Abstract
Plasmacytoid dendritic cells (pDCs) play an important primary role for antiviral innate immunity by rapidly producing large amounts of type 1 interferon (IFN) upon viral infection. To study pDC biology, we generated a monoclonal antibody, termed 2E6, that recognizes pDCs. Molecular cloning of a cDNA encoding the 2E6 antigen revealed that it is a type II C-type lectin, Ly49Q, that consists of 247 amino acids with high homology to the natural killer (NK) receptor family Ly49, with an immunoreceptor tyrosine-based inhibitory motif in the cytoplasmic domain. Ly49Q is expressed on pDCs but not on NK cells or myeloid dendritic cells. B220+, CD11c+, CD11b– pDCs in bone marrow were divided into Ly49Q+ and Ly49Q– subsets. While both subsets produced IFN-α upon cytosine-phosphate-guanosine (CpG) and herpes simplex virus stimulation, Ly49Q– pDCs responded poorly to influenza virus. In addition, Ly49Q– pDCs produced inflammatory cytokines such as interleukin 6 (IL-6), IL-12, and tumor necrosis factor α (TNF-α) upon stimulation at lower levels than those produced by Ly49Q+ pDCs. In contrast to bone marrow, Ly49Q+ pDCs were only found in peripheral blood, lymph nodes, and spleen. These results indicate that Ly49Q is a specific marker for peripheral pDCs and that expression of Ly49Q defines 2 subsets of pDCs in bone marrow.
Introduction
Dendritic cells (DCs) are highly motile cells that operate at the interface of innate and acquired immunity. They are specialized for the uptake, transport, processing, and presentation of antigens to T cells. Among DC subsets, plasmacytoid DC (pDC) or type I interferon-producing cells (IPCs) have been reported as immature (precursor) DCs and high producers of type I interferon (IFN) in response to viral infection and other stimuli such as bacterial cytosine-phosphate-guanosine (CpG) in both humans and mice.1-5 The pDCs control myeloid DCs and activate macrophages and natural killer (NK) cells through release of cytokines.6 In addition, upon viral infection, pDCs have the ability to differentiate into mature DCs, which are capable of inducing naive T-cell proliferation toward T-helper 1 (Th1) cells and expansion of interleukin 10 (IL-10)–producing regulatory T cells.7-10 Thus, pDCs initiate an immune response by linking innate and acquired immunity upon activation. The frequency of pDCs in blood and secondary lymphoid organs is low in both humans and mice. It is therefore difficult to study the nature of this DC subset. Blood dendritic cell antigen 2 (BDCA-2) has recently been identified as a specific marker for pDCs in humans and it was suggested that this C-type lectin receptor may participate in antigen presentation.11 Two antibodies have recently been reported as specific to murine pDCs in mice.12,13 However, none of the antigens specific to murine pDCs have been molecularly cloned.
The function of DCs is mediated in part by the expression of specialized surface receptors. For example, CD205 (DEC-205) expressed on the DC surface binds and internalizes carbohydrate-bearing antigens by receptor-mediated endocytosis, thus playing a role in antigen uptake and presentation.14 This receptor belongs to the type I Ca2+-dependent (C-type) lectin family that contains multiple Ca2+-dependent extracellular carbohydrate-recognition domains (CRDs) on their NH2 termini. Another family of C-type lectin molecules, termed type II, has a single CRD at the COOH terminal.15 Dectin-1 has recently been reported as a member of this type II lectin family that acts as a receptor for fungal-derived carbohydrates, β-glucans. Dectin-1 contains an immunoreceptor tyrosine-based activation motif (ITAM) in the cytoplasmic region that is phosphorylated upon ligand (such as bacteria and fungi) binding, then recruited into phagosomes together with Toll-like receptors (TLRs), and thus induces DC activation upon infection.16,17
In order to identify the molecules that control pDC functions, we describe a murine pDC-specific monoclonal antibody (mAb), designated 2E6, and the molecular cloning of the cDNA encoding Ly49Q18 recognized by this newly generated mAb. Ly49Q encodes a type II C-type lectin membrane–associated polypeptide of 247 amino acids containing a single CRD motif at the COOH terminal and an immunoreceptor tyrosine-based inhibition motif (ITIM) in its cytoplasmic region. By using 2E6 Ab, we found that previously defined pDCs (B220+, CD11c+, CD11b–) in bone marrow can be divided into 2 groups.
Materials and methods
Reagents
CD3, CD11b, CD11c, CD14, CD19, CD45RB, DX5, and B220 Abs were purchased from Becton Dickinson (BD) Biosciences (San Jose, CA). fms-like kinase 3 ligand (Flt-3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were purchased from R&D Systems (Minneapolis, MN). Influenza virus strain PR8 and herpes simplex virus (HSV) strain KOS were kindly provided by Drs Kawaoka (Institute of Medical Science University of Tokyo [IMSUT], Tokyo, Japan) and Yanagi (National Institute of Infectious Diseases, Tokyo, Japan), respectively.
Generation of DC-specific mAb
A 4-week-old female Wistar rat was immunized in both hind footpads with 1 × 106 to 2 × 106 pDCs (purified by cell sorting for B220, CD11c+, CD11b– cells from cultured bone marrow cells with Flt-3L for 10 days) in the presence of Freund complete adjuvant (Yatoron, Tokyo, Japan) on days 0, 4, and 11. On day 12, cells from hind popliteal lymph nodes were fused to p3X myeloma cells by polyethylene glycol as previously described,19 and fused cells were plated onto 96-well flat-bottom plates. Hybridoma supernatants were screened for negative reaction to spleen cells (< 1%) and positive reaction to pDCs by flow cytometry. Hybridoma 2E6 was chosen based on the specific staining pattern for pDCs. The 2E6 mAb was obtained from culture supernatant and purified using a protein G column. The isotype of the 2E6 mAb was determined using a rat immunoglobulin G (IgG) isotyping kit (Zymed Labs, South San Francisco, CA). Biotinylated antibody was prepared in accordance with the manufacturer's instruction (Pierce Biotechnology, Rockford, IL).
Cell preparation
Bone marrow cells were prepared from tibiae and femora of 4- to 6-week-old female BALB/c mice. After red cell lysis, cells were cultured with RPMI 1640 supplemented with 10% fetal calf serum (FCS), 1 mM sodium pyruvate, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), l-glutamine, penicillin, streptomycin, 2-mercaptoethanol, and murine Flt-3L (10 ng/mL) for 10 days, as described previously.20 On day 10, cells were harvested and stained with B220, CD11c, and CD11b markers, and then B220, CD11c+, CD11b– cells were sorted by fluorescence-activated cell sorter (FACS) Vantage (BD Biosciences) as pDCs. In the case of purifying myeloid DCs, freshly isolated bone marrow cells were cultured in the presence of GM-CSF (10 ng/mL) for 10 days. To purify 2E6+ cells from spleen, cells were selected by auto magnetic-activated cell separation (MACS) using biotinylated 2E6 antibody followed by avidin-conjugated beads (Miltenyi Biotec, Bergisch Gladbach, Germany). For enrichment of pDCs from spleen, bone marrow, and lymph nodes, T cells, B cells, monocytes, NK cells, granulocytes, and mature DCs were depleted by using anti-CD3, CD19, CD14, DX-5, and CD11b Abs by Auto MACS. Cells were then stained with anti-CD11c, B220, and 2E6 Abs and analyzed by FACS.
In vitro stimulation of pDCs and quantification of cytokine production
The 2E6+ or 2E6– cells were purified from BALB/c bone marrow, spleen, blood, and lymph nodes and plated onto 96-well round-bottom plates. They were then stimulated with virus (influenza virus PR8 [10 plaque-forming units {pfu's}] or HSV [2 pfu's]) or 0.1 μM of phosphorothioate CpG oligodeoxynucleotide (ODN) 1668 (5′-TCCATGACGTTCCTGATGCT-3′; MWG Biotech, Munich, Germany) for 20 to 24 hours at 37°C. Supernatants were harvested and cytokines were measured by enzyme-linked immunosorbent assay (ELISA).
IFN-α production from the supernatants was determined by ELISA. In brief, 96-well microtiter plates were coated with a rat anti–mouse IFN-α Ab (PBL Biomedical Laboratory, Piscataway, NJ) at 4°C overnight. They were then sequentially incubated with samples or a recombinant mouse IFN-α standard for 2 hours, with polyclonal rabbit anti–mouse IFN-α antibody (PBL Biomedical Laboratory) for 1 hour, and with horseradish peroxidase–conjugated goat anti–rabbit IgG (BD Biosciences) for 1 hour. This was followed by peroxidase-substrate (BD Biosciences) incubation for 20 minutes. Samples were then identified by measuring their optical density at 450 nm. ELISA for IL-12p70, TNF-α, and IL-6 was performed according to manufacturer's protocol (R&D Systems).
Cloning of the gene encoding the protein recognized by 2E6 mAb
Total RNA was extracted from cultured bone marrow cells in the presence of Flt-3L for 10 days by the standard phenol/guanidium thiocyanate method, and mRNA was then purified with an oligo-dT column (Micro-Fast Track mRNA isolation kit; Invitrogen, Carlsbad, CA). Double-stranded cDNA primed with oligo (dT) and random hexamer was synthesized (SuperScript Choice System kit; Invitrogen) according to the manufacturer's protocol and ligated into the EcoRI site of pME18S expression vector. Escherichia coli DH10B cells (Invitrogen) were transformed with ligated DNA by electroporation. Aliquots of 2 × 106 independent clones were used for transfection. COS7 cells were transfected with plasmid DNA using Effectine transfection reagent (Qiagen, Hilden, Germany). Three days after transfection, cells were harvested and reacted with binding biotinylated 2E6 and then with magnetic beads conjugated with streptavidin or fluorescein isothiocyanate (FITC) conjugated with streptavidin. After magnetic sorting by Auto MACS, plasmid DNA was extracted from positive cells by the improved Hirt method.21 The plasmid DNA was introduced into E coli DH10B cells by electroporation and then applied to the next round of transfection. After 4 rounds of magnetic sorting and an additional 2 rounds of sorting by FACS Vantage, plasmid DNA from the positive clone was purified and sequenced. Database search was performed using BLAST.22
RT-PCR analysis
Sets of cDNA (Mouse Multiple Tissue cDNA Mouse I and Mouse II; BD Biosciences Clontech, Palo Alto, CA) were used for reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of Ly49Q mRNA expression in various tissues. PCR amplification for Ly49Q mRNA was performed (95°C for 30 s; 55°C for 30 s; 72°C for 1 min; 30 cycles) using sense (5′-AAGCACCCAGGTGGAAGCCTGG-3′) and antisense (5′-TTAACTGTTGTTGGGGAGCGA-3′) oligonucleotide primers. PCR amplification for G3PDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was performed (95°C for 30 seconds; 55°C for 30 seconds; 72°C for 1 minute; 30 cycles) using sense (5′-ACCACAGTCCATGCCATCAC-3′) and antisense (5′-TCCACCACCCTGTTGCTGTA-3′) primers. The PCR products were size fractionated by agarose gel electrophoresis and analyzed after ethidium bromide staining.
Results
Ab reactivity with cultured bone marrow cells and freshly isolated spleen cells
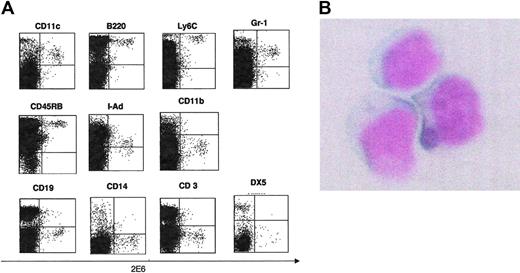
First, mAbs were screened by staining spleen cells from BALB/c mice. Since pDCs constitute a rare population in spleen and bone marrow, we selected mAbs that stained less than 5% of splenocytes. Second, the selected mAbs were then tested for their reactivity on pDCs derived from cultured bone marrow cells with Flt-3L by flow cytometry. The 2E6+ cells from bone marrow cells cultured in the presence of Flt-3L for 10 days are B200+CD11c+Ly6c+CD11b– (Figure 1A). This staining pattern was compatible with the reported pDC phenotype.3-5 To determine whether the antigen recognized by 2E6 is expressed on other DC subsets, we tested 2E6 expression on CD11b+, CD11c+ myeloid DCs isolated from cultured bone marrow cells in the presence of GM-CSF for 10 days. This antibody did not stain conventional myeloid DCs (Figure 1B). Third, we performed double-color staining with various cell surface antigens and 2E6 on freshly isolated splenic lymphocytes. Cells positively stained by 2E6 antibody were B220+, CD11c+, Ly6C+, Gr-1dull+, CD45RB+, I-Ad dull+, CD11b–, CD3–, CD14–, CD19–, and DX5– (Figure 2). This indicates that 2E6+ cells were not T cells, B cells, monocytes, macrophages, or NK cells. The staining pattern of bone marrow cells was similar to that of splenocytes.
Ab reactivity with cultured bone marrow cells and freshly isolated spleen cells. (A) The 2E6+ cells are compatible with pDCs. Bone marrow cells from BALB/c mice were cultured in the presence of Flt-3L for 10 days to generate pDCs in vitro. Cells were then stained with 2E6, CD11c, CD11b, B220, and Ly6C. Profiles showed 2E6+ gate (R1). (B) The 2E6 does not express on myeloid DCs. Bone marrow cells from BALB/c mice were cultured in the presence of GM-CSF for 10 days and then stained with CD11b, CD11c, and 2E6 Ab. At day 10, the majority of the cells (58%) became CD11b+CD11c+ myeloid DCs. Percentages in quadrants indicate the percentage of positive cells in that quadrant.
Ab reactivity with cultured bone marrow cells and freshly isolated spleen cells. (A) The 2E6+ cells are compatible with pDCs. Bone marrow cells from BALB/c mice were cultured in the presence of Flt-3L for 10 days to generate pDCs in vitro. Cells were then stained with 2E6, CD11c, CD11b, B220, and Ly6C. Profiles showed 2E6+ gate (R1). (B) The 2E6 does not express on myeloid DCs. Bone marrow cells from BALB/c mice were cultured in the presence of GM-CSF for 10 days and then stained with CD11b, CD11c, and 2E6 Ab. At day 10, the majority of the cells (58%) became CD11b+CD11c+ myeloid DCs. Percentages in quadrants indicate the percentage of positive cells in that quadrant.
Staining with various cell surface antigens and 2E6 on freshly isolated splenic lymphocytes. (A) Staining of freshly isolated splenocytes with 2E6 mAb. Freshly isolated splenocytes from BALB/c mice were stained with various Abs (CD11c, B220, Ly6C, CD45RB, Gr-1, I-Ad, DX5, CD19, CD14, CD11b, and CD3) in combination with 2E6 mAb. (B) Morphologic features of 2E6+ cells. CD3+, CD19+, CD11b+, CD14+, and DX5+ cells were depleted from freshly isolated spleen cells by Auto MACS. The 2E6+ cells were then sorted by cell sorter (BD Biosciences). Cells were plated onto slides by centrifugation and stained with May-Giemsa (original magnification, × 1000). Micrography was performed using a Nikon OPTIPHOT-2 microscope equipped with a Plan 40 objective lens, the numerical aperture of which was 0.70 (Nikon, Tokyo, Japan). Images were acquired using a COOLPIX 990 3.34-megapixel camera (Nikon) and Preview software 2.1.0 (Apple Computer, Cupertino, CA).
Staining with various cell surface antigens and 2E6 on freshly isolated splenic lymphocytes. (A) Staining of freshly isolated splenocytes with 2E6 mAb. Freshly isolated splenocytes from BALB/c mice were stained with various Abs (CD11c, B220, Ly6C, CD45RB, Gr-1, I-Ad, DX5, CD19, CD14, CD11b, and CD3) in combination with 2E6 mAb. (B) Morphologic features of 2E6+ cells. CD3+, CD19+, CD11b+, CD14+, and DX5+ cells were depleted from freshly isolated spleen cells by Auto MACS. The 2E6+ cells were then sorted by cell sorter (BD Biosciences). Cells were plated onto slides by centrifugation and stained with May-Giemsa (original magnification, × 1000). Micrography was performed using a Nikon OPTIPHOT-2 microscope equipped with a Plan 40 objective lens, the numerical aperture of which was 0.70 (Nikon, Tokyo, Japan). Images were acquired using a COOLPIX 990 3.34-megapixel camera (Nikon) and Preview software 2.1.0 (Apple Computer, Cupertino, CA).
The 2E6+ cells are pDCs and produce IFN-α upon viral stimulation
To verify the cell types recognized by this antibody, we purified 2E6+ cells from splenocytes. CD3+, CD19+, CD11b+, CD14+, and DX5+ cells were depleted by Auto MACS from fresh splenocytes, and 2E6+ cells were then isolated by cell sorting. The 2E6+ cells showed a plasmacytoid morphology that was characterized by a round shape and an eccentric nucleus without dendrites (Figure 2B).
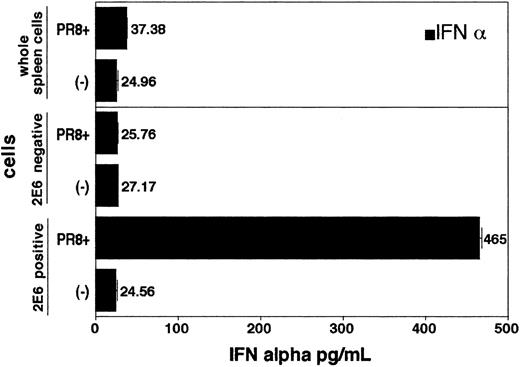
In order to prove that 2E6 recognizes the cell surface antigen specifically expressed on pDCs, we purified 2E6+ cells from freshly isolated spleen cells by Auto MACS. While 2E6+ spleen cells produced large amounts of IFN-α upon stimulation with influenza virus PR8, neither 2E6– cells nor whole spleen cells produced IFN-α upon stimulation (Figure 3). Moreover, addition of 2E6 antibody into the culture (up to 5 μg/mL) of bone marrow–derived pDCs had no effect on either IFN-α production analyzed by ELISA or proliferation of pDCs induced by various stimuli in vitro as detected by [3H]-thymidine incorporation (data not shown).
The 2E6+cells from spleen produce large amount of IFN-α upon influenza virus (strain PR8) stimulation. Spleen cells were stained with biotinylated 2E6 mAb followed by avidin-conjugated magnet beads. The 2E6+ and 2E6– cells were separated using Auto MACS. Each group (2E6+, 2E6–, whole spleen cells) was plated onto 96-well round-bottom plates (5 × 104 cells/well). Experiments were performed in triplicate. Then, either medium alone or influenza virus PR8 were added to the wells to a total volume of 200 μL and incubated at 37°C for 24 hours. IFN-α in supernatants was measured by ELISA. Values represent mean ± SD from triplicate samples.
The 2E6+cells from spleen produce large amount of IFN-α upon influenza virus (strain PR8) stimulation. Spleen cells were stained with biotinylated 2E6 mAb followed by avidin-conjugated magnet beads. The 2E6+ and 2E6– cells were separated using Auto MACS. Each group (2E6+, 2E6–, whole spleen cells) was plated onto 96-well round-bottom plates (5 × 104 cells/well). Experiments were performed in triplicate. Then, either medium alone or influenza virus PR8 were added to the wells to a total volume of 200 μL and incubated at 37°C for 24 hours. IFN-α in supernatants was measured by ELISA. Values represent mean ± SD from triplicate samples.
Taken together, we concluded that 2E6 mAb recognizes the antigen that was expressed on the surface of pDCs. Thus, 2E6 mAb will be a useful tool for detection and purification of pDCs.
Expression cloning of cDNAs recognized by 2E6 mAb
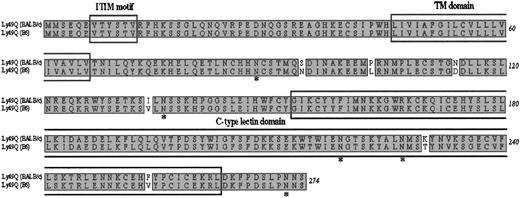
To characterize the molecular nature of the antigen recognized by 2E6, we performed expression cloning in COS7 cells. COS7 cells, transfected with a cDNA library prepared from Flt-3L–derived bone marrow pDCs, were allowed to be bound by biotinylated 2E6 followed by magnetic beads conjugated with streptavidin. Cells bound by biotinylated 2E6 were enriched by Auto MACS. After 4 rounds of enrichment by Auto MACS, 2 further rounds of enrichment by sorting were performed. We thereby obtained a plasmid carrying 1.2-kilobase (kb) cDNA. We sequenced this cDNA insert and found an 825-bp open reading frame encoding a protein of 274 amino acids. A BLAST22 homology search revealed that this molecule is highly similar to mouse Ly49Q (about 98% similarity, 6 amino acids differences) as reported by Toyama-Sorimachi et al18 (GenBank accession no. AB033769; Figure 4). Since there is considerable diversity in the Ly49 gene family among mouse strains, and they cloned this molecule from C57Black 6 mice, the 6–amino acid difference may be due to the different mouse strains used. In addition, our antibody also reacted with pDCs from the C57/B6 mice (data not shown). We concluded that the molecule encoded in cloned cDNA is Ly49Q. Analysis of the predicted amino acid sequence of Ly49Q indicated that Ly49Q belongs to the type II Ca2+-dependent (C-type) lectin family with the presence of CRD in the extracellular domain and ITIM in the intercellular domain. In addition, the putative extracellular domain contains 5 potential N-linked glycosylation sites.
Amino acid sequence alignment of the type II C-type lectin Ly49Q18 derived from either BALB/c or C57/B6. There is a difference of 6 amino acids between the 2 strains. Ly49Q belongs to the Ly49 family that has a CRD in the extracellular domain and an ITIM in the intercellular domain. *Potential N-linked glycosylation sites in the putative extracellular domain. TM indicates transmembrane.
Amino acid sequence alignment of the type II C-type lectin Ly49Q18 derived from either BALB/c or C57/B6. There is a difference of 6 amino acids between the 2 strains. Ly49Q belongs to the Ly49 family that has a CRD in the extracellular domain and an ITIM in the intercellular domain. *Potential N-linked glycosylation sites in the putative extracellular domain. TM indicates transmembrane.
Expression of Ly49Q mRNA in tissue
The tissue expression of Ly49Q mRNA was examined by RT-PCR using first-strand cDNA from multiple tissues. The primers used for PCR were Ly49Q specific and did not detect any other members of Ly49 family. Ly49Q transcripts were detected in the greatest quantity in spleen and bone marrow and at low levels in lung. Ly49Q mRNA was not detected in heart, brain, liver, muscle, kidney, testis, eye, prostate, uterus, or stomach (Figure 5). Taken together, these data indicate a preferential expression of Ly49Q mRNA in immune tissues where a relatively large number of pDCs may exist. Furthermore, the absence of Ly49Q mRNA expression on lymph nodes and thymus may be due to the low percentage of pDCs residing in those tissues.
Ly49Q expression in various tissues examined by RT-PCR. Lane 1, heart; lane 2, brain; lane 3, spleen; lane 4, lung; lane 5, liver; lane 6, skeletal muscle; lane 7, kidney; lane 8, testis; lane 9, bone marrow; lane 10, eye; lane 11, lymph node; lane 12, smooth muscle; lane 13, prostate; lane 14, thymus; lane 15, uterus; lane 16, stomach; and lane 17, no template.
Ly49Q expression in various tissues examined by RT-PCR. Lane 1, heart; lane 2, brain; lane 3, spleen; lane 4, lung; lane 5, liver; lane 6, skeletal muscle; lane 7, kidney; lane 8, testis; lane 9, bone marrow; lane 10, eye; lane 11, lymph node; lane 12, smooth muscle; lane 13, prostate; lane 14, thymus; lane 15, uterus; lane 16, stomach; and lane 17, no template.
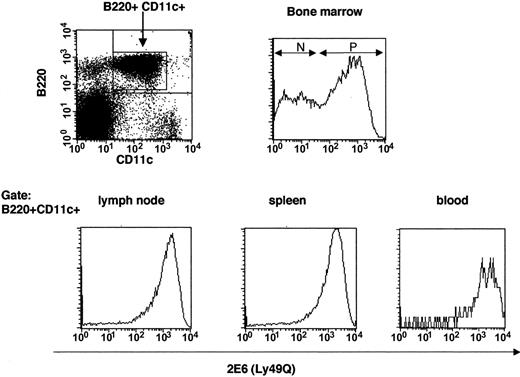
Ly49Q– pDCs exist in bone marrow and respond poorly to influenza virus compared with Ly49Q+ pDCs
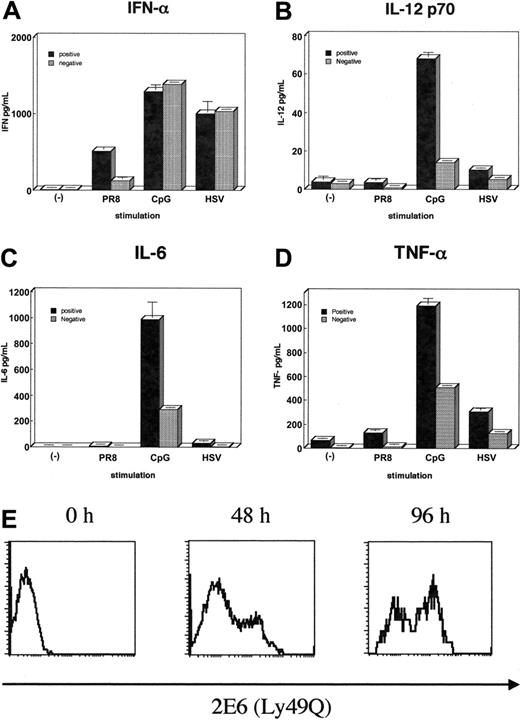
As shown in Figure 6, we found that depending on Ly49Q expression, there are 2 subsets of pDCs in bone marrow. To investigate any differences between Ly49Q+ and Ly49Q– pDCs, we purified these cells populations from bone marrow by sorting within the previously defined pDC gate (B220+CD11c+). All other pDC cell surface makers and levels of IFN-α production upon stimulation with both HSV and CpG were similar between Ly49Q+ and Ly49Q– pDCs. However, when cells were stimulated with influenza virus PR8, Ly49Q– cells poorly produced IFN-α compared with Ly49Q+ cells (Figure 7A). Moreover, the production of inflammatory cytokines, such as IL-12p70, TNF-α, and IL-6, induced by various stimuli was severely reduced in Ly49Q– pDCs (Figure 7B-D). These data indicate that 2 functionally different classes of pDCs exist in bone marrow within previously defined pDCs.
Ly49Q– pDCs exist in bone marrow but not in peripheral lymphoid organs. Cells were harvested from various lymphoid organs (bone marrow, spleen, lymph nodes, and peripheral blood lymphocytes) and then depleted of CD3+, CD19+, CD11b+, and DX5+ cells by Auto MACS. Next, negatively selected cells were stained with B220, CD11c, and 2E6 Abs. B220+CD11c+ cells were gated and analyzed for 2E6 expression. N indicates 2E6–;P,2E6+.
Ly49Q– pDCs exist in bone marrow but not in peripheral lymphoid organs. Cells were harvested from various lymphoid organs (bone marrow, spleen, lymph nodes, and peripheral blood lymphocytes) and then depleted of CD3+, CD19+, CD11b+, and DX5+ cells by Auto MACS. Next, negatively selected cells were stained with B220, CD11c, and 2E6 Abs. B220+CD11c+ cells were gated and analyzed for 2E6 expression. N indicates 2E6–;P,2E6+.
Differential cytokine production in 2 subsets of pDCs. (A-D) After depletion of CD3+, CD19+, CD11b+, and DX5+ cells from BALB/c bone marrow, cells were stained with B220, CD11c, and 2E6 Abs. The 2E6+ (▪) or 2E6– (▦) cells were sorted within the gate of B220+CD11c+ cells and then 4 × 104 cells/well of each group were plated onto 96-well round-bottom plates. These cells were variously stimulated (influenza virus PR8, CpG-ODN, or HSV) at 37°C for 24 hours, then supernatants of IFN-α, TNF-α, IL-6, and IL-12p70 were measured by ELISA. Experiments were performed at least 3 times with similar results. Values represent the mean ± SD from triplicate samples. (E) 2E6– pDCs acquire the expression of Ly49Q. Sorted 2E6– pDCs derived from bone marrow were cultured in the presence of Flt-3L (100 ng/mL) for 0, 2, and 4 days. Cells were then stained with 2E6 mAb and analyzed by FACS.
Differential cytokine production in 2 subsets of pDCs. (A-D) After depletion of CD3+, CD19+, CD11b+, and DX5+ cells from BALB/c bone marrow, cells were stained with B220, CD11c, and 2E6 Abs. The 2E6+ (▪) or 2E6– (▦) cells were sorted within the gate of B220+CD11c+ cells and then 4 × 104 cells/well of each group were plated onto 96-well round-bottom plates. These cells were variously stimulated (influenza virus PR8, CpG-ODN, or HSV) at 37°C for 24 hours, then supernatants of IFN-α, TNF-α, IL-6, and IL-12p70 were measured by ELISA. Experiments were performed at least 3 times with similar results. Values represent the mean ± SD from triplicate samples. (E) 2E6– pDCs acquire the expression of Ly49Q. Sorted 2E6– pDCs derived from bone marrow were cultured in the presence of Flt-3L (100 ng/mL) for 0, 2, and 4 days. Cells were then stained with 2E6 mAb and analyzed by FACS.
In order to identify the lineage relationship between Ly49Q– and Ly49Q+ pDCs, Ly49Q– pDCs were purified from freshly isolated bone marrow cells and cultured in the presence of Flt-3L for a few days. As shown in Figure 7E, Ly49Q– pDCs acquired the expression of Ly49Q. Thus, it is suggested that Ly49Q– pDCs might be the precursors of Ly49Q+ pDCs.
Discussion
In the present study, we generated a mAb against pDCs and found that the gene recognized by this antibody encoded a type II C-type lectin, Ly49Q. Recently, 2 antibodies specific to murine pDCs (120G8 and 440c) have been reported.12,13 Since the function of DCs is mediated in part by the expression of specialized surface receptors, it is also important to characterize the molecules expressed on the cell surface of pDCs, such as BDCA-2 in humans.11 However, none of the antigens specific to pDCs have yet been reported in a murine system. This is the first report describing molecular cloning of the antigen expressed on murine pDCs. Since Ly49Q expressed on pDCs belongs to type II C-type lectin and contains a potential inhibitory signaling motif, ITIM, in the cytoplasm, it may play a role in the regulation of pDC function.
Ly49 belongs to the NK receptor family that is known to bind to major histocompatibility complex (MHC) class I proteins and to regulate NK cell activation, either positively or negatively through receptor signaling.23,24 The Ly49 family cluster is located on chromosome 6 in a region designated as the NK gene complex.25,26 In the case of inhibitory Ly49 receptors, each subunit contains an ITIM. Upon receptor engagement, the tyrosine residues of the ITIM motifs of NK inhibitory receptors become phosphorylated and recruit src homology domain–containing tyrosine phosphatase 1 (SHP-1). The phosphatase activity of the SHP-1 is activated upon Ly49 binding, and SHP-1 dephosphorylates tyrosine-phosphorylated proteins involved in the NK activation cascade, thereby blocking response.23 For example, the Ly49A inhibitory receptor engages H-2Dd class I MHC molecules on normal cells and suppresses NK cell–mediated lysis of target cells.27,28 This type of inhibition is diminished when tumor cells or virally infected cells with down-regulated class I MHC expression are encountered,29 and NK-mediated target cell lysis generally ensues. In summary, during NK contact with healthy cells, inhibitory receptor signals are dominant, preventing autoaggression and thus maintaining NK self-tolerance. Moreover, Inngjerdingen et al30 reported another function of inhibitory Ly49A that down-regulates chemotactic responses to either CXCL10 or CXCL12 in NK cells.
Ly49Q– pDCs are present in the bone marrow. Both Ly49Q+ and Ly49Q– pDCs in bone marrow were able to produce IFN-α induced by various stimuli such as bacterial CpG-ODN and viruses, although Ly49Q– pDCs respond poorly to influenza virus in terms of IFN-α production. Moreover, the levels of other inflammatory cytokines such as IL-12p70, IL-6, and TNF-α were severely reduced in Ly49Q– pDCs upon any stimulation. These data indicate that the ability to respond to various infectious organisms by Ly49Q– pDCs is less potent compared with Ly49Q+ pDCs. It has been reported that both CpG-ODN and DNA virus HSV stimulate IFN-α production of pDCs through the TLR9/myeloid differentiation factor 88 (MyD88) pathway.31 However, stimulation of pDCs by RNA virus (influenza) is TLR9 independent when analyzed in TLR9-deficient mice.32 The stimulation of pDCs by CpG-ODN or DNA virus such as HSV is considered to be triggered by the interaction of the TLR9 with the DNA in the endosomal compartment after internalization of virus or CpG-DNA.31 Recent investigations33-35 report that RNA viruses activate pDCs via TLR7 but not TLR9. The distinct responses against RNA viruses might depend on differential expression of TLR/adapter molecules within Ly49Q+ and Ly49Q– pDCs in the bone marrow.
In contrast to IFN-α, inflammatory cytokines were all reduced in Ly49Q– pDCs upon CpG and HSV stimulation. It has been reported that the same TLR ligand induces distinct cytokines, IFN-α and IL-12 in pDCs and myeloid DCs, respectively.36 Our data suggest that the induction of these inflammatory cytokines might require additional signals that are not necessary for the induction of IFN-α and are present in Ly49Q+ pDCs but not in Ly49Q– pDCs. In summary, Ly49Q– pDCs in the bone marrow have not yet acquired the full sets of cell surface and/or adapter signaling molecules that are necessary for appropriate responses against various infectious organisms. The reason for the absence of Ly49Q– pDCs in peripheral lymphoid organs is unknown. Since Ly49Q– pDCs exist in bone marrow but not in the periphery, have less ability to produce inflammatory cytokines, and become Ly49Q+ pDCs after few days culture with Flt-3L, we speculate that Ly49Q– pDCs might be premature pDCs that stay in bone marrow. They may migrate into the periphery after full maturation. However, further experimental study is necessary to confirm this hypothesis.
Our data provide the first evidence that previously defined murine pDCs can be divided into either Ly49Q+ or Ly49Q– subsets that differentially respond to RNA virus and induce distinct inflammatory cytokines. Thus, the mAb 2E6 against Ly49Q will be a useful marker to distinguish peripheral pDCs and a good tool for the separation of pDCs, since 2E6 did not affect IFN-α production in vitro. In addition, Ly49Q also expresses on activated macrophages, as reported by Toyama-Sorimachi et al,18 and cross-linking of Ly49Q causes morphologic change in IFN-γ–treated macrophages. However cross-linking of Ly49Q by 2E6 mAb did not cause any morphologic change in pDCs. Moreover, IFN-γ treatment did not cause up-regulation of Ly49Q in pDCs (data not shown). Therefore, Ly49Q expression on pDCs may be differentially regulated from macrophages. The function of Ly49Q in pDCs is not yet known. However, since Ly49Q contains an ITIM motif in cytoplasm, it may play a role in terminating pDC activation or preventing autoreactive responses by pDCs upon ligand binding. It is consequently necessary to study how Ly49Q regulates pDC function and investigate its ligand in future studies.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-09-3388.
Supported in part by Ginkgo Biomedical Research Institute.
Four of the authors (Y.K.-S., J.O., S.N., N.A.) are employed by a company (Ginkgo Biomedical Research Institute) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful for the generous gift of HSV from Dr Yanagi at NIH Japan and of influenza virus from Dr Kawaoka at IMSUT Japan. We thank Drs S. Miyatake, A. Miyajima, and P. Todd for critical reading of the manuscript. We also thank Dr N. Toyama-Sorimachi for constructive discussion.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal