to a concentration of 107 cells/mL. Cells (106; 0.1 mL) were injected per site under the skin using a 25-gauge needle. A time course of tumor progression was determined, and 7 days was chosen as optimal to generate tumors between 1 mm and 3 mm. Mice were euthanized and tumors were excised and fixed in IHC zinc fixative (BD Biosciences). CD31 monoclonal antibody from BD Biosciences and hematoxylin and eosin–stained sections were prepared (Histoserv, Germantown, MD). Photographs of tumors were taken using a Nikon DXM 1200 digital camera on a Zeiss Axiovert 100M microscope equipped with a 32×/0.40 Achrostigmat objective.
Results
CD97 elicits an angiogenic response in vivo
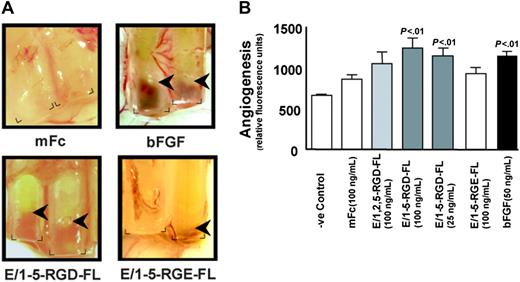
Based on structural considerations and expression patterns, we sought to investigate whether CD97 affects angiogenesis. To obtain purified CD97 protein, recombinant CD97 constructs were engineered to include the entire extracellular domain up to the proteolytic processing site at amino acid 531, coupled to a mouse Fc fragment (Figure 1). Alternatively spliced forms containing EGF-like repeats 1, 2, and 5 (E/1,2,5-RGD-FL) or 1-5 (E/1-5-RGD-FL) were constructed. Recombinant CD97 was expressed and purified from COS cell supernatants. We assayed purified CD97 using the DIVAA, which allows a quantitative assessment of angiogenesis in vivo.30 DIVAA uses an implanted angioreactor, consisting of a silicone tube that is opened at one end and contains an angiogenic factor polymerized in matrigel, to measure vectorial blood vessel development. Shown in Figure 2A are angioreactors containing mFc control chimera protein, basic fibroblast growth factor (bFGF), or E/1-5-RGD-FL, excised 9 days after subcutaneous implantation in mice. The development of blood vessels growing into the open end of the tubes containing bFGF and E/1-5-RGD-FL was apparent. Blood vessel development was quantitated by injecting mice with FITC-dextran, which measures vessel volume, and subsequently excising the angioreactors and determining fluorescence intensity. Statistically significant angiogenic responses to E/1-5-RGD-FL were observed at 25 ng/mL and 100 ng/mL, which was comparable to the response elicited by bFGF, whereas there was a trend toward angiogenic responses to E/1,2,5-RGD-FL. As is typical for FITC-dextran quantifications, there was a background with Matrigel alone or control mFc protein, even though there was no apparent new vessel development.30 Because human CD97 is acting across species to stimulate angiogenesis, this implies that the CD97 interaction with CD55 is not necessary, since this has been shown to be a species-specific interaction.11,13
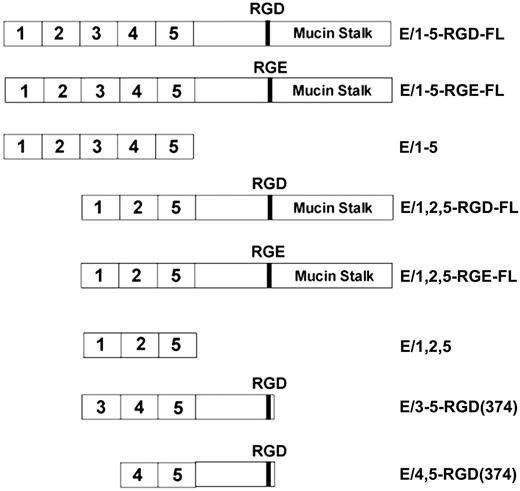
Structure of CD97 soluble proteins. Constructs encompassing the extracellular domain of CD97 up to the natural proteolysis processing site at amino acid 531 are designated full length (FL). Isoforms containing 3 (E/1,2,5) or 5 (E/1-5) EGF-like repeats, an RGD motif, and mucin stalk, as well as RGD to RGE and various deletion mutants, were used in this study.
Structure of CD97 soluble proteins. Constructs encompassing the extracellular domain of CD97 up to the natural proteolysis processing site at amino acid 531 are designated full length (FL). Isoforms containing 3 (E/1,2,5) or 5 (E/1-5) EGF-like repeats, an RGD motif, and mucin stalk, as well as RGD to RGE and various deletion mutants, were used in this study.
DIVAA analysis of purified recombinant CD97 proteins. (A) Angioreactors were prepared as described and recovered after 9 days.30 Shown are angioreactors containing Matrigel and either the mFc backbone protein, bFGF, E/1-5-RGD-FL (100 ng/mL), or E/1-5-RGE-FL (100 ng/mL). The corners at the open end of the angioreactors are marked, and arrows indicate the depth of invasion by new vessel formation. Image acquisition was performed as described elsewhere.30 (B) DIVAA was quantitated by intravenous injection of FITC-dextran, killing of the mice after 20 minutes, and recovery of the angioreactors, dispase digestion, and fluorescence spectroscopy. One representative experiment from a total of 3 that were performed is shown. Results are expressed in fluorescence units as the average plus or minus the standard error of the mean (SEM), n = 4. Student t test comparison of results for bFGF versus Matrigel alone and for E/1-5-RGD-FL at 100 ng/mL and 25 ng/mL versus mFc protein was significant (P < .01). The E/1-5-RGE-FL sample was assayed separately from the others and is the average of 4 angioreactors. Florescence units have been normalized to mFc protein (100 ng/mL) performed in the same experiment.
DIVAA analysis of purified recombinant CD97 proteins. (A) Angioreactors were prepared as described and recovered after 9 days.30 Shown are angioreactors containing Matrigel and either the mFc backbone protein, bFGF, E/1-5-RGD-FL (100 ng/mL), or E/1-5-RGE-FL (100 ng/mL). The corners at the open end of the angioreactors are marked, and arrows indicate the depth of invasion by new vessel formation. Image acquisition was performed as described elsewhere.30 (B) DIVAA was quantitated by intravenous injection of FITC-dextran, killing of the mice after 20 minutes, and recovery of the angioreactors, dispase digestion, and fluorescence spectroscopy. One representative experiment from a total of 3 that were performed is shown. Results are expressed in fluorescence units as the average plus or minus the standard error of the mean (SEM), n = 4. Student t test comparison of results for bFGF versus Matrigel alone and for E/1-5-RGD-FL at 100 ng/mL and 25 ng/mL versus mFc protein was significant (P < .01). The E/1-5-RGE-FL sample was assayed separately from the others and is the average of 4 angioreactors. Florescence units have been normalized to mFc protein (100 ng/mL) performed in the same experiment.
HUVECs adhere to the extracellular domain of CD97, the minimal composition of which is 3 EGF-like repeats and an RGD sequence, via an interaction with integrin α5β1 and αvβ3
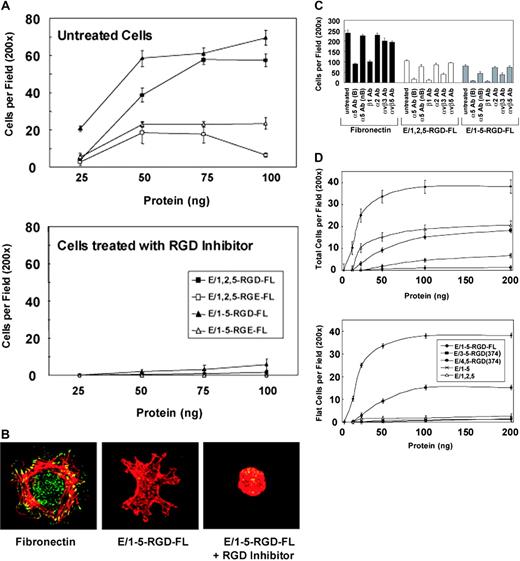
An important question is which counterreceptors are engaged by CD97 on endothelial cells. Based on the presence of EGF-like repeat modules and an RGD sequence, we hypothesized that CD97 may interact with integrin receptors. The interaction of purified CD97 with HUVECs was assayed using a solid support adhesion assay. Adhesion of HUVECs to CD97, spotted on polystyrene at concentrations between 1 μg/mL and 40 μg/mL, was assayed, and cells were found to bind at concentrations as low as 1 μg/mL, with the optimum at 10 μg/mL (Figure 3A). HUVECs bound similarly to the full-length extracellular region of CD97 containing 3 EGF-like repeats (E/1,2,5-RGD-FL) or 5 EGF-like repeats (E/1-5-RGD-FL). The CD97 EGF-like repeats 2 through 5 bind calcium, and as expected, HUVEC binding was calcium dependent (not shown). Cells did not flatten and adhere as firmly to CD97 as to fibronectin, but instead, demonstrated ruffles and filopodia. Representative cells bound to fibronectin or CD97 are shown in Figure 3B.
CD97 binds to HUVECs through α5β1 and αvβ3. (A) HUVEC attachment to increasing amounts of plate-bound purified CD97 are shown. CD97 splice variants, possessing an Asp(D) to Glu(E) mutation in the RGD motif, also were used in the adhesion assay (top panel). In addition, cells were treated with a competitive inhibitor of RGD (300 μm) to determine the necessity of the RGD sequence in HUVEC interaction with CD97 (bottom panel). ▪ indicates E/1, 2, 5-RGD-FL; □ indicates E/1, 2, 5-RGE-FL; ▴ indicates E/1-5-RGD-FL; ▵ indicates E/1-5-RGE-FL. (B) HUVECs adhere to CD97 with a distinct morphology. Representative cells are shown following their attachment and spreading on coverslips coated with fibronectin or 10 μg/mL E/1-5-RGD-FL. Fixed cells were observed by staining cellular actin with Texas Red phalloidin and focal adhesions with antivinculin antibody followed by an FITC-conjugated secondary antibody. Image acquisition was performed as described elsewhere.30 (C) Adhesion of HUVECs to CD97 involves α5β1 and αvβ3 integrins. HUVEC binding to fibronectin or 10 μg/mL soluble 3EGF(E/1,2,5-RGD-FL) or 5EGF(E/1-5-RGD-FL) CD97 splice variants was determined. To assess the role of integrins on adhesion, cells were treated with function-blocking anti-integrin monoclonal antibodies (20 μg/mL) for 30 minutes before being added to the adhesion assay plate. (D) Deletion mutants of E/1-5-RGD-FL were used in the adhesion assay. Total attached cells are shown in the top panel and nonrounded cells are shown in the lower panel. E/3-5-RGD-374 containing EGF repeats 3, 4, and 5, as well as the RGD motif, stimulated HUVEC binding, whereas a similar construct containing EGF repeats 4 and 5 and the RGD motif did not. EGF-like repeats in the absence of the RGD motif resulted in only rounded cells binding.  indicates E/1-5-RGD-FL; ▪ indicates E/3-5-RGD(374); • indicates E/4, 5-RGD(374); × indicates E/1-5; ▵ indicates E/1, 2, 5. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field. Error bars represent ± standard error of the mean; n = 5.
indicates E/1-5-RGD-FL; ▪ indicates E/3-5-RGD(374); • indicates E/4, 5-RGD(374); × indicates E/1-5; ▵ indicates E/1, 2, 5. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field. Error bars represent ± standard error of the mean; n = 5.
CD97 binds to HUVECs through α5β1 and αvβ3. (A) HUVEC attachment to increasing amounts of plate-bound purified CD97 are shown. CD97 splice variants, possessing an Asp(D) to Glu(E) mutation in the RGD motif, also were used in the adhesion assay (top panel). In addition, cells were treated with a competitive inhibitor of RGD (300 μm) to determine the necessity of the RGD sequence in HUVEC interaction with CD97 (bottom panel). ▪ indicates E/1, 2, 5-RGD-FL; □ indicates E/1, 2, 5-RGE-FL; ▴ indicates E/1-5-RGD-FL; ▵ indicates E/1-5-RGE-FL. (B) HUVECs adhere to CD97 with a distinct morphology. Representative cells are shown following their attachment and spreading on coverslips coated with fibronectin or 10 μg/mL E/1-5-RGD-FL. Fixed cells were observed by staining cellular actin with Texas Red phalloidin and focal adhesions with antivinculin antibody followed by an FITC-conjugated secondary antibody. Image acquisition was performed as described elsewhere.30 (C) Adhesion of HUVECs to CD97 involves α5β1 and αvβ3 integrins. HUVEC binding to fibronectin or 10 μg/mL soluble 3EGF(E/1,2,5-RGD-FL) or 5EGF(E/1-5-RGD-FL) CD97 splice variants was determined. To assess the role of integrins on adhesion, cells were treated with function-blocking anti-integrin monoclonal antibodies (20 μg/mL) for 30 minutes before being added to the adhesion assay plate. (D) Deletion mutants of E/1-5-RGD-FL were used in the adhesion assay. Total attached cells are shown in the top panel and nonrounded cells are shown in the lower panel. E/3-5-RGD-374 containing EGF repeats 3, 4, and 5, as well as the RGD motif, stimulated HUVEC binding, whereas a similar construct containing EGF repeats 4 and 5 and the RGD motif did not. EGF-like repeats in the absence of the RGD motif resulted in only rounded cells binding.  indicates E/1-5-RGD-FL; ▪ indicates E/3-5-RGD(374); • indicates E/4, 5-RGD(374); × indicates E/1-5; ▵ indicates E/1, 2, 5. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field. Error bars represent ± standard error of the mean; n = 5.
indicates E/1-5-RGD-FL; ▪ indicates E/3-5-RGD(374); • indicates E/4, 5-RGD(374); × indicates E/1-5; ▵ indicates E/1, 2, 5. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field. Error bars represent ± standard error of the mean; n = 5.
To determine whether HUVEC adhesion to CD97 was dependent on an interaction with integrins, we tested function-blocking antibodies directed toward various integrins in the cell attachment assay. As shown in Figure 3C, HUVEC binding to CD97 was almost completely blocked by monoclonal antibodies IIA1 and 13 directed to the ligand-binding region of α5 and β1, respectively.26 Antibody to a nonligand binding region of α5, VC5, partially blocked binding, whereas α2β1- and αvβ5-specific antibodies had relatively little effect. Antibody directed to αvβ3, the least discriminatory RGD-ligand binding receptor, also significantly blocked binding to CD97. As expected, HUVEC binding to fibronectin appeared to be mediated almost entirely by α5β1.
We produced purified CD97 in which the RGD sequence had been mutated to RGE. Cell attachment to the RGE-containing forms of CD97 was reduced in number by approximately 70% (Figure 3A). Consistent with this, competitive inhibition with excess RGD peptide entirely inhibited binding of HUVECs to E/1-5-RGD-FL, E/1,2,5-RGD-FL (Figure 3A), E/1-5-RGE-FL, and E/1,2,5-RGE-FL (not shown) and converted the normally spread and ruffled morphology of bound cells to a rounded morphology (Figure 3B). Consistent with the decreased binding affinity for HUVECs, recombinant E/1-5-RGE-FL protein stimulated a substantially reduced angiogenic response in the DIVAA assay (Figure 2A-B).
We also determined whether the various combinations of EGF-like repeats alone, in the absence of adjacent sequence and the RGD tripeptide (Figure 1), would bind cells in the attachment assay (Figure 3D). Previous studies have demonstrated low affinity interactions of the CD97 EGF-like repeats with either CD55 or chondroitin sulfate.11,14 Following binding to EGF-like repeats alone, either 1, 2, 5 or 1-5, there was a 50% to 90% reduction in total cell attachment relative to full-length CD97, and importantly, a complete absence of attached cells with a spread, activated cell morphology (Figure 3D, compare top and bottom panels). Those cells that were attached had a rounded morphology. Inclusion of RGD peptide in the adhesion assay had no effect on attachment to the EGF-like repeats alone (not shown). Because antibodies directed against CD55 inhibited HUVEC binding to E/1-5 and E/1,2,5, regardless of the ability of such antibodies to block the interaction of CD55 with CD97, we were unable to assess to what extent CD97 binding to CD55 was responsible for the non–RGD-dependent binding (not shown).
We further analyzed the role of the CD97 EGF-like domains in the context of the RGD sequence. We produced amino terminally truncated forms of the 5 EGF-like repeat isoform of CD97 containing either 3 EGF-like repeats (3, 4, and 5) or 2 repeats (4 and 5) and terminating 5 amino acids carboxy-terminal to the RGD sequence at amino acid 374 (Figure 1). As shown in Figure 3D, E/3-5-RGD-374 was clearly functional in that attached cells had an activated morphology, although the total number of cells was reduced by about 50%. By contrast, CD97 with 2 EGF-like repeats adjacent to the RGD was entirely inactive. As expected, HUVEC binding was inhibited by antibody IIA1 and mAb13 directed to α5 and β1, respectively (not shown). These data show that the CD97 RGD sequence alone is not sufficient for binding and an additional interaction is required. However, EGF-like repeat 5, adjacent to the RGD sequence and shared between the 2 isoforms of CD97, in combination with RGD is not sufficient. One possibility is that 3 EGF-like repeats may be required for proper conformation since EGF repeats are known to interact via interdomain linkages. Alternatively, the 2 isoforms of CD97 may bind an auxiliary site or sites through different primary sequences. CD55 binding by CD97 is not obligatory since EGF-like repeats 1 and 2 have been shown to interact with CD55.
To address the possibility that endogenous fibronectin contamination of CD97 was responsible for HUVEC binding, we included mAb 16G3, which blocks fibronectin attachment to α5β1, in the adhesion assay.25 Although mAb 16G3 completely blocked binding of HUVECs to fibronectin spotted at 2.5 μg/mL, it had no effect on binding to CD97 (not shown).
The requirement for α5β1 receptors in binding CD97 was verified using isogenic HT29 colon carcinoma cell lines that differentially express integrin α5β132 (not shown). The parental HT29-1 cell line, which expresses β1 but not α5, did not adhere to CD97 or to the fibronectin control. Clone HT29-30, transfected with α5, was adherent to both CD97 isoforms and to fibronectin. HT29-30 cells had a less spread morphology as compared with HUVECs upon binding to fibronectin or CD97. Because HT29-30 does not express β3, this shows that adhesion to CD97 does not require binding to αvβ3.
CD97 is a chemoattractant for HUVECs
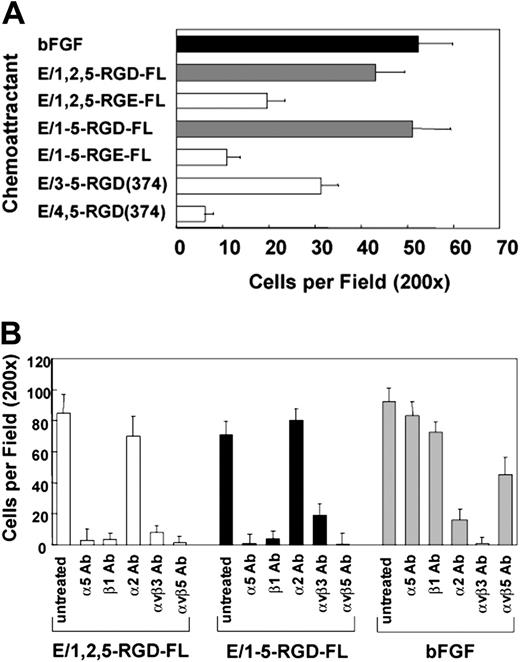
We determined that soluble CD97 is a chemoattractant, with potency comparable to bFGF, when used in migration assays. As shown in Figure 4A, similar to the adhesion data, both isoforms of full-length CD97 were active as chemoattractants, whereas the truncated E/3,4,5-RGD-374 fragment was less active and E/4,5-RGD-374 was inactive. Paralleling the cell attachment data, the RGE mutated versions of the full-length CD97 isoforms had weaker chemoattractant activity, suggesting that the glutamic acid substitution results in a lower binding affinity and/or less functional activation of the binding integrins. The EGF-like repeats truncated prior to the RGD sequence (E/1,2,5 or E/1-5; Figure 1) had no chemoattractant activity (not shown).
CD97 is a chemoattractant for HUVECs. (A) CD97-stimulated HUVEC chemotaxis is RGD-dependent and requires at least 3 EGF repeats. Wild-type, RGE mutants, and EGF-like domain deletion fragments were used as chemoattractants in a HUVEC migration assay. Basic fibroblast growth factor (bFGF) was used as a positive control. (B) HUVEC migration to CD97 involves α5β1, αvβ3, and αvβ5 integrins. HUVECs were treated with 20 μg/mL of various function-blocking anti-integrin monoclonal antibodies before being placed into the migration chamber. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
CD97 is a chemoattractant for HUVECs. (A) CD97-stimulated HUVEC chemotaxis is RGD-dependent and requires at least 3 EGF repeats. Wild-type, RGE mutants, and EGF-like domain deletion fragments were used as chemoattractants in a HUVEC migration assay. Basic fibroblast growth factor (bFGF) was used as a positive control. (B) HUVEC migration to CD97 involves α5β1, αvβ3, and αvβ5 integrins. HUVECs were treated with 20 μg/mL of various function-blocking anti-integrin monoclonal antibodies before being placed into the migration chamber. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
Incubation of HUVECs with blocking antibodies to α5 or β1, αvβ3, or αvβ5 inhibited their migration to CD97, whereas antibodies to α2 did not (Figure 4B). Integrin αvβ3- and α2β1-specific, but not α5β1- or αvβ5-specific, antibodies blocked HUVEC migration to bFGF on gelatin-coated filters. Also, antibodies directed toward αvβ5 did not inhibit binding (Figure 3C) but did inhibit migration, suggesting an indirect involvement of αvβ5 following CD97 binding. Monoclonal antibody 16G3 directed against fibronectin blocked migration stimulated by soluble fibronectin but had no effect on CD97-stimulated chemotaxis (not shown).
The binding to HUVECs of CD97 containing 5 EGF-like repeats stimulates an invasive phenotype, which requires chondroitin sulfate expression
The capacity of soluble CD97 to stimulate invasion through a Matrigel-coated filter was investigated with transwell assays in which CD97 in the bottom chamber served as a chemoattractant for HUVECs in the upper chamber (Figure 5A). Interestingly, E/1-5-RGD-FL induced significant invasion of HUVECs, comparable to bFGF, whereas E/1,2,5-RGD-FL stimulated less than half as much invasion, and fibronectin did not stimulate invasion. The RGE versions of both isoforms and E/3-5-RGD-374 on average were slightly stimulatory for invasion, to about the same extent as E/1,2,5-RGD-FL. These data indicate that the longer isoform differentially activates a response leading to invasion, since both full-length isoforms containing the 1, 2, 5 and 1-5 EGF-like repeats stimulated equivalent migration. As shown in Figure 5B, consistent with the expected specificities for receptor-dependent activation, invasion stimulated by E/1-5-RGD-FL was inhibited by excess RGD peptide and by antibodies directed against α5β1, αvβ5, and αvβ3.
The 5 EGF-like isoform of CD97 stimulates invasion. (A) E/1-5-RGD-FL is a potent chemoattractant for HUVEC invasion through Matrigel. The use of RGD to RGE mutants demonstrates that an intact RGD sequence in CD97 contributes to maximal stimulation of HUVEC migration through Matrigel. All 5 EGF repeats are also necessary. Basic FGF was used as a positive control. (B) α5β1, αvβ3, and αvβ5 integrins play a role in HUVEC invasion to E/1-5-RGD-FL. Cells were treated with integrin function-blocking antibodies for 30 minutes before being added to the invasion chamber. Alternatively, cells were treated with 300 μM RGD peptide inhibitor for 30 minutes before initiation of the invasion assay. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
The 5 EGF-like isoform of CD97 stimulates invasion. (A) E/1-5-RGD-FL is a potent chemoattractant for HUVEC invasion through Matrigel. The use of RGD to RGE mutants demonstrates that an intact RGD sequence in CD97 contributes to maximal stimulation of HUVEC migration through Matrigel. All 5 EGF repeats are also necessary. Basic FGF was used as a positive control. (B) α5β1, αvβ3, and αvβ5 integrins play a role in HUVEC invasion to E/1-5-RGD-FL. Cells were treated with integrin function-blocking antibodies for 30 minutes before being added to the invasion chamber. Alternatively, cells were treated with 300 μM RGD peptide inhibitor for 30 minutes before initiation of the invasion assay. Results shown are representative of at least 10 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
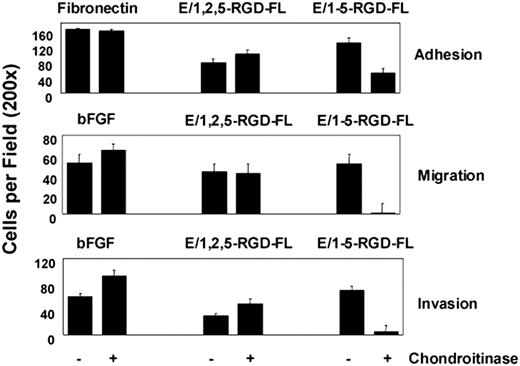
Since EGF-like repeat 4 has been shown to bind CS, we investigated whether the engagement of CS on the surface of HUVECs contributed to the activity of the E/1-5-RGD-FL isoform.14 HUVECs were treated with chondroitinase ABC and then were assayed for adhesion, migration, and invasion in response to E/1,2,5-RGD-FL, E/1-5-RGD-FL, and either fibronectin or bFGF controls (Figure 6). The loss of CS from the HUVEC surface reduced by more than 50% their adhesion to the 5 but not the 3 EGF-like–containing isoform, suggesting that CS-mediated binding contributes to multivalent adhesion mediated by the isoform containing EGF repeat 4. Chondroitinase treatment specifically inhibited migration and invasion to E/1-5-RGD-FL in agreement with the reduced binding affinity. Similar results were obtained whether the HUVECs were washed free of chondroitinase or left in its presence during incubation of the invasion assay (not shown). These and other data suggest that the different isoforms of CD97 have distinct conformations (see “Discussion”). We suggest that coengagement of α5β1 and a CS-containing cell surface protein is synergistic for stimulating invasion following binding of the longest CD97 isoform.
Chondroitinase treatment inhibits adhesion, migration, and invasion of HUVECs to the 5 EGF (E/1-5-RGD-FL) but not the 3 EGF (E/1,2,5-RGD-FL) splice variant of CD97. Assays were designed as outlined in previous figures. Cells were left untreated or exposed to 150 mU/mL chondroitinase ABC (Sigma-Aldrich, Saint Louis, MO) for 30 minutes at 37°C before being loaded into the assay system. Results shown are representative of at least 5 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
Chondroitinase treatment inhibits adhesion, migration, and invasion of HUVECs to the 5 EGF (E/1-5-RGD-FL) but not the 3 EGF (E/1,2,5-RGD-FL) splice variant of CD97. Assays were designed as outlined in previous figures. Cells were left untreated or exposed to 150 mU/mL chondroitinase ABC (Sigma-Aldrich, Saint Louis, MO) for 30 minutes at 37°C before being loaded into the assay system. Results shown are representative of at least 5 replicates. Data show mean number of cells per ×200 microscopic field plus or minus SEM.
CD97 expression on the surface of tumor cells stimulates angiogenesis in developing tumors
CD97 expression has been shown to be elevated in various types of carcinomas as compared with corresponding normal tissue.8-10 To determine whether CD97 expressed on the surface of tumor cells could influence vascularization of a developing tumor, we assayed vessel genesis in isogenic sets of 3T3-derived tumor cells expressing no CD97 or permanently transfected with the 3 or 5 EGF-like repeat isoforms. 3T3 cells transformed with a constitutively active form of MEK (MEKDD) were used because they form relatively poorly vascularized subcutaneous tumors. Similar tumor growth rates were observed for the various cell lines (not shown). At approximately 7 days after inoculation of the tumor cells, the tumors ranged from 1 mm to 3 mm in diameter, with comparable variability in tumor size for the 3 cell lines. The area of greatest vessel growth for all of the tumors was on the surface adjacent to the dermis. Vessels more than 50 μm in size were identified by CD31 staining and enumerated (Figure 7A). There was statistically significant greater vessel density in the tumors derived from CD97-expressing cells, with the 5 EGF-like form being the most robust in stimulating angiogenesis (Figure 7B).
CD97 promotes angiogenesis in vivo. Subcutaneous tumors from MEKDD-parental, MEKDD-CD97/5EGF, and MEKDD-CD97/3EGF cells were generated and stained for endothelial cells as described in “Materials and methods.” (A) Representative fields of blood vessels stained with CD31 were photographed. Arrows indicate CD31-stained endothelial cells. Image acquisition was performed as described elsewhere.30 (B) Five ×32 microscopic fields were photographed and blood vessels more than 50 μm were counted. The results represent the mean of 5 fields for each of 4 tumors plus or minus SEM; n = 20; P values shown were determined by an unpaired t test comparing each CD97-expressing cell line to the parental.
CD97 promotes angiogenesis in vivo. Subcutaneous tumors from MEKDD-parental, MEKDD-CD97/5EGF, and MEKDD-CD97/3EGF cells were generated and stained for endothelial cells as described in “Materials and methods.” (A) Representative fields of blood vessels stained with CD31 were photographed. Arrows indicate CD31-stained endothelial cells. Image acquisition was performed as described elsewhere.30 (B) Five ×32 microscopic fields were photographed and blood vessels more than 50 μm were counted. The results represent the mean of 5 fields for each of 4 tumors plus or minus SEM; n = 20; P values shown were determined by an unpaired t test comparing each CD97-expressing cell line to the parental.
Discussion
We show here for the first time that the extracellular domain of CD97 binds to the angiogenic integrins α5β1 and αvβ3, and acts as a proangiogenic factor by stimulating endothelial cell migration and invasion. CD97 is expressed on the surface of inflammatory myeloid and lymphoid leukocytes, and soluble CD97 has been found in fluids extracted from inflammatory sites, such as synovial fluid from rheumatoid arthritic joints.3,33,34 Thus, CD97 may act as a cell-bound counterreceptor for integrins on local endothelial cells or in a chemotactic gradient as a soluble or matrix-associated form. Inflammation and angiogenesis are closely linked in nonpathogenic situations such as wound healing and in numerous pathogenic conditions including rheumatoid arthritis, diabetic retinopathy, and tumorigenesis, among others.35 In addition, CD97 is expressed on a variety of carcinomas including thyroid, gastric, colorectal, and pancreatic cancers.8 In the case of thyroid cancer, CD97 expression levels are elevated with increased tumor stage.9 It also has been suggested that CD97 expression is correlated with histologically defined invasiveness in colorectal cancers.10
Angiogenesis is a multifactoral process, which is regulated by a balance of proangiogenic and antiangiogenic soluble factors and ECM components.36 Integrins have attracted attention as targets of antiangiogenic therapy for cancer and some chronic inflammatory diseases. To this end, it is important to dissect the variety and functional consequences for ligands, now suggested to include CD97, which act through integrins to control angiogenesis.21,24
Integrin α5β1 clearly plays a role in angiogenesis. α5β1 is up-regulated on endothelium during angiogenesis, and antibody and peptide antagonists directed at this integrin inhibit growth factor as well as tumor-stimulated angiogenesis and metastasis.22,37 Integrin α5β1 was previously thought to have narrow ligand specificity for fibronectin, but additional ligands including fibrinogen and fibrillin now have been described.38,39 Fibronectin, which is a component of provisional ECM found during physiologic remodeling responses, has been shown to enhance angiogenesis.22 The ligation of α5β1 by fibronectin in comparison to CD97 results in distinct morphologic differences as assayed in HUVECs (Figure 3B). Fibronectin and CD97 may contribute complementary functions to angiogenesis that are regulated with respect to, for example, the initiating stimulus or the timing of the response.
It is apparent from their effects on morphology that fibronectin and CD97 binding to endothelial cells initiate distinct intracellular signaling cascades, suggesting that their binding properties are different. CD97 binds αvβ3, and although such binding is not required for cell attachment of HT-29 cells, αVβ3 may play a role in signaling. With regard to α5β1, fibronectin binding is known to require 2 sites within fibronectin, the RGD domain in the fibronectin type III tenth repeat and a synergy site in the ninth repeat.40 The RGD sequence binding at the αβ interface is thought to activate and align the α5β1 fibronectin interaction, while the synergy site provides mechanical strength.41 Similarly, deletional analysis of CD97 binding to endothelial cells, which stimulates an activated morphology and is α5β1 dependent, revealed that the RGD sequence and at least 3 EGF-like repeats, either repeats 1, 2, 5 or 3, 4, 5, are required (Figure 3A,D). The specific auxillary binding site or sites within CD97 are not known, and there may be binding sites within more than one repeat. Alternatively, a common binding site in EGF-like repeat 5 could require interaction of the other EGF-like repeats, which are known to stack relative to one another, for the conformational presentation of the binding site.42 It is perhaps significant that like CD97, fibrillin binding to α5β1 on mesenchymal cells requires an RGD sequence and an adjacent calcium-binding EGF-like repeat, and such binding by human dermal fibroblasts produces a motile morphology.38 In summary, a hypothetical difference between fibronectin and CD97 binding lies in an additional site within α5β1, which ultimately could affect the conformation and outside-in signaling of α5β1 through effects on clustering or other yet-less-well defined mechanisms.
We observed a difference in the functional consequences of binding the 2 isoforms of CD97 to HUVECs. The isoform containing EGF-like repeats 1, 2, 5 stimulated migration but little invasion of HUVECs, whereas the isoform containing repeats 1-5 stimulated migration and invasion (Figure 5A), suggesting that additional signaling pathways coupled to the invasive phenotype were being engaged. Because it has been established previously that EGF-like repeat 4 binds CS, we investigated whether CS binding was involved in the induction of invasiveness. We determined that chondroitinase ABC pretreatment of HUVECs specifically reduced binding and functional responses to the isoform of CD97, which contains EGF-like repeat 4 (Figure 6).14
Why is CS binding necessary for the adhesive and migratory activity of the isoform containing EGF repeat 4, yet the short isoform missing EGF-4 has adhesive and migratory activity? Previous data analyzing CD97 interaction with CD55 have suggested that interdomain linkages affect the conformation of adjacent EGF repeats.42 For example, EGF-1 and -2 appear to have distinct binding properties for CD55 dependent on whether they are adjacent to EGF-3 or -5. Therefore, we suggest that the different context of identical EGF repeats in the 2 isoforms leads to nonidentical affinity interactions. The data imply that one or more identical EGF repeats in the long isoform relative to the short isoform have reduced affinity for its counterreceptor and that CS binding contributes to the total affinity of the long isoform.
We hypothesize that CD97-mediated coengagement of α5β1 and/or αvβ3 and a cell surface proteoglycan containing CS leads to an invasive phenotype. Two major classes of cell surface proteoglycans are the glypican and syndecan families. Syndecan-4, one of the 4 known members of the syndecan family of proteoglycans, has been found in focal adhesions, and coengagement of syndecan-4 with α5β1 by the central cell-binding domain of fibronectin has been shown to be required for focal adhesion formation.43 The binding of CS proteoglycans by multivalent CD97 EGF-like repeats, in the absence of an RGD sequence, has demonstrated a lack of specificity for core proteins.14 However, it is possible that the cooperativity provided by binding to α5β1 could provide specificity for syndecan-4. Our attempts to demonstrate specificity for syndecan-4 as a coreceptor by using blocking antibodies have been frustrated by a general inhibitory effect of such antibodies on migration stimulated by several chemoattractants. Clearly, future experiments using other methods or systems to modulate syndecan-4 expression are of interest.
The work described here has focused on the activity of CD97 as a ligand for angiogenic integrins. However, the structure of CD97 and other members of the adhesion-linked G protein–coupled receptors suggests that binding of the extracellular α subunit to cellular or ECM ligands will induce intracellular signaling cascades dependent on the 7-transmembrane β subunit.3 The lack of high-affinity ligands or counterreceptors known to bind the adhesion G protein receptors has been a limitation to investigating their downstream signaling cascades and regulation. Whether CD97 binding to α5β1 initiates signaling by CD97β is a fascinating question with implications for 2-way communication circuits between inflammatory and endothelial cells.
Prepublished online as Blood First Edition Paper, December 2, 2004; DOI 10.1182/blood-2004-07-2878.
Supported by the Center for Cancer Research, National Cancer Institute, Bethesda, MD.
T.W. and Y.W. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hsi-Hsien Lin and Martin Stacey for providing pSec-mFc-bio vectors and plasmids encoding E/1-5 and E/1,2,5. Dr Kenneth Yamada is thanked for his generous gifts of monoclonal antibodies 13, 16, and 16G3. We thank Judith Varner for sharing HT29 clones. We thank Dave Roberts and Kenneth Yamada for their valuable comments during the preparation of this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal