Abstract
We generated a murine monoclonal antibody (B28p) detecting an antigenic determinant shared by the immunoglobulin superfamily receptor translocation-associated 1 (IRTA1) receptor (the immunogen used to raise B28p) and an unrelated 28-kDa protein that was subsequently subjected to extensive characterization. The expression of the 28-kDa protein in normal lymphohematopoietic tissues was restricted to B cells and plasma cells and clearly differed from that expected for IRTA1 (selectively expressed by mucosa-associated lymphoid tissue [MALT] marginal zone B cells). Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)/mass-spectrometry analysis identified the 28-kDa protein as human tumor protein D52 (TPD52), whose expression had been previously described only in normal and neoplastic epithelia. Specific B28p reactivity with TPD52 was confirmed by immunostaining/immunoblotting of TPD52-transfected cells. TPD52 expression pattern in normal and neoplastic B cells was unique. In fact, unlike other B-cell molecules (paired box 5 [PAX5], CD19, CD79a, CD20, CD22), which are down-regulated during differentiation from B cells to plasma cells, TPD52 expression reached its maximum levels at the plasma cell stage. In the Thiel myeloma cell line, TPD52 bound to annexin VI in a Ca2+-dependent manner, suggesting that these molecules may act in concert to regulate secretory processes in plasma cells, similarly to what was observed in pancreatic acinar cells. Finally, the anti-TPD52 monoclonal antibody served as a valuable tool for the diagnosis of B-cell malignancies.
Introduction
Differentiation of B cells to plasma cells, in which a crucial role is played by the plasma cell transcription factors B-lymphocyte–induced maturation protein 1 (Blimp1), X-box–binding protein 1 (XBP-1), and multiple myeloma oncogene-1 protein/interferon regulatory factor 4 (MUM1/IRF4),1,2 results in down-regulation of B-cell–associated proteins and dramatic but still poorly understood changes in the phenotypic profile.1,2 Better understanding of these changes is relevant to the study of normal B-cell ontogenesis as well as of multiple myeloma and lymphoma subtypes characteristically associated with plasma cell differentiation.3,4 Proteins commonly used as immunohistochemical markers of plasma cell differentiation include, in addition to intracytoplasmic immunoglobulins, CD138/syndecan-1 (a collagen-1–binding proteoglycan possibly involved in plasma cell homing properties),5 the p63 rough endoplasmic reticulum protein (antibody VS38c),6,7 and MUM1/IRF4 (a transcription factor expressed by a late germinal center B-cell subset and plasma cells).8
In the course of immunizations aimed at producing a monoclonal antibody (mAb) against the intracytoplasmic portion of the human immunoglobulin superfamily receptor translocation-associated 1 (IRTA1) protein,9 a cell surface receptor we previously showed to be selectively expressed by mucosa-associated lymphoid tissue (MALT) marginal zone B cells and monocytoid B cells,10 we generated a murine mAb (named B28p) that detects an antigenic determinant shared by IRTA1 and an unrelated 28-kDa protein. In this paper, we describe the characteristics of this 28-kDa molecule that by 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE)/mass spectrometry was identified as the tumor protein D52 (TPD52)11 and by immunohistochemistry showed a previously unrecognized12,13 strong expression in all mature B cells, reaching its maximum level at the plasma cell stage. Moreover, in the Thiel myeloma cell line, TPD52 coimmunoprecipitated with annexin VI in a Ca2+-dependent manner, suggesting that these molecules may act in concert to regulate secretory processes of plasma cells, similarly to what was previously reported in pancreatic acinar cells.14 These findings are of biologic relevance and also indicate that TPD52 can serve as a new tool for the diagnosis of B-cell malignancies.
Materials and methods
Generation of recombinant GST-IRTA1 protein
A cDNA fragment encoding the intracellular portion of the human IRTA1 protein was subcloned into pGEX 3X (Pharmacia Biotech, Piscataway, NJ) bacterial expression vector, in frame to glutathione S-transferase (GST), and confirmed by sequencing. The GST-IRTA1 fusion protein was then expressed in BL21 competent bacteria and purified by affinity chromatography following the manufacturer's instructions.
Production of the B28p monoclonal antibody
BALB/c mice were injected intraperitoneally (4 times, at 10-day intervals) with 150 μg of the GST-IRTA1 fusion protein plus Freund adjuvant, as described previously.8 The hybridoma supernatants were screened by the alkaline phosphatase antialkaline phosphatase (APAAP) technique15 on tonsil paraffin sections. A hybridoma (named B28p), producing an immunoglobulin G1 (IgG1) mAb that reacted strongly with all B cells and plasma cells and weakly with tonsil epithelium, was cloned by a limiting dilution technique and selected for further studies.
Other antibodies
Monoclonal antibodies against the IRTA1 extracellular portion (M-IRTA1)10 and the MUM1/IRF4 protein8 were generated in one author's laboratory (B.F.). The anti-CD79a mAb was kindly provided by Professor D. Y. Mason (Oxford, United Kingdom). Other antibodies were obtained from the following sources: mAb anti-CD138 (DBA Italia, Segrate, Milano, Italy), mAb anti-CD20 and polyclonal anti-CD3 (DakoCytomation, Glostrup, Denmark), mAb anti–paired box 5 (anti-PAX5; Transduction Laboratories, San Diego, CA), polyclonal antiactin (Santa Cruz Biotechnology, Santa Cruz, CA). An affinity-purified rabbit polyclonal antibody recognizing human and murine TPD52 was produced in one author's laboratory (J.A.B.).16 A secondary tetramethylrhodamine-isothiocyanate (TRITC)–conjugated antimouse antibody was purchased from DakoCytomation.
Purified cell populations and cell lines
B cells and T cells were purified from peripheral blood of healthy donors by Ficoll gradient centrifugation followed by MidiMACS (Miltenyi Biotec, Bergish Gladbach, Germany) CD19 or CD3 selection. B-cell and T-cell purity of greater than 95% was confirmed by 2-color (CD20+/CD3- or CD20-/CD3+, respectively) flow cytometry. Bone marrow myeloma cells were isolated by MidiMACS CD138 selection, and plasma cell purity of greater than 95% was confirmed on cytospins by morphologic criteria and MUM1/IRF4 immunolabeling.
The Thiel myeloma cell line (provided by one of the authors; K.P., Oxford, United Kingdom) and the HeLa epithelial and the U937 myeloid cell lines (American Type Culture Collection [ATCC], Manassas, VA) were cultured in RPMI containing 10% fetal bovine serum plus penicillin (100 IU/mL) and streptomycin (100 μg/mL).
Cell transfection
COS cells (simian fibroblasts) were transiently transfected with pMT2–IRTA1-hemagglutin (HA) and pMT2-HA (as negative control) expression vectors using the diethylamino ethyl (DEAE)–dextran method. Phoenix cells (human embryonic kidney cells) were transiently transfected with cDNAs encoding for TPD52, TPD52L1 (previously named hD53), TPD52L2 (previously named hD54), and murine TPD52 (Tpd52) using the pTL1 expression vector and the standard calcium-phosphate method; the empty pTL1 vector served as negative control. Cells were grown in Dulbecco modified essential medium (DMEM) containing 10% bovine calf serum, penicillin (100 IU/mL), and streptomycin (100 μg/mL); lysed; and analyzed by Western blotting. Cells were also cytocentrifuged or grown on chambered slides, air-dried overnight, acetone-fixed for 10 minutes, and immunostained with B28p.
Western blotting
Western blotting with the mAbs B28p and M-IRTA1 was performed on transfected COS or Phoenix cells, purified normal tonsil B and T cells, a case of follicular lymphoma, 2 multiple myeloma cases, and the Thiel and U937 cell lines. The lysis buffer's composition was 1.5 mM MgCl2; 50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 1% Triton X-100; 5 mM EGTA (ethyleneglycotetraacetic acid), pH 7.5; 50 mM NaF, pH 8.0; and 10% glycerol plus a protease inhibitor cocktail (leupeptin, aprotinin, pepstatin A, and phenylmethylsulfonyl fluoride). Following 10% sodium dodecyl sulfate (SDS)–PAGE, Western blotting was performed as previously described10 using the primary mAbs as undiluted culture supernatants in an overnight incubation.
Coimmunoprecipitation studies
The Thiel and U937 cell lines were lysed in ice-cold lysis buffer containing 0.2% Triton X-100 and either 5 mM EGTA or 1 mM CaCl2. For immunoprecipitation, lysates were incubated with protein G–Sepharose beads (Amersham Biosciences, Milan, Italy), followed by the B28p mAb. After washing, the immunoprecipitates were loaded onto 10% SDS-PAGE. Filters were immunostained with mAbs against annexin VI (BD Transduction Laboratories, San Jose, CA; at 1:5000 dilution) and B28p (undiluted culture supernatant) and revealed as described in “Western blotting.”
Tissue and cell samples
The B28p reactivity was assessed in paraffin sections from normal and reactive palatine tonsils (n = 20), lymph nodes (n = 20), spleens (n = 10), and bone marrows (n = 20). We also investigated 180 lymphoid neoplasms representative of most Revised European American Lymphoma/World Health Organization (REAL/WHO) categories3,4 (Table 1), 40 acute myeloid leukemias, and 18 carcinomas (6 breast, 6 colon, and 6 lung).
Expression of TPD52 protein in paraffin sections of lymphomas
Type . | Cases, N = 180 . | TPD52 expression by tumor cells* . |
|---|---|---|
| B-cell derived | ||
| Acute lymphoblastic leukemia | 30 | 6/30 |
| Chronic lymphocytic leukemia | 8 | 7/8 |
| Hairy cell leukemia | 10 | 10/10 |
| Mantle cell lymphoma | 5 | 5/5 |
| Marginal zone lymphoma | 5 | 4/5 |
| FCC lymphoma | 15 | 15/15 |
| DLCL-B | 20 | 15/20 |
| Burkitt lymphoma | 2 | 2/2 |
| Plasma cell tumors | 20 | 20/20† |
| T-cell derived | ||
| Lymphoblastic/T-ALL | 15 | 6/15 |
| Anaplastic large cell lymphoma | 10 | 0/10 |
| Other PTCL | 16 | 0/16 |
| Hodgkin lymphoma | ||
| Nodular sclerosis | 14 | 6/14‡ |
| Mixed cellularity | 9 | 3/9‡ |
| NLPHD | 1 | 1/1 |
Type . | Cases, N = 180 . | TPD52 expression by tumor cells* . |
|---|---|---|
| B-cell derived | ||
| Acute lymphoblastic leukemia | 30 | 6/30 |
| Chronic lymphocytic leukemia | 8 | 7/8 |
| Hairy cell leukemia | 10 | 10/10 |
| Mantle cell lymphoma | 5 | 5/5 |
| Marginal zone lymphoma | 5 | 4/5 |
| FCC lymphoma | 15 | 15/15 |
| DLCL-B | 20 | 15/20 |
| Burkitt lymphoma | 2 | 2/2 |
| Plasma cell tumors | 20 | 20/20† |
| T-cell derived | ||
| Lymphoblastic/T-ALL | 15 | 6/15 |
| Anaplastic large cell lymphoma | 10 | 0/10 |
| Other PTCL | 16 | 0/16 |
| Hodgkin lymphoma | ||
| Nodular sclerosis | 14 | 6/14‡ |
| Mixed cellularity | 9 | 3/9‡ |
| NLPHD | 1 | 1/1 |
The expression of TPD52 protein in paraffin sections of lymphomas was assessed using the B28p mAb. FCC indicates follicular lymphoma; DLCL-B, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; and NLPHD, nodular lymphocyte predominance Hodgkin lymphoma.
In all positive cells reactivity was cytoplasmic
Multiple myeloma (n = 16); solitary plasmocytoma of bone (n = 4)
Twenty percent to 80% positive tumor cells. One mixed-cellularity case was previously misdiagnosed as ALK-negative anaplastic large cell lymphoma (see “Results” and “Discussion”)
Tissue specimens were fixed either in buffered formalin (overnight) or B5 (3-4 hours) and processed routinely. Bone marrow biopsies were fixed in B5 (2 hours) and decalcified for 5 hours in ethylenediaminetetraacetic acid (EDTA) before processing.
B28p reactivity was also investigated on nonfixed and nonpermeabilized cell suspensions from purified B and T cells and the Thiel myeloma cell line by flow cytometry.
Immunostaining procedures
Paraffin sections were subjected to microwaving (750 W × 3 cycles of 5 minutes each) using 1 mM EDTA buffer, pH 8.0, and immunostained by the 3-stage APAAP technique15 (when using mAb) or the 4-stage APAAP technique8 (when using polyclonal antibodies). Following substrate development, slides were counterstained in hematoxylin (5 minutes) and mounted in Kaiser glycerol gelatin.
Double staining for B28p/MUM1-IRF4, B28p/PAX5, B28p/CD68, and B28p/CD3 was performed using a sequential immunoperoxidase/APAAP technique, as previously described.17 Slides were mounted in Kaiser gelatin without counterstain.
Images were captured from stained slides using an Olympus BX61 microscope equipped with 20×/0.7, 40×/0.85, and 100×/1.3 objective lenses and an Olympus Camedia C-3030–zoom camera (Olympus, Tokyo, Japan). Following acquisition with DP-Soft version 3.2 software (Olympus), images were further processed with Adobe Photoshop version 4.01 (Adobe, San Jose, CA).
Flow cytometry analysis on cell suspensions was performed using an indirect immunofluorescence procedure (the mAb B28p followed by a TRITC-conjugated antimouse antibody).
Two-dimensional gel electrophoresis and mass spectrometry
Two-dimensional PAGE. Cells from a B28p-reactive follicular lymphoma patient and from the B28p-unreactive U937 cells (as negative control) were lysed in 8 M urea, 4% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid), 40 mM Tris-hydroxymethyl-aminomethane, and 65 mM dithiothreitol (DTT).
Protein isoelectrofocusing was carried out for 85 000 volts per hour (Vh) on immobilized linear pH gradient strips, pH 4 to 7 (Bio-Rad, Hercules, CA). Strips were incubated for 10 minutes with 6 mL equilibration buffer (0.375 M Tris-HCl, pH 8.8; 6 M urea; 20% glycerol; 2% SDS) containing 130 mM DTT and 10 minutes with 6 mL equilibration buffer containing 135 mM iodoacetamide. The second dimension was run on 12% acrylamide/piperazine-diacrylamide gel, according to Hochstrasser et al.18
Western blotting. Proteins were blotted onto Immobilon-NC membranes (Millipore, Bedford, MA), stained with Bio-Safe Coomassie (Bio-Rad), and scanned using an Agfa Duoscan T 1200 scanner (AGFA, Milan, Italy). Western blotting was then performed as previously described,10 and developed films were scanned as described in “Western blotting.” Immunoreactive spots were identified in Coomassie-stained blots by matching with Western blot images using the Bio-Rad PDQuest software (Bio-Rad Laboratories, Milano, Italy). Two reactive spots (S1 and S2) present in the follicular lymphoma sample but not in the U937 sample were excised from Coomassie blue–stained gels and in-gel trypsin digested according to Moritz et al.19
Protein identification by tandem mass-spectrometry (MS/MS). Peptides were eluted from gel fragments using 20 μL of 0.1% trifluoroacetic acid (TFA), and 5 μL of peptide-containing solution was injected on a home-made C18 reverse-phase column of 75 μm inner diameter (YMS-ODS-AQ200; Michrom Bioresource, Auburn, CA). Peptides were eluted with an acetonitrile (AcN) gradient in the presence of 0.1% formic acid using SunFlow pumps (SunChrom, Friderichsdorf, Germany) and a flow splitter to decrease the flow rate from 200 to 0.4 μL/minute. Peptides were analyzed with a quadrupole time-of-flight (Q-TOF) mass spectrometer (Micromass, Wythenshawe, United Kingdom), applying a 2700 V potential on the nanoelectrospray capillary (New Objective, Woburn, MA) and using argon as the collision gas. MS/MS spectra were acquired by automatic switching between MS and MS/MS mode. Acquired MS/MS data were converted into a compatible format (.DTA files) by ProteinLynx software (Micromass) and analyzed using v1.8 of the Mascot search engine20 against Swiss-Prot,21 TrEMBL,22 NCBInr,23 and EST24 databases.
Protein identification by peptide mass fingerprinting (PMF). MS measurements were conducted with a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometer, Voyager super STR (PerSeptive Biosystems, Framingham, MA) equipped with a 337-nm nitrogen laser. Spectra were obtained by summation of 150 consecutive laser shots. Peak masses were extracted from the spectra and used for protein identification with Mascot20 v1.8 and the SmartIdent peptide mass fingerprint tool25 against Swiss-Prot21 and TrEMBL22 databases.
Results
The protein recognized by B28p is expressed in mature B cells with the highest levels in plasma cells
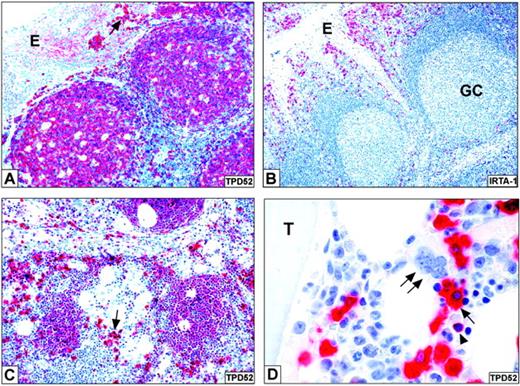
In palatine tonsils, in addition to the epithelium (weakly positive), the antibody labeled the cytoplasm of all peripheral B-cell subsets (mantle, germinal center, intraepithelial/subepithelial, and interfollicular B cells), the most intense positivity being observed in plasma cells (Figure 1A). Cytoplasmic localization of the protein recognized by B28p was also supported by the negative immunostaining in nonfixed/nonpermeabilized purified B cells and the Thiel myeloma cell line at flow cytometry (not shown).
The protein recognized by B28p is expressed in mature B cells and plasma cells. (A) In normal tonsil, B28p reacts with B cells and, more strongly, with plasma cells (arrow); notice the weak positivity of tonsil epithelium (E). (B) The expression pattern of IRTA1 in normal tonsil (as revealed by the M-IRTA1 mAb) clearly differs from that of TPD52, labeling being restricted to a subset of B cells located within and beneath the tonsil epithelium (E). GC indicates germinal center. (C) The expression pattern of TPD52 in a reactive lymph node is similar to that observed in the tonsil; the arrow points to an aggregate of strongly positive plasma cells. (D) In a reactive bone marrow, TPD52 is strongly expressed in plasma cells (arrow) and B cells (arrowhead), while hematopoietic precursors are negative. The double arrow points to a TPD52-negative megakaryocyte. T indicates a bone trabecula. The APAAP technique was used with hematoxylin counterstain. Original magnifications, × 200 (A-C) and × 1000 (D).
The protein recognized by B28p is expressed in mature B cells and plasma cells. (A) In normal tonsil, B28p reacts with B cells and, more strongly, with plasma cells (arrow); notice the weak positivity of tonsil epithelium (E). (B) The expression pattern of IRTA1 in normal tonsil (as revealed by the M-IRTA1 mAb) clearly differs from that of TPD52, labeling being restricted to a subset of B cells located within and beneath the tonsil epithelium (E). GC indicates germinal center. (C) The expression pattern of TPD52 in a reactive lymph node is similar to that observed in the tonsil; the arrow points to an aggregate of strongly positive plasma cells. (D) In a reactive bone marrow, TPD52 is strongly expressed in plasma cells (arrow) and B cells (arrowhead), while hematopoietic precursors are negative. The double arrow points to a TPD52-negative megakaryocyte. T indicates a bone trabecula. The APAAP technique was used with hematoxylin counterstain. Original magnifications, × 200 (A-C) and × 1000 (D).
The B-cell nature of the B28p+ cells was confirmed by the finding that all of them coexpressed the B-cell–specific nuclear transcription factor PAX5 (with the exception of those clearly morphologically recognizable as plasma cells and therefore expected to be PAX5 negative). Conversely, B28p+ cells were negative for CD3 and the macrophage marker CD68 (not shown). Notably, the staining pattern of B28p clearly differed from that expected for the IRTA1 protein, whose expression in tonsil is restricted to a B-cell subset located within and immediately beneath the tonsil epithelium10 (Figure 1B).
That plasma cells were, among B cells, those containing the highest amount of the B28p-reactive protein was particularly evident at increasingly higher dilution of the B28p mAb, when its reactivity with B cells markedly decreased or disappeared while that with plasma cells remained strong (not shown). In serial tonsil sections, the number of B28p+ and CD138+ plasma cells was comparable (not shown), although the subcellular labeling pattern of B28p (cytoplasmic) differed from that of the CD138 protein (restricted to the cell surface). As expected, plasma cells coexpressed B28p and MUM1/IRF4 (not shown). Reactivity with mature B cells and, most abundantly, plasma cells was also observed in reactive lymph nodes (Figure 1C), spleens (not shown), and bone marrows (Figure 1D), where erythroid and myeloid cells as well as megakaryocytes were B28p negative.
Outside the lymphohematopoietic system, B28p reacted with glandular epithelia from different organs, including stomach, pancreas, and colon (not shown), that were in turn negative for IRTA1. The staining was usually weaker than that observed in B cells and plasma cells.
These findings provide evidence that B28p reacts with a protein expressed in mature B cells (with the highest level in plasma cells), an expression pattern that clearly differs from that of IRTA1.
The B28p mAb recognizes an epitope shared by IRTA1 and an unrelated 28-kDa protein
The B28p mAb identified a strong band of 70 to 75 kDa in Western blots of COS cells transfected with a vector encoding for the full-length IRTA1 protein (not shown).
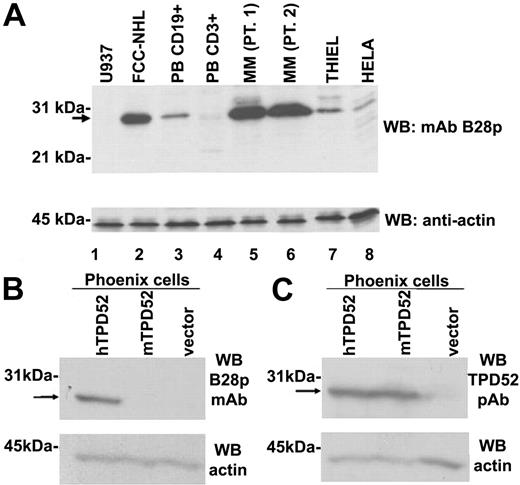
Apart from this cross-reactivity with IRTA1, B28p mAb detected a band of about 28 kDa in lysates from a follicular lymphoma (Figure 2A, lane 2) lacking IRTA1 protein both at Western blotting and immunohistochemistry (not shown). The B28p mAb also identified a band of the same molecular weight (about 28 kDa) in lysates from normal B cells purified from the peripheral blood (Figure 2A, lane 3), from 2 multiple myeloma cases (Figure 2A, lanes 5-6), from the Thiel myeloma cell line (Figure 2A, lane 7), and from the epithelial HeLa cell line (Figure 2A, lane 8). In contrast, the 28-kDa band was undetectable in the U937 myeloid cell line and barely detectable in normal T cells purified from the peripheral blood (Figure 2A, lanes 1 and 4, respectively), the latter finding being likely accounted for by the residual B cells contaminating the greater than 95% pure T-cell sample.
Western blotting of various primary samples and cell lines. (A) The B28p mAb recognizes a 28-kDa protein expressed in B cells and plasma cells. B28p identifies a band of approximately 28 kDa (arrow) in a Western blot of lysates from the NHL-FCC patient sample (lane 2), peripheral blood (PB) purified B cells (lane 3), 2 multiple myeloma (MM) patients (lanes 5 and 6), the Thiel myeloma cell line (lane 7), and the HeLa cell line (lane 8). The 28-kDa band was undetectable in the U937 cell line (lane 1) and barely detectable in normal T cells purified from peripheral blood (lane 4). Similar amounts of total protein lysates were loaded in each lane (see antiactin Western blot, bottom). None of the samples showed IRTA1 expression at immunohistochemical and Western blot analyses using the M-IRTA1 mAb (not shown). (B-C) B28p mAb and an anti-TPD52 polyclonal antibody recognize the same band of about 28 kDa in TPD52-transfected Phoenix cells. A band (arrow) of the expected molecular weight of TPD52 (about 28 kDa) is selectively detected by the B28 mAb (A) in cells transfected with human TPD52 (hTPD52) but not in cells transfected with murine TPD52 (mTPD52) or with the empty vector. A rabbit polyclonal antibody (here abbreviated as TPD52 pAb), known to recognize both hTPD52 and mTPD52, detects (C) the same band of about 28 kDa (arrow) in cells transfected with hTPD52 or with mTPD52 but not in cells transfected with the empty vector. An antiactin Western blot is shown as loading control in the bottom part of the 2 panels.
Western blotting of various primary samples and cell lines. (A) The B28p mAb recognizes a 28-kDa protein expressed in B cells and plasma cells. B28p identifies a band of approximately 28 kDa (arrow) in a Western blot of lysates from the NHL-FCC patient sample (lane 2), peripheral blood (PB) purified B cells (lane 3), 2 multiple myeloma (MM) patients (lanes 5 and 6), the Thiel myeloma cell line (lane 7), and the HeLa cell line (lane 8). The 28-kDa band was undetectable in the U937 cell line (lane 1) and barely detectable in normal T cells purified from peripheral blood (lane 4). Similar amounts of total protein lysates were loaded in each lane (see antiactin Western blot, bottom). None of the samples showed IRTA1 expression at immunohistochemical and Western blot analyses using the M-IRTA1 mAb (not shown). (B-C) B28p mAb and an anti-TPD52 polyclonal antibody recognize the same band of about 28 kDa in TPD52-transfected Phoenix cells. A band (arrow) of the expected molecular weight of TPD52 (about 28 kDa) is selectively detected by the B28 mAb (A) in cells transfected with human TPD52 (hTPD52) but not in cells transfected with murine TPD52 (mTPD52) or with the empty vector. A rabbit polyclonal antibody (here abbreviated as TPD52 pAb), known to recognize both hTPD52 and mTPD52, detects (C) the same band of about 28 kDa (arrow) in cells transfected with hTPD52 or with mTPD52 but not in cells transfected with the empty vector. An antiactin Western blot is shown as loading control in the bottom part of the 2 panels.
These findings demonstrate that the B28p mAb reacts with an epitope shared by IRTA1 (whose cytoplasmic portion represented the immunogen used to generate B28p) and an unrelated protein of about 28 kDa associated to B cells and plasma cells, thus confirming the immunohistochemical results.
Two-dimensional PAGE and mass spectrometry identifies the 28-kDa protein as tumor protein D52 (TPD52)
To further characterize the nature of the 28-kDa protein, we performed mass spectrometry analysis on 2 closely located protein spots (S1 and S2) that were detected by B28p in 2D gels/Western blots from the B28p-reactive follicular lymphoma (Figure 3A-B) but not from the U937 myeloid cell line (not shown). The S1 and S2 spots had an isoelectric point of pH 5.2 and pH 5.1, respectively, and a molecular weight of 24 kDa and 25 kDa, respectively (Figure 3A-B). The minor differences in estimation of the molecular weight of the protein in 1D-Western blot (1D-WB; 28 kDa) compared with the 2D-WB (24-25 kDa) are likely due to the different molecular weight standards and different electrophoresis conditions used in these experiments.
2D-PAGE and mass spectrometry identify the 28-kDa protein as TPD52. (A-B) Spots S1 and S2 (corresponding to the 28-kDa band in 1D-Western blots in Figure 1) are shown in a silver-stained 2D gel of B28p-positive lymphoma cells (A) and in the corresponding 2D-Western blot probed with the B28 mAb (B). Spot S3, which represented an unspecific reaction also detected in the negative control (U937 cell line; not shown), was not subjected to mass spectrometry analysis. (C) Peptide spectra. Protein S2 was isolated from a Coomassie-stained 2D gel and digested with trypsin. The mass of the resulting fragments was assessed by MALDI-TOF spectrometry. Peptide masses corresponding to TPD52 in S2 are denoted with asterisks: 1222.644 Da, 1454 Da, 1657 Da, and 2099 Da. (D-E) B28p strongly stains Phoenix cells transfected with a construct encoding human TPD52 (D). In contrast, no reactivity is seen toward cells transfected with any of the constructs encoding TPD52L2 (E) or TPD52L1, murine Tpd52, and the empty vector (not shown). The APAAP technique was used with hematoxylin counterstain. Original magnification, × 1000.
2D-PAGE and mass spectrometry identify the 28-kDa protein as TPD52. (A-B) Spots S1 and S2 (corresponding to the 28-kDa band in 1D-Western blots in Figure 1) are shown in a silver-stained 2D gel of B28p-positive lymphoma cells (A) and in the corresponding 2D-Western blot probed with the B28 mAb (B). Spot S3, which represented an unspecific reaction also detected in the negative control (U937 cell line; not shown), was not subjected to mass spectrometry analysis. (C) Peptide spectra. Protein S2 was isolated from a Coomassie-stained 2D gel and digested with trypsin. The mass of the resulting fragments was assessed by MALDI-TOF spectrometry. Peptide masses corresponding to TPD52 in S2 are denoted with asterisks: 1222.644 Da, 1454 Da, 1657 Da, and 2099 Da. (D-E) B28p strongly stains Phoenix cells transfected with a construct encoding human TPD52 (D). In contrast, no reactivity is seen toward cells transfected with any of the constructs encoding TPD52L2 (E) or TPD52L1, murine Tpd52, and the empty vector (not shown). The APAAP technique was used with hematoxylin counterstain. Original magnification, × 1000.
Both tandem mass spectrometry analysis on protein spot S1 (not shown) and peptide mass fingerprinting on protein spot S2 (Figure 3C) identified the respective spot as tumor protein D52 (Swiss-Prot accession no. P55327).
Independent evidence that the B28p mAb recognizes TPD52 was provided by its selective reactivity with TPD52-transfected (Figure 3D) but not TPD52L2-transfected (Figure 3E), TPD52L1-transfected, or empty vector–transfected Phoenix cells (not shown). The reactivity was specific for the human TPD52, since Phoenix cells transfected with murine Tpd52 were unreactive (not shown). The specificity of B28p for human TPD52 was also confirmed by Western blotting that showed the expected 28-kDa band only in lysates from TPD52-transfected but not from TPD52L1-, TPD52L2-, or murine Tpd52–transfected Phoenix cells (Figure 2B). The same band of 28 kDa was selectively detected in TPD52-transfected cells by a specific anti-TPD52 rabbit polyclonal antibody16 (Figure 2C) serving as positive control.
To further confirm that the immunoreactivity of B28p with B cells/plasma cells in tissues was due to the expression of TPD52 protein, we also stained tonsil and marrow paraffin sections with the anti-TPD52 rabbit polyclonal antibody.16 The staining pattern was identical to that observed with the B28p mAb, although the background labeling was higher (not shown). This confirms also in lymphoid tissues the overlapping reactivities toward TPD52 of the B28p mAb and the anti-TPD52 polyclonal antibody.
These findings demonstrate that (i) the B-cell–associated 28-kDa protein recognized by the B28p mAb is human TPD52; and (ii) B28p recognizes TPD52 both at Western blotting and immunocytochemistry.
Ca2+-dependent binding of TPD52 to annexin VI in the Thiel myeloma cell line
Previous findings, showing that in rat pancreatic acinar cells CRHSP28 (the rat orthologue of human TPD52) binds annexin VI in a Ca2+-dependent manner and that this molecular complex plays a role in digestive enzyme secretion,14 prompted us to expand these studies to plasma cells. A rationale for proceeding in this direction also emerged from our recent finding that normal and neoplastic plasma cells express annexin VI protein both at Western blotting and immunohistochemistry (E.T. and B.F., unpublished results, August 2003).
To determine whether TPD52 associates to annexin VI in plasma cells, lysates of the Thiel human myeloma cell line (treated either with 5 mM EGTA or 1 mM CaCl2) were immunoprecipitated with B28p (anti-TPD52) and Western blotted with an anti–annexin VI mAb (Figure 4). Interestingly, a TPD52–annexin VI complex was barely detectable when lysates were prepared in the absence of Ca2+ (Figure 4 lane 5), whereas inclusion of Ca2+ in the lysis buffer caused the 2 proteins to strongly coimmunoprecipitate (Figure 4 lane 6). This Ca2+-dependent association of annexin VI with TPD52 was not due to unspecific binding of annexin VI to protein G–Sepharose beads (Figure 4 lanes 7-8) or to the B28p mAb used in the immunoprecipitation, because, either with or without Ca2+, no annexin VI was immunoprecipitated by B28p from lysates of the annexin VI–positive/TPD52-negative U937 myeloid cell line (not shown).
Ca2+-dependent coimmunoprecipitation of annexin VI with TPD52 in the Thiel myeloma cell line. Following immunoprecipitation (IP) with mAb B28p and protein G–Sepharose beads (Prt-G), proteins were analyzed by immunoblotting with murine mAbs against TPD52 (B28p, bottom panel) and annexin VI (top panel; bands in lanes 3-6 indicated by the arrowhead correspond to the heavy chains of the murine mAb B28p, detected by the secondary antimouse antibody). Coimmunoprecipitation of annexin VI (arrow in top panel) with TPD52 (arrow in bottom panel) was dependent on the presence of Ca2+ in the lysate (compare lanes 5 and 6).
Ca2+-dependent coimmunoprecipitation of annexin VI with TPD52 in the Thiel myeloma cell line. Following immunoprecipitation (IP) with mAb B28p and protein G–Sepharose beads (Prt-G), proteins were analyzed by immunoblotting with murine mAbs against TPD52 (B28p, bottom panel) and annexin VI (top panel; bands in lanes 3-6 indicated by the arrowhead correspond to the heavy chains of the murine mAb B28p, detected by the secondary antimouse antibody). Coimmunoprecipitation of annexin VI (arrow in top panel) with TPD52 (arrow in bottom panel) was dependent on the presence of Ca2+ in the lysate (compare lanes 5 and 6).
TPD52 expression in hematologic malignancies
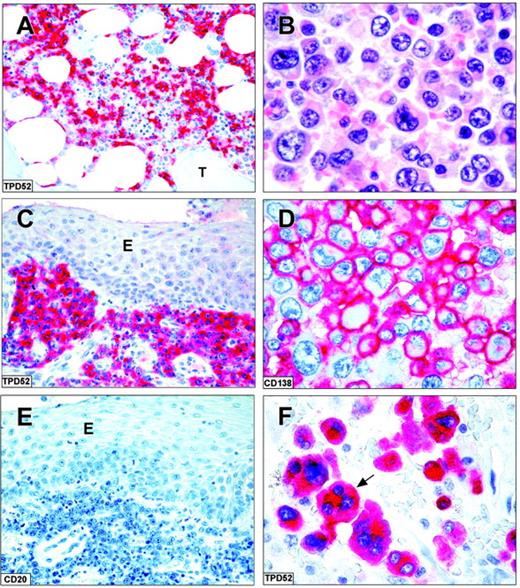
The immunostaining results in 180 lymphoid neoplasms are summarized in Table 1. TPD52 was mostly absent in tumors derived from early precursor B cells with weak positivity being observed in only 20% of acute lymphoblastic leukemias. In contrast, lymphomas derived from peripheral B cells showed consistent TPD52 expression (Figure 5A,C,E). About 25% of diffuse large B-cell lymphomas were TPD52 negative. Some diffuse large B-cell lymphomas with plasma cell differentiation showed TPD52 expression in the absence of CD20 (Figure 5C,E). The strongest TPD52 expression was seen in plasma cell tumors (Figure 5B,D,F).
TPD52 expression in lymphomas and myeloma. Strong cytoplasmic expression of TPD52 in a case of hairy cell leukemia (A; paraffin section from bone marrow trephine; original magnification, × 200). T indicates a bone trabecula. Plasmoblastic lymphoma in an HIV-infected patient (paraffin sections): tumor cells express TPD52 (C; original magnification, × 400) but are CD20- (E; original magnification, × 400); E indicates oral epithelium. The APAAP technique was used with hematoxylin counterstain. (B,D,F) Tumor cells in a case of undifferentiated myeloma (B; hematoxylin-eosin; original magnification, × 1000). The myeloma cells strongly express CD138 on the cell surface (D; APAAP technique; hematoxylin counterstain; original magnification, × 1000) and TPD52 in the cytoplasm (F; APAAP technique; hematoxylin counterstain; original magnification, × 1000); the arrow points to a TPD52-positive multinucleated myeloma cell. All panels represent paraffin sections from B5-fixed/EDTA decalcified bone marrow trephines.
TPD52 expression in lymphomas and myeloma. Strong cytoplasmic expression of TPD52 in a case of hairy cell leukemia (A; paraffin section from bone marrow trephine; original magnification, × 200). T indicates a bone trabecula. Plasmoblastic lymphoma in an HIV-infected patient (paraffin sections): tumor cells express TPD52 (C; original magnification, × 400) but are CD20- (E; original magnification, × 400); E indicates oral epithelium. The APAAP technique was used with hematoxylin counterstain. (B,D,F) Tumor cells in a case of undifferentiated myeloma (B; hematoxylin-eosin; original magnification, × 1000). The myeloma cells strongly express CD138 on the cell surface (D; APAAP technique; hematoxylin counterstain; original magnification, × 1000) and TPD52 in the cytoplasm (F; APAAP technique; hematoxylin counterstain; original magnification, × 1000); the arrow points to a TPD52-positive multinucleated myeloma cell. All panels represent paraffin sections from B5-fixed/EDTA decalcified bone marrow trephines.
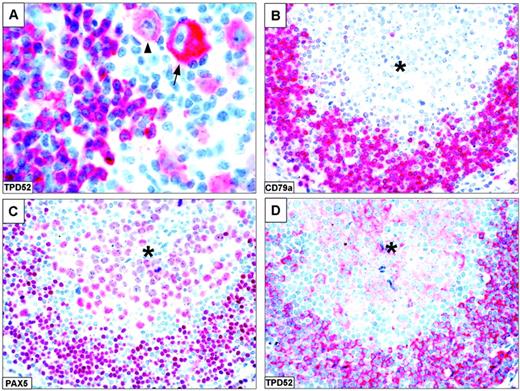
In about 40% of classical Hodgkin lymphoma samples (especially those fixed in B5), a variable percentage of tumor cells (ranging from 20% to 80%) showed weak to moderate cytoplasmic expression of TPD52 (Figure 6A) in the absence of the B-cell molecules CD20 and CD79a. Notably, in one case received in consultation as anaplastic lymphoma kinase (ALK)–negative anaplastic large-cell lymphoma with null phenotype, the diagnosis was changed into that of tumor cell–rich Hodgkin lymphoma, according to the expression of PAX5 and TPD52 (Figure 6B-D).
TPD52 expression in Hodgkin lymphoma. (A) Mixed cellularity Hodgkin lymphoma. Mononuclear variants show strong (arrow) and moderate (arrowhead) TPD52 positivity (original magnification × 800). (B-D) Tumor cell–rich Hodgkin lymphoma (original magnification × 400). A nodule of tumor cells (*) that are CD30+ (not shown) and CD79a- (B) displays nuclear staining for PAX5 (C) and cytoplasmic labeling for TPD52 (D). The rim of residual normal B cells at the periphery of the nodule served as positive control. Lymph node paraffin sections. The APAAP technique was used with hematoxylin counterstain.
TPD52 expression in Hodgkin lymphoma. (A) Mixed cellularity Hodgkin lymphoma. Mononuclear variants show strong (arrow) and moderate (arrowhead) TPD52 positivity (original magnification × 800). (B-D) Tumor cell–rich Hodgkin lymphoma (original magnification × 400). A nodule of tumor cells (*) that are CD30+ (not shown) and CD79a- (B) displays nuclear staining for PAX5 (C) and cytoplasmic labeling for TPD52 (D). The rim of residual normal B cells at the periphery of the nodule served as positive control. Lymph node paraffin sections. The APAAP technique was used with hematoxylin counterstain.
No TPD52 expression was observed in peripheral T-cell lymphomas (Table 1). In contrast, weak TPD52 expression was detected in about 40% of T-lymphoblastic lymphomas/leukemias (Table 1), the percentage of positive neoplastic cells ranging from 20% to 100%. Since a similar fraction of precursor T-cell malignancies is known to aberrantly express CD79a,26,27 we investigated the 6 T-lymphoblastic lymphomas/T-acute lymphoblastic leukemias (T-ALLs) that were TPD52 and/or CD79a positive and found the following expression patterns: TPD52+/CD79a+ (2 cases), TPD52+/CD79a- (2 cases), TPD52-/CD79a+ (2 cases). This finding may reflect some yet unknown function of these B-cell proteins in early neoplastic T cells.
All 40 acute myeloid leukemias were TPD52 negative (not shown). As expected,11 about 50% of carcinomas of breast, colon, and lung expressed TPD52 (not shown).
The expression pattern of TPD52 differs from that of other B-cell–associated molecules
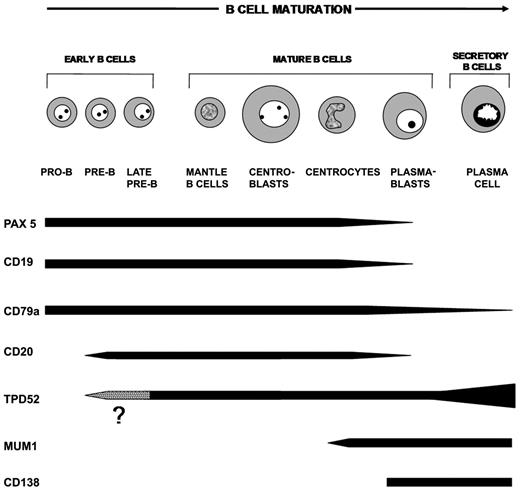
Based on immunostaining results on normal and neoplastic lymphohematopoietic tissues, it is clear that TPD52 up-regulation in plasma cells makes the expression pattern of this protein markedly different from that of other known B-cell–associated proteins (eg, PAX5, CD79a, CD19, CD22, CD20; Figure 7 and “Discussion”), which are all down-regulated at variable extents upon plasma cell differentiation, or from that of other plasma cell–associated proteins (eg, CD138, MUM1/IRF4), which are expressed almost exclusively in plasma cells (Figure 7 and “Discussion”).
Expression of TPD52 during normal B-cell ontogenesis. Differences in TPD52 expression compared with other B-cell/plasma cell–associated proteins are highlighted by the corresponding bars. It is not yet determined at which stage of normal early B-cell differentiation expression of the TPD52 first occurs (dotted area).
Expression of TPD52 during normal B-cell ontogenesis. Differences in TPD52 expression compared with other B-cell/plasma cell–associated proteins are highlighted by the corresponding bars. It is not yet determined at which stage of normal early B-cell differentiation expression of the TPD52 first occurs (dotted area).
Discussion
The TPD52 gene was initially identified as being overexpressed in human breast, lung, and prostate carcinomas16,28,29 and it is currently recognized as a candidate target gene at chromosome 8q21,16,28,29 a region gained in many types of cancer. The encoded TPD52 protein is a member of a highly conserved family30 (comprising TPD52, TPD52L1,30 and TPD52L231,32 ) of small hydrophilic polypeptides of approximately 200 residues that share about 50% sequence identity but bear little similarity to proteins of known function in any species.32 These proteins share in common a conserved coiled-coil motif that supports homomeric and heteromeric interactions among family members.33
The finding that all members of the TPD52 family may interact in a heteromeric fashion with MAL2,13 a new member of the MAL proteolipid family that is required for transcytosis in HepG2 cells,34 implies a potential involvement of TPD52-like proteins in vesicle transport. Indeed, TPD52L1 has been shown to localize in an endosomal compartment in PC12 cells and to stabilize the association of synaptobrevin 2 and syntaxin 1 complexes in vitro, implicating TPD52L1 in the promotion of soluble N-ethylmaleimide–sensitive fusion protein (NSF) attachment protein receptor (SNARE) complex formation and, thus, in the facilitation of membrane fusion events.35 Moreover, an alternatively spliced isoform of TPD52L1 has been described to specifically interact with 14-3-3 proteins,36 which have been implicated in membrane trafficking–related processes, including exocytosis and cytoskeleton dynamics.37,38
In this paper, we provide novel data about TPD52 protein expression in normal and neoplastic human tissues as revealed by a newly raised mAb (B28p), which 2D-PAGE/mass spectrometry analysis found to recognize TPD52 (in addition to IRTA1, the immunogen used in the generation of this antibody). In fact, using Northern blotting and in situ hybridization, TPD52 transcripts were originally found to be expressed at high levels in tumor cell lines and in primary biopsies from breast, prostate, pancreas, kidney, colon, and cervical carcinomas,11,12 as well as in many normal adult and fetal tissues such as kidney, prostate, small intestine, and pancreas.12 In contrast, only low levels of transcripts were detected in normal lymphohematopoietic tissues (spleen, thymus, peripheral mononuclear cells) and germ cell tissues (testis and ovary).12 The present study demonstrates for the first time that normal and neoplastic B cells and particularly plasma cells represent the human cell populations that express the TPD52 protein most consistently and at high levels. This unrecognized finding in previous studies is likely due to the lower sensitivity of the methods employed (Northern blotting) and/or to the possible lack of strict correspondence between TPD52 mRNA and protein levels and/or to the fact that B-cell/plasma cell–rich tissues (lymph nodes and tonsil) were not analyzed.11,12 Whether expression in normal lymphohematopoietic tissues also applies to other members of the TPD52 family is currently unknown. Interestingly, TPD52L2 mRNA emerged as being associated to the mixed lineage leukemia (MLL) subtype of B-cell acute lymphoblastic leukemias from a gene expression profiling study.39 Moreover, from a gene expression analysis of normal B-cell populations and B-cell lymphomas40 it clearly emerged that TPD52 and TPD52L2 are expressed in all stages of mature B cells (naive, germinal center, and memory) and in all categories of B-cell lymphomas with the exception of some diffuse large B-cell lymphomas (Riccardo Dalla Favera, Institute for Cancer Genetics, New York, NY; personal written communication, February 2003).
In spite of its cross-reactivity with the cytoplasmic portion of IRTA1, B28p can be regarded in practical terms as a specific anti-TPD52 mAb suitable for immunohistochemical work, since IRTA1+ normal and neoplastic B cells are part of the TPD52-positive B-cell populations. This is also supported by the finding that in normal lymphoid tissues the polyclonal antibody against TPD52 (not cross-reacting with IRTA1) produces the same immunohistologic pattern as B28p. However, it may be advisable to compare IRTA1 and TPD52 stainings in order to ascertain whether any given B-cell population expresses one or both proteins.
The B28p mAb identifies TPD52 as a new B-cell–associated protein whose subcellular location and expression pattern during B-cell development clearly differs from that of previously characterized B-cell antigens (eg, PAX5, CD79a, CD19, CD20, and CD22) or plasma cell antigens (eg, MUM1/IRF4, CD138; Figure 7).
Selective expression of TPD52 in mature B cells and plasma cells suggests that this protein may play an important role during B-cell maturation. Because of the unrecognized expression of TPD52 in lymphoid B cells, previous studies on TPD52 function have been confined to epithelial cells. Rabbit (CSPP28) and rat (CRHSP28) TPD52 orthologues were shown to be phosphorylated in response to cholinergic stimulation of gastric parietal cells41 and to cholecystekinin stimulation of pancreatic acinar cells,42-44 respectively. These studies concluded that CSPP28/CRHSP28 may represent an intermediate within calcium-sensitive signaling pathways in a variety of tissues. Indeed, CRHSP28 has been recently shown to be serine phosphorylated upon increasing Ca2+ levels in epithelial cells.45 Finally, it has been reported that in rat pancreatic acinar cells, CRHSP28 can bind annexin VI in a calcium-dependent way and that this molecular complex plays an important role in acinar cell membrane trafficking events that are essential for digestive enzyme secretion.14 Our finding—that in the Thiel human myeloma cell line, TPD52 associates to annexin VI in a Ca2+-dependent manner—raises the intriguing possibility that these molecules may act in concert to regulate secretory processes also in plasma cells. When considering that TPD52 reaches the maximum expression level in plasma cells, its involvement in processes related to immunoglobulin secretion would be an appealing hypothesis. Further studies are warranted to clarify this issue.
Because of its reactivity pattern that is different from that of other B-cell molecules and the preservation of its target epitope in routine paraffin sections, TPD52 represents a new tool for the diagnosis of lymphomas associated with plasma cell differentiation, including transplantation-associated lymphomas, plasmablastic lymphomas of the oral cavity,46 or pleural-effusion lymphomas (PELs).3,4 Such tumors are in fact characterized by minimal or absent expression of B-cell lymphoma 6 (BCL6) and CD2046 and weak to moderate positivity for CD79a.
Another interesting area of application is Hodgkin lymphoma. The finding of weak to moderate TPD52 expression in a variable proportion of Hodgkin and Reed-Sternberg cells in about 40% classical Hodgkin lymphoma cases (negative for CD20 and CD79a) is in keeping with gene expression profile analysis of Hodgkin lymphoma cell lines.47,48 In fact, the latter shows that, unlike other B-cell–associated transcripts such as CD19, CD20, CD22, CD79a, and IgM,47,48 TPD52 appears to be expressed in tumor cells of Hodgkin lymphoma (Riccardo Dalla Favera, personal written communication, February 2003). These data have diagnostic implications. Since TPD52 is not expressed in normal and neoplastic peripheral T cells, it could be useful, in combination with PAX549 for the differential diagnosis between tumor cell–rich Hodgkin lymphoma and ALK-negative anaplastic large-cell lymphoma (2 neoplasms requiring different therapeutic approaches). An example of such an application is depicted in Figure 6, and studies are in progress in a larger number of cases.
Moreover, due to its lack of reactivity with normal hematopoietic cells, the B28p mAb can also be used in combination with anti-CD13850 to quantify plasma cells in the bone marrow. This is of value in the differential diagnosis between multiple myeloma and monoclonal gammopathy of “undetermined” significance (MGUS) and in the assessment of minimal residual disease after conventional therapy or peripheral stem cell transplantation.
Prepublished online as Blood First Edition Paper, December 2, 2004; DOI 10.1182/blood-2004-07-2630.
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC). E.T. was supported by a “Livia Benedetti” grant. A.P. and R.B. were supported by Federazione Italiana per la Ricerca sul Cancro (FIRC). K.P. and H.R. were supported by the Leukemia Research Fund, grant no. 9970.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Riccardo Dalla Favera for reading the manuscript and making available the gene expression profiling data. We also thank Mrs Barbara Verducci, Alessia Tabarrini, Federica Frenguelli, and Francesca Brachelente for the excellent technical assistance and Mrs Claudia Tibidò for the secretarial assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal