Abstract
Mechanisms accounting for gender dimorphism during immune responses are still poorly understood. Since invariant natural killer T (iNKT) cells exert important regulatory functions through their capacity to produce both T helper 1 (Th1) and Th2 cytokines, we addressed the question of whether these activities could be modulated by sexual hormones. We found that in vivo challenge with the specific ligand of iNKT cells, α-galactosylceramide (α-GalCer), induced significantly higher concentrations of interferon γ (IFN-γ) in the serum of female than in that of male mice, while interleukin 4 (IL-4) production was not modified. In support of a crucial role of ovarian hormones in this phenomenon, a significant decrease of serum IFN-γ concentrations occurred in ovariectomized females, in response to treatment with α-GalCer, while orchidectomy affected neither IFN-γ nor IL-4 serum concentrations in males. The implication of estrogens in this selective enhancement of IFN-γ production by iNKT cells was demonstrated by (1) the increased α-GalCer–induced IFN-γ synthesis by iNKT cells upon both in vitro and in vivo exposure to estradiol and (2) the abolition of the sex-linked difference in α-GalCer–induced IFN-γ release in estrogen receptor α-deficient mice. These results provide the first evidence that estrogens influence iNKT cells leading to this gender dimorphism in their cytokine production profile.
Introduction
Both genetic and hormonal factors are thought to participate in sexual dimorphism affecting the incidence and the severity of various infectious and autoimmune diseases.1,2 Since the influence of gender on immune responsiveness usually becomes apparent after sexual maturity, a crucial role in this process has been attributed to sex steroid hormones, such as estrogens and androgens.3,4 Compared with males, female mice display increased T-cell proliferative responses5 and higher interferon γ (IFN-γ) production.6,7 Because estradiol (E2) has the ability to promote IFN-γ production by effector CD4+ T cells after in vivo antigenic challenge8 and upon in vitro activation,9,10 its implication as a modulator of the immune response seems plausible. However, it is still unknown whether the effect of estrogens on cytokine production is restricted to conventional effector T cells or may also concern immunoregulatory T cells such as invariant natural killer T (iNKT) cells.
iNKT cells constitute a population of T cells, which share some characteristics of NK cells. In this respect, iNKT cells occupy a strategic position halfway between adaptive and innate immunity.11 In mice, this subset includes CD4+ and CD4-CD8- T cells, characterized by the usage of an invariant Vα14-Jα18 T-cell receptor (TCR)–α-chain.12 The TCR/CD3 complex allows iNKT cells to sense their antigenic environment with a predilection for lipids and glycolipid ligands presented by the nonpolymorphic major histocompatibility complex (MHC) class I–like molecule CD1d.13-15 Although natural ligand(s) of iNKT cells remain unknown, α-galactosylceramide (α-GalCer), a synthetic glycolipid that binds to CD1d, strongly stimulates iNKT cell functions: namely, cytokine production.
The hypothesis that iNKT cells are targeted and functionally affected by estrogens is relevant. Indeed, it is generally acknowledged that the various regulatory functions they can accomplish during the effector phase of the immune responses proceed from their unique capacity to promptly release large amounts of IFN-γ and interleukin 4 (IL-4) upon TCR engagement.12,16 It is also clear that iNKT cells participate in situations for which sex dimorphism is well documented: namely, autoimmune conditions.12,16,17 Furthermore, the influence of estrogens on the incidence and the course of autoimmune diseases has been clearly established in mouse models of systemic lupus erythematosus,18 multiple sclerosis, and autoimmune encephalomyelitis (EAE).19
Starting from our observation that female mice injected with the specific iNKT cell ligand α-GalCer generated higher serum IFN-γ concentrations than males, we investigated whether this sex difference in iNKT cell function could be ascribed to estrogens. To this end, we examined the respective effect of ovariectomy, continuous administration of E2, and deficiency in estrogen receptor α on the IFN-γ/IL-4 cytokine production profile of iNKT cells. From our results, it can be concluded that the gender dimorphism in IFN-γ production by iNKT cells is totally due to estrogens.
Materials and methods
Mice and surgical procedure
Male and female C57BL/6J mice were bred in our animal facilities under specific pathogen-free conditions. Estrogen receptor α-deficient (ER-α-/-) mice, which were kindly provided by P. Chambon's group (Strasbourg, France), were bred in our animal facilities and screened by polymerase chain reaction (PCR) genotyping as previously described.20 All procedures were performed in accordance with the recommendations of the European Accreditation of Laboratory Animal Care. In some experiments, male and female mice were either gonadectomized or sham-operated at 4 weeks of age. To study the specific effect of E2, ovariectomized female mice were given either placebo or E2 60-day time-release pellets (0.1 mg estradiol-17β, releasing 80 μg · kg-1 · d-1; Innovative Research of America, Sarasota, FL) implanted subcutaneously in the scapular region with a sterile trochar. They were killed at 8 to 9 weeks of age, after a 4- to 5-week placebo or E2 treatment period, and wet uterine weight was systematically recorded. Sera were prepared and immediately frozen until cytokine assays. Liver and spleen were carefully removed from exsanguinated mice for mononuclear cell preparation.
In vivo challenge with α-GalCer and IL-12/IL-18
α-GalCer was synthesized by the Pharmaceutical Research Laboratory of Kirin Brewery (Gunma, Japan) as described previously.21 Mice received a single injection of either α-GalCer (1 μg intravenously + 1 μg intraperitoneally) or mrIL-12 (0.2 μg intravenously; R&D Systems, Abingdon, United Kingdom) plus mrIL-18 (1 μg intravenously; R&D Systems) diluted in NaCl. They were killed 90 minutes later when serum, spleen cells, and liver mononuclear cells were recovered as previously described.16,22 In control mice that received an identical volume of vehicle solution alone, cytokines were not detectable (< 20 pg/mL).
Cell preparation
Spleen cell suspensions were prepared using a homogenizer, and red blood cells (RBCs) were lysed in hemolysis buffer. Liver was perfused with phosphate-buffered saline (PBS), then pressed through a 70-μm cell strainer. Total liver cells were resuspended in a 40% isotonic Percoll solution (Amersham Biosciences Europe, Orsay, France) underlain with a 70% isotonic Percoll solution. After centrifugation for 20 minutes at 900g, mononuclear cells were isolated at the 40% to 70% interface before RBCs were lysed. For cytokine intracellular staining experiments, total splenocytes were enriched for CD4+ and CD4-CD8- T cells.23 For in vitro experiments, splenocytes were enriched for CD4+ T cells using anti-CD4–coated magnetic beads (Miltenyi Biotech, Bergisch-Gladbach, Germany).22 Purity of enriched-CD4+ cell fractions was 85% to 95% after reanalysis. For cell sorting, liver mononuclear cells were stained with fluorescein isothiocyanate (FITC)–anti-αβ-TCR, and allophycocyanin (APC)–-CD1d/α-GalCer-tetramer. Then, αβTCR+ CD1d/α-GalCer-tetramer+ cells (iNKT) were sorted using a fluorescence activated cell sorting (FACS) Vantage sorter (Becton Dickinson, Mountain View, CA). Purity was more than 98% after reanalysis.
Antibodies and flow cytometry analysis
Fluorochrome or biotin-conjugated anti-NK1.1 (clone PK136), anti–αβ-TCR (clone H57-597), anti–IFN-γ (clone XMG1.2), anti–IL-4 (clone 11B11), and corresponding isotype controls were purchased from BD Pharmingen (San Diego, CA), as well as Cy-chrome and APC-conjugated streptavidin. The FcγR blocking monoclonal antibody (mAb; 24G2.3 clone) was obtained from DNAX (Palo Alto, CA). Tetramers were prepared in our laboratory from the mCD1d/mβ2m expression vector constructed by Kronenberg's group (Matsuda et al24 ), then loaded or not with α-GalCer. Membrane labeling22,23 as well as intracellular cytokine staining16 was performed as previously described. The proportion of IL-4– or IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone. Cells were analyzed on a FACScalibur cytometer using Cellquest software (Becton Dickinson). Dead cells were excluded by forward and side scatter characteristics. Statistics presented are based on at least 1500 events gated on the population of interest.
Cytokine assays by specific ELISA
IL-4 and IFN-γ in sera and supernatants were quantified using standard sandwich enzyme-linked immunosorbent assays (ELISA) as previously described.22 IL-4 and IFN-γ concentrations are expressed in nanogram per milliliter, as calculated from calibration curves from serial dilutions of mouse recombinant standards (R&D Systems) in each assay. The sensitivity of both IL-4 and IFN-γ assays was 20 pg/mL.
In vitro E2 treatment and α-GalCer–induced activation of CD4+ T cells
To obtain antigen-presenting cells (APCs), cells isolated from spleens of 6-week-old female mice were incubated for 2 hours at 37°C, 5% CO2 with 200 ng/mL α-GalCer or with the vehicle only, resuspended in PBS at 2 × 106/mL, then fixed with 0.001% glutaraldehyde (Sigma, St Louis, MO) at room temperature for 15 minutes. CD4+ splenocytes from 6- to 8-week-old female mice were washed and suspended in phenol-red–free RPMI 1640 supplemented with steroid-free serum (10%; Hyclone, Logan, UT). The cells (2 × 105/well) were seeded into 96-well round-bottom cultured plates and incubated in the presence of mrIL-2 (50 U/mL; Genzyme, Cambridge, MA) for 60 hours. The cells were reincubated in phenol-red–free RPMI 1640 supplemented with 10% steroid-free serum in the presence or not of E2 (10-8 M, estradiol-17β; Sigma) for 20 hours. The cells were then harvested and incubated again at 2 × 105 per well with α-GalCer–pulsed or unloaded fixated APCs (5 × 105 cells/well) for an additional 60 hours period. The culture supernatants were collected for cytokine determination.
Reverse transcriptase (RT)–PCR analysis of estrogen receptor-α expression
Total RNA was extracted from αβ-TCR+ CD1d/α-GalCer-tetramer+ (iNKT) sorted cells (2-4 × 105 cells) of C57BL/6 wild-type female mice. TRIzol reagent (Invitrogen, Carlsbad, CA) was used according to the manufacturer's instructions. RNA from Total RNA was reverse-transcribed as previously described10 and submitted to PCR. The following gene-specific primers were used: ER-α sense (5′-ATAGCCCTGCCTTGTCCTTGAC-3′) and antisense (5′-TCATGCGGAACCGACTTGA-3′), corresponding to sequences in exon 9 and 13, respectively; β-actin sense (5′-TCACGCCATCCTGCGTCTGGACCT-3′) and antisense 5′-CCGGACTCATCGTACTCCTGCTTG-3′), corresponding to sequences in exon 4 and 6, respectively. Amplified products were separated by agarose gel electrophoresis.
Statistics
Data were expressed as means ± SEMs. Mean differences between mice groups were evaluated using Student t test. To analyze the in vitro effect of E2, nonparametric paired comparisons were performed using the Mann and Whitney test. P less than .05 was considered statistically significant.
Results
Differential α-GalCer–induced IFN-γ production in male and female C57BL/6 mice
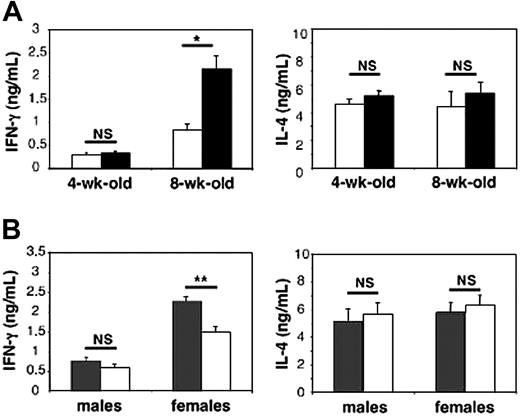
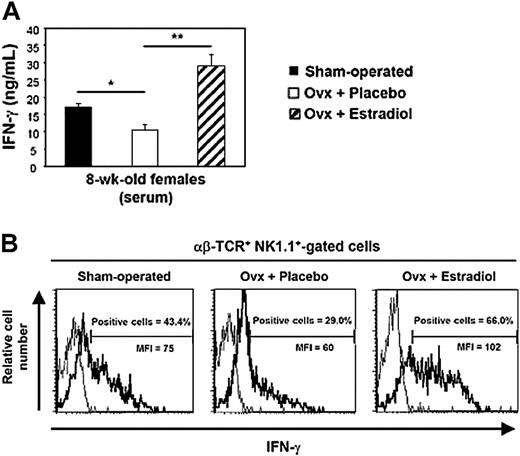
One of the original features of iNKT cells consists of their capacity to release large amounts of both T helper 1 (Th1) and Th2 cytokines, mainly IFN-γ and IL-4, following in vivo treatment with their cognate ligand α-GalCer.16,22 To assess the influence of gender on the production of these cytokines, iNKT cells were stimulated in vivo by their specific ligand α-GalCer in 4- and 8-week-old male and female C57BL/6 mice, which received a single injection of the antigen. They were killed 90 minutes later, when cytokine concentrations were determined in the serum. In recently weaned 4-week-old animals, IFN-γ and IL-4 concentrations were not influenced by gender (Figure 1A). By contrast, at 8 weeks of age, female mice produced significantly more IFN-γ (2.6-fold increase, P < .001) than males, while IL-4 production was similar in both genders (Figure 1A). The differences between sexes were even more pronounced at 16 weeks of age when IFN-γ production in females reached its maximum (4.6-fold increase, P < .001), while IL-4 remained unchanged (data not shown).
Sexual dimorphism in serum IFN-γ release following in vivo challenge with the iNKT cell ligand α-GalCer: contribution of ovarian hormones. Male and female mice received a single injection of α-GalCer and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were determined by ELISA and expressed as means ± SEMs (6-10 mice per group from 3 separate experiments). In control mice that received an identical volume of vehicle solution alone, both cytokines were not detectable (< 20 pg/mL). (A) IFN-γ but not IL-4 serum release is higher in female (▪) than in male (□) adult mice; *P < .001. (B) Ovariectomy leads to decreased IFN-γ serum concentrations in females while orchidectomy is ineffective in males. Mice were gonadectomized (□) or sham-operated (▦) at 4 weeks of age, then challenged with α-GalCer at 8 weeks of age; **P < .01.
Sexual dimorphism in serum IFN-γ release following in vivo challenge with the iNKT cell ligand α-GalCer: contribution of ovarian hormones. Male and female mice received a single injection of α-GalCer and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were determined by ELISA and expressed as means ± SEMs (6-10 mice per group from 3 separate experiments). In control mice that received an identical volume of vehicle solution alone, both cytokines were not detectable (< 20 pg/mL). (A) IFN-γ but not IL-4 serum release is higher in female (▪) than in male (□) adult mice; *P < .001. (B) Ovariectomy leads to decreased IFN-γ serum concentrations in females while orchidectomy is ineffective in males. Mice were gonadectomized (□) or sham-operated (▦) at 4 weeks of age, then challenged with α-GalCer at 8 weeks of age; **P < .01.
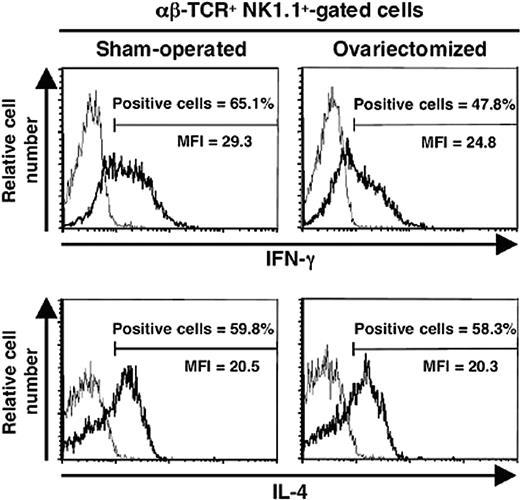
Since gender dimorphism in α-GalCer–induced IFN-γ production became apparent after sexual maturation, it was likely that sexual steroid hormones were involved in this process. To test this assumption, we gonadectomized or sham-operated 4-week-old male and female C57BL/6J mice and injected them 4 to 5 weeks later with α-GalCer. No significant differences were observed between sham-operated and orchidectomized males in terms of circulating IFN-γ and IL-4 (Figure 1B). By contrast, IFN-γ concentrations were substantially diminished after ovariectomy, which did not affect IL-4 production (Figure 1B). This differential effect of ovariectomy on IFN-γ rather than IL-4 secretion by iNKT cells was confirmed at the single cell level, by intracellular staining (Figure 2). Indeed, this treatment decreased both the percentage of IFN-γ–producing cells in the spleen (62.6% ± 3.7% for sham-operated versus 47.2% ± 5.1% for ovariectomized mice; P < .05) and the amount produced per cell, as assessed by the lower mean fluorescence intensity (MFI). In contrast, ovariectomy changed neither the percentage of IL-4–producing iNKT cells nor the intensity of intracellular staining.
Ovariectomy leads to decreased IFN-γ production by iNKT cells. Female mice were ovariectomized or sham-operated at 4 weeks of age, then challenged with α-GalCer at 8 weeks of age, and killed 90 minutes later. Spleen cells were analyzed in the αβ-TCR+ NK.1.1+ cell gate for IFN-γ and IL-4 synthesis by intracytoplasmic staining (bold line). As controls, cells were stained with irrelevant isotype-matched anti-immunoglobulin G (IgG) mAb (thin line). Histograms are representative of 3 separate experiments, each carried out with 2 mice per group. The proportion of IL-4– or IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone. MFI indicates mean fluorescence intensity.
Ovariectomy leads to decreased IFN-γ production by iNKT cells. Female mice were ovariectomized or sham-operated at 4 weeks of age, then challenged with α-GalCer at 8 weeks of age, and killed 90 minutes later. Spleen cells were analyzed in the αβ-TCR+ NK.1.1+ cell gate for IFN-γ and IL-4 synthesis by intracytoplasmic staining (bold line). As controls, cells were stained with irrelevant isotype-matched anti-immunoglobulin G (IgG) mAb (thin line). Histograms are representative of 3 separate experiments, each carried out with 2 mice per group. The proportion of IL-4– or IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone. MFI indicates mean fluorescence intensity.
Estradiol promotes α-GalCer–induced IFN-γ production by iNKT cells both in vitro and in vivo
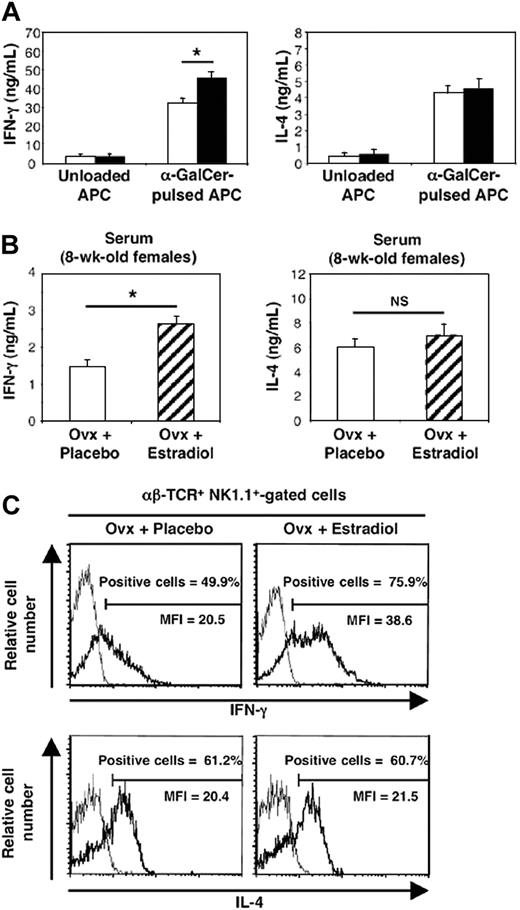
Before examining the effect of E2 on cytokine production by stimulated iNKT cells in vivo, we wanted to make sure that this female hormone could influence the functional capacities of these cells in vitro. To avoid the contribution of endogenous or contaminating estrogens during culture, we used CD4+ spleen cells that had been incubated for 2 days in IL-2–containing phenol-red–free medium supplemented with steroid-free serum. These cells were cultured further for 20 hours with or without E2 before stimulation with α-GalCer–pulsed APCs for 60 hours. In this experimental setup, we found that E2 treatment significantly increased IFN-γ production by α-GalCer–activated cells (43%), while IL-4 concentrations remained unchanged (Figure 3A).
Both in vitro and in vivo estradiol exposure promotes α-GalCer–induced IFN-γ production by iNKT cells. (A) E2 treatment in vitro enhances IFN-γ but not IL-4 production by α-GalCer–activated iNKT cells. After a first set of culture with IL-2, CD4+ spleen cells were incubated in the presence (▪) or not (□) of E2 (10-8 M) for 20 hours, then harvested, and incubated with unloaded or α-GalCer–pulsed fixated APCs for an additional 60-hour period. IFN-γ and IL-4 concentrations in culture supernatants were expressed as means ± SEMs from 4 separate experiments. *P = .02, Mann and Whitney test. (B) E2 administration in vivo increases serum IFN-γ concentration induced by α-GalCer challenge. Placebo- (□) and E2-treated (▨) ovariectomized (Ovx) females received a single injection of α-GalCer (10 mice per group) or vehicle (5 mice per group) and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were expressed as means ± SEMs. *P = .002. (C) E2 administration in vivo selectively promotes IFN-γ production by iNKT cells. Spleen cells from placebo- or E2-treated mice challenged with α-GalCer were analyzed for IFN-γ and IL-4 synthesis in the αβ-TCR+ NK.1.1+ cell gate by intracytoplasmic staining (bold line). Staining with irrelevant isotype-matched anti-IgG mAb was used as control (thin line). Histograms are representative of 3 separate experiments, each carried out with 2 mice per group. The proportion of IL-4– or IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone.
Both in vitro and in vivo estradiol exposure promotes α-GalCer–induced IFN-γ production by iNKT cells. (A) E2 treatment in vitro enhances IFN-γ but not IL-4 production by α-GalCer–activated iNKT cells. After a first set of culture with IL-2, CD4+ spleen cells were incubated in the presence (▪) or not (□) of E2 (10-8 M) for 20 hours, then harvested, and incubated with unloaded or α-GalCer–pulsed fixated APCs for an additional 60-hour period. IFN-γ and IL-4 concentrations in culture supernatants were expressed as means ± SEMs from 4 separate experiments. *P = .02, Mann and Whitney test. (B) E2 administration in vivo increases serum IFN-γ concentration induced by α-GalCer challenge. Placebo- (□) and E2-treated (▨) ovariectomized (Ovx) females received a single injection of α-GalCer (10 mice per group) or vehicle (5 mice per group) and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were expressed as means ± SEMs. *P = .002. (C) E2 administration in vivo selectively promotes IFN-γ production by iNKT cells. Spleen cells from placebo- or E2-treated mice challenged with α-GalCer were analyzed for IFN-γ and IL-4 synthesis in the αβ-TCR+ NK.1.1+ cell gate by intracytoplasmic staining (bold line). Staining with irrelevant isotype-matched anti-IgG mAb was used as control (thin line). Histograms are representative of 3 separate experiments, each carried out with 2 mice per group. The proportion of IL-4– or IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone.
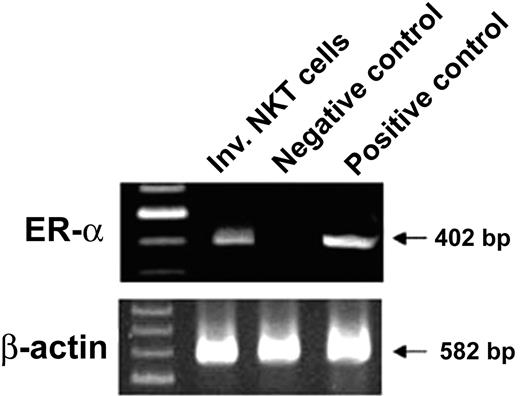
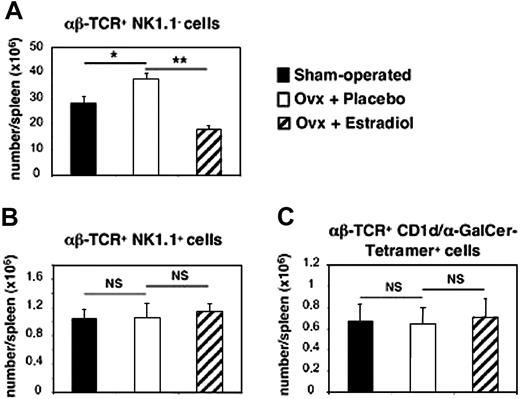
This result, together with the expression of estrogen receptor-α transcripts in purified iNKT cells (Figure 4), prompted us to further investigate the differential effect of E2 in vivo. To this end, 4-week-old ovariectomized female mice were administered during 4 to 5 weeks either with placebo or E2 at physiologic and constant doses.8,19 The efficacy of hormone administration was systematically verified by uterine weight measurement (< 20 mg in all placebo-treated mice versus 148 ± 11 mg in E2-treated mice). As shown in Figure 3B, this treatment resulted in a significant increase in serum IFN-γ concentrations following in vivo challenge with α-GalCer, whereas changes in serum IL-4 concentrations were not significant between placebo- and E2-treated mice. To verify whether iNKT cells were responsible for this differential effect, we analyzed IFN-γ and IL-4 production by iNKT cells on the single-cell level, using intracellular staining. As shown in Figure 3C, E2 administration augmented not only the percentage of IFN-γ–positive cells among iNKT spleen cells (47.9% ± 4.4% for placebo-treated versus 68.9% ± 3.3% for E2-treated ovariectomized mice, P < .05) but also the amounts produced per cell, as assessed by the enhanced MFI of IFN-γ–producing iNKT cells. By contrast, the same treatment affected neither the percentage of IL-4–producing iNKT cells nor their MFI (Figure 3C). Since E2 treatment diminished the number of spleen cells, mainly because of the loss of B cells (data not shown) and T conventional cells, namely, αβ-TCR+ NK1.1- lymphocytes (Figure 5A), we examined whether the number of iNKT cells was also affected by E2. We found no significant difference in the total number of iNKT lymphocytes, namely αβ-TCR+ NK1.1+ (Figure 5B) or αβ-TCR+ CD1d/α-GalCer-tetramer+ cells (Figure 5C) per spleen between untreated, placebo-, and E2-treated ovariectomized mice. Thus, E2 specifically promotes the production of IFN-γ by iNKT cells following TCR engagement by enhancing their functional capacities rather than their number.
iNKT cells express estrogen receptor-α (ER-α) mRNA. Liver mononuclear cells were isolated from 8-week-old female mice, and αβ-TCR+ CD1d/α-GalCer-tetramer+ (iNKT cells) cells were sorted using a FACS Vantage sorter. Purity was more than 98% after reanalysis. Total cellular RNA was extracted and subjected to RT-PCR using specific primers for ER-α (40 cycles) and β-actin (30 cycles). RNAs from uterine extracts of C57BL/6 wild-type and ER-α-/- mice were used as positive and negative controls, respectively. bp indicates base pair.
iNKT cells express estrogen receptor-α (ER-α) mRNA. Liver mononuclear cells were isolated from 8-week-old female mice, and αβ-TCR+ CD1d/α-GalCer-tetramer+ (iNKT cells) cells were sorted using a FACS Vantage sorter. Purity was more than 98% after reanalysis. Total cellular RNA was extracted and subjected to RT-PCR using specific primers for ER-α (40 cycles) and β-actin (30 cycles). RNAs from uterine extracts of C57BL/6 wild-type and ER-α-/- mice were used as positive and negative controls, respectively. bp indicates base pair.
Spleen iNKT cell number is not affected by E2 treatment. Sham-operated (▪) and either placebo- (□) or E2-treated (▨) ovariectomized (ovx) female mice were killed at 8 weeks of age. Splenocytes were labeled with fluorochrome-conjugated anti–αβ-TCR and anti-NK.1.1 Abs and with either CD1d/α-GalCer-tetramer or CD1d/vehicle-tetramer, then analyzed by flow cytometry. Absolute number of conventional T (αβ-TCR+ NK.1.1-) lymphocytes (A) and iNKT cells— namely, αβ-TCR+ NK.1.1+ (B) and αβ-TCR+ CD1d/α-GalCer-tetramer positive cells (C)—are given as means ± SEMs from 16 individual mice per group. In each group, control staining with CD1d/vehicle-tetramer was less than 0.05%. *P < .05; **P < 01. NS indicates not significant.
Spleen iNKT cell number is not affected by E2 treatment. Sham-operated (▪) and either placebo- (□) or E2-treated (▨) ovariectomized (ovx) female mice were killed at 8 weeks of age. Splenocytes were labeled with fluorochrome-conjugated anti–αβ-TCR and anti-NK.1.1 Abs and with either CD1d/α-GalCer-tetramer or CD1d/vehicle-tetramer, then analyzed by flow cytometry. Absolute number of conventional T (αβ-TCR+ NK.1.1-) lymphocytes (A) and iNKT cells— namely, αβ-TCR+ NK.1.1+ (B) and αβ-TCR+ CD1d/α-GalCer-tetramer positive cells (C)—are given as means ± SEMs from 16 individual mice per group. In each group, control staining with CD1d/vehicle-tetramer was less than 0.05%. *P < .05; **P < 01. NS indicates not significant.
Estradiol enhances IFN-γ production by iNKT cells following IL-12 plus IL-18 in vivo challenge
iNKT cells are known to participate not only in acquired but also in innate immune responses, since these cells can be fully activated and produce large amounts of IFN-γ in the absence of TCR engagement, after exposure to the proinflammatory cytokine IL-12 together with IL-18.16 To determine whether E2 influences this TCR-independent function of iNKT cells, sham-operated and ovariectomized female mice treated or not with E2 received a single intravenous injection of IL-12 plus IL-18 and were killed 90 minutes later. Serum IFN-γ concentrations were significantly decreased in ovariectomized mice in comparison with their sham-operated controls (Figure 6A). We then demonstrated that iNKT cells directly participate in this phenomenon even though NK cells and conventional T cells produce IFN-γ production under IL-12 plus IL-18 challenge. Indeed, intracellular staining clearly showed that both the percentage of IFN-γ–positive cells among liver iNKT cells and the level of IFN-γ production by IFN-γ–producing iNKT cells were lower in ovariectomized than in sham-operated females (Figure 6B). In addition, a normal IFN-γ production could be restored in ovariectomized mice having received the treatment with E2, both in terms of circulating IFN-γ (Figure 6A) and intracytoplasmic staining (Figure 6B). From our study, it can, therefore, be concluded that E2 promotes IFN-γ synthesis by iNKT cells in response to both proinflammatory cytokine stimulation and TCR engagement.
Estradiol promotes IFN-γ production by iNKT cells following in vivo IL-12/IL-18 challenge. Sham-operated (▪) and either placebo- (□) or E2-treated (▨) ovariectomized (Ovx) female mice received or not a single intravenous injection of IL-12 plus IL-18 and were killed 90 minutes later. Two separate experiments each including 3 mice per group are considered. (A) Serum IFN-γ concentrations are expressed as means ± SEMs. *P < .01; **P < .005. (B) Total liver mononuclear cells were analyzed in the αβ-TCR+ NK.1.1+ cell gate for IFN-γ synthesis by intracytoplasmic staining (bold line). As controls, cells were stained with irrelevant isotype-matched anti-IgG mAb (thin line). Mean percentages of IFN-γ–positive cells among iNKT cells: 45.6 ± 2.7 for sham-operated mice, 27.0 ± 2.0 for placebo-treated Ovx mice (P < .05 versus sham-operated mice), and 64.6 ± 4.0 for E2-treated Ovx mice (P < .01 versus placebo-treated mice). The proportion of IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone.
Estradiol promotes IFN-γ production by iNKT cells following in vivo IL-12/IL-18 challenge. Sham-operated (▪) and either placebo- (□) or E2-treated (▨) ovariectomized (Ovx) female mice received or not a single intravenous injection of IL-12 plus IL-18 and were killed 90 minutes later. Two separate experiments each including 3 mice per group are considered. (A) Serum IFN-γ concentrations are expressed as means ± SEMs. *P < .01; **P < .005. (B) Total liver mononuclear cells were analyzed in the αβ-TCR+ NK.1.1+ cell gate for IFN-γ synthesis by intracytoplasmic staining (bold line). As controls, cells were stained with irrelevant isotype-matched anti-IgG mAb (thin line). Mean percentages of IFN-γ–positive cells among iNKT cells: 45.6 ± 2.7 for sham-operated mice, 27.0 ± 2.0 for placebo-treated Ovx mice (P < .05 versus sham-operated mice), and 64.6 ± 4.0 for E2-treated Ovx mice (P < .01 versus placebo-treated mice). The proportion of IFN-γ–positive cells in the αβ-TCR+ NK.1.1+ gate was less than 0.5% in mice injected with vehicle alone.
Sexual dimorphism in α-GalCer–induced IFN-γ release is abolished in estrogen receptor α-deficient mice
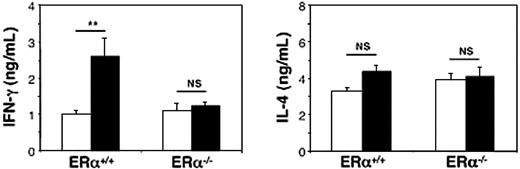
Finally, we determined to which extent endogenous estrogens could explain the sexual dimorphism in IFN-γ production by iNKT cells. To this end, 10-week-old estrogen receptor α-deficient (ERα-/-) and wild-type littermate (ERα+/+) male and female mice were challenged with α-GalCer, and serum cytokine release was measured 90 minutes later as described in “Differential α-GalCer–induced IFN-γ production in male and female C57BL/6 mice.” As shown in Figure 7, the sexual dimorphism in α-GalCer–induced serum IFN-γ concentrations, which was confirmed in ERα+/+ mice, was totally abolished in ERα-/- mice. In ERα-/- mice as well as in littermate ERα+/+ mice, changes in IL-4 concentrations were not significantly different between males and females (Figure 7). These observations definitely demonstrate that the sexual dimorphism in IFN-γ production by iNKT cells can be attributed to endogenous estrogens.
Sexual dimorphism in serum IFN-γ release following in vivo challenge with α-GalCer is abolished in ER-α–deficient mice. Ten-week-old male (□) and female (▪) ER-α-/- mice received a single injection of α-GalCer and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were determined by ELISA and expressed as means ± SEMs (4-6 mice per group from 2 separate experiments). In control mice that received an identical volume of vehicle solution alone, both cytokines were not detectable (< 20 pg/mL). **P < .01. NS indicates not significant.
Sexual dimorphism in serum IFN-γ release following in vivo challenge with α-GalCer is abolished in ER-α–deficient mice. Ten-week-old male (□) and female (▪) ER-α-/- mice received a single injection of α-GalCer and were killed 90 minutes later. Serum IFN-γ and IL-4 concentrations were determined by ELISA and expressed as means ± SEMs (4-6 mice per group from 2 separate experiments). In control mice that received an identical volume of vehicle solution alone, both cytokines were not detectable (< 20 pg/mL). **P < .01. NS indicates not significant.
Discussion
It is generally acknowledged that the regulatory functions of iNKT cells are explained by their apparent self-reactivity and their capacity to rapidly secrete large amounts of both Th1 and Th2 cytokines, including IFN-γ and IL-4. However, how these contrasting functional activities are controlled remains poorly understood. Although genetic background,25 cytokine environment,12,16 and costimulatory factors26 have been recognized as important factors in this context, it is also quite obvious by now that mouse iNKT cells are not easily polarized toward the classic Th1 or Th2 phenotype.27
By demonstrating that the female hormone E2 promotes IFN-γ production by iNKT cells, we show that the range of molecules influencing iNKT cell functions can be extended to hormonal factors. Several lines of evidence support this conclusion. First, E2 administration to ovariectomized female mice resulted in a striking increase in serum IFN-γ concentrations following in vivo treatment with the cognate α-GalCer ligand for iNKT cells. Second, this effect of E2 in vivo occurred likewise after exposure to the proinflammatory cytokines IL-12 plus IL-18, known for their particular efficiency in activating IFN-γ production by iNKT cells. Third, the frequency of IFN-γ–producing iNKT cells from mice challenged either with α-GalCer ligand or with IL-12 plus IL-18 was selectively increased in E2-treated mice. Finally, the effect of E2 on IFN-γ production by α-GalCer–reactive iNKT cells was confirmed in vitro. These in vitro results, together with the expression of ER-α transcripts in purified iNKT cells support the possibility of a direct effect of E2.
From our data, it can also be assumed that E2 exerts its action on iNKT cells qualitatively by enhancing the synthesis of IFN-γ but has no influence on the absolute number of iNKT cells. Accordingly, on the basis of CD1d–α-GalCer-tetramer specificity, the absolute number of iNKT cells in spleen remained unmodified after E2 administration, in contrast to conventional T-cell populations. Moreover, no preferential proliferation was observed when iNKT cells were exposed to E2 in vitro (data not shown).
To the best of our knowledge, this is the first demonstration that a hormonal factor influences biologic activities of iNKT cells. Overall, our data support the notion that E2 exerts differential effects on Th1 and Th2 cytokine secretion by iNKT cells. Indeed, our in vitro and in vivo experiments are in perfect agreement to assert that E2 exposure enhances α-GalCer–induced IFN-γ production but not that of IL-4 by iNKT cells. This finding is in agreement with previous observations demonstrating that E2 markedly increases IFN-γ production by both mouse and human T cells to reinforce the concept that E2 may favor Th1 responses.8,9,28 Since in vitro studies of human T cells have suggested that E2 may increase IFN-γ secretion at low concentrations but enhance IL-10 production by the same cells at higher supraphysiologic concentrations (> 5000 pg/mL),28 it is important to note that our experiments were all performed with physiologic doses of the hormone. The significant decrease in IFN-γ production by iNKT cells from ovariectomized mice challenged either with IL-12 plus IL-18 or with α-GalCer ligand, as compared with sham-operated controls, lends further support to a critical role of endogenous estrogens. Conversely, orchidectomy did not affect α-GalCer–induced cytokine production in males, supporting the notion that estrogens but not androgens promote sexual dimorphism in iNKT cell activity. Moreover, the sex-linked difference in α-GalCer–induced IFN-γ release was abolished in ER-α-/- mice, providing further evidence for this crucial influence of endogenous estrogens on iNKT cell functions.
Further investigations are now needed to determine to what extent E2 contributes to sex-linked differences in immunity by targeting iNKT cells. Indeed, the regulatory effect of estrogens on iNKT cell functions may be of pathophysiologic interest since this lymphocyte subset is well recognized for its pivotal role during anti-infection and antitumor immune responses.12,16,17 It is tempting to speculate that the influence of E2 on such immune responses could be at least in part mediated through the enhancement of iNKT-derived IFN-γ. Consistent with this idea, we found that in vivo E2 administration indirectly enhances NK cell activation through its capacity to amplify IFN-γ production by α-GalCer–activated iNKT cells (data not shown). In addition, the present results may help to understand how gender or E2 administration influences the incidence and/or the course of autoimmune diseases, such as EAE,19 type 1 diabetes,4,19 or concanavalin A–induced hepatitis,29 the development of which involves iNKT cells. Finally, regarding gestational immune surveillance, it should be mentioned that human decidual CD4+ iNKT cells exhibit a striking Th1-like bias, characterized by IFN-γ production.30 This latter observation, along with data obtained in mice showing that iNKT cells play a unique role in the defense against pathogens in the pregnant uterus in a mechanism, which involves IFN-γ,31 support the hypothesis of a critical role of estrogens in this phenomenon.
In conclusion, our study provides the first evidence for the influence of a hormonal factor on a regulatory T-cell subset and contributes to elucidate how the contrasting activities of iNKT cells are differentially regulated. Because of the crucial role attributed to iNKT cells in immune regulation, it seems highly probable that estrogens participate in the sexual dimorphism associated with autoimmunity and infectious diseases, at least in part, by targeting iNKT cells and modulating their functions.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-07-2819.
Supported by INSERM, the Ministère de la Recherche et de la Technologie (ACI 2001 and 2003), the Fondation de France, and the European Vascular Genomics Network no 503254; by a grant from INSERM (Poste d'accueil 2001-2003) (P.G.); by personal grants from INSERM (Poste vert 2001-2003) (L.A.) and from the Association pour la Recherche sur le Cancer (R.Z.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to E. Schneider (CNRS UMR 8147, Paris, France) for critical review of the manuscript. We are also specially indebted to Pharmaceutical Research Laboratory; Kirin Brewery Co, Ltd (Gunma, Japan) for providing α-GalCer; and to M. Kronenberg, P. Van Endert, and J.M. Fourneau for providing plasmid containing CD1d and β2m genes and assisting in the preparation of CD1d/α-GalCer-tetramer, respectively.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal