Abstract
Signal-regulatory proteins (SIRPs) are transmembrane glycoproteins belonging to the immunoglobulin (Ig) superfamily that are expressed in the immune and central nervous systems. SIRPα binds CD47 and inhibits the function of macrophages, dendritic cells, and granulocytes, whereas SIRPβ1 is an orphan receptor that activates the same cell types. A recently identified third member of the SIRP family, SIRPβ2, is as yet uncharacterized in terms of expression, specificity, and function. Here, we show that SIRPβ2 is expressed on T cells and activated natural killer (NK) cells and, like SIRPα, binds CD47, mediating cell-cell adhesion. Consequently, engagement of SIRPβ2 on T cells by CD47 on antigen-presenting cells results in enhanced antigen-specific T-cell proliferation.
Introduction
Signal-regulatory proteins (SIRPs) comprise a family of transmembrane glycoproteins expressed in the immune and central nervous system (CNS).1-3 SIRPs are characterized by 3 homologous extracellular immunoglobulin (Ig)–like domains (D1-D3) but have distinct transmembrane and cytoplasmic domains that transduce different signals. The prototypical member of the SIRP family, SIRPα, is expressed in macrophages, dendritic cells (DCs), granulocytes, neurons, and astrocytes. SIRPα binds CD47,4-7 or integrin-associated protein, which is ubiquitously expressed and functions in cell adhesion and migration.8,9 SIRPα-CD47 binding, stimulation of cells with various growth factors, and cell-cell adhesion induce phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in the cytoplasmic domain of SIRPα. Phosphorylated ITIMs recruit the SH2 domain-containing protein tyrosine phosphatases SHP-21,10 and SHP-1,11,12 which inhibit tyrosine kinase-coupled signaling pathways. Thus, SIRPα is an inhibitory receptor that modulates macrophage and DC function,13-15 as well as signaling pathways induced by growth factors and cell adhesion.16-19 In addition, SIRPα mediates cell-cell adhesion in the immune system and CNS, supporting fusion of macrophages19 ; DC–T-cell interactions7 ; migration of DCs, monocytes, and neutrophils20-22 ; and neurite extension and synapse formation.17,18
A second member of the SIRP receptor family, SIRPβ1 is also expressed in myeloid cells.7 However, it does not bind CD47 and lacks cytoplasmic ITIMs capable of recruiting phosphatases and mediating inhibitory signals. In fact, SIRPβ1 contains a single basic lysine residue within the hydrophobic transmembrane domain that mediates association with an adapter protein, DAP12 or KARAP, which contains a cytoplasmic tyrosine-based activating motif (ITAM).23-26 Thus, engagement of SIRPβ1 activates myeloid cells, leading to cytokine release, tyrosine phosphorylation, and calcium (Ca2+) mobilization.
A third member of the SIRP family, SIRPβ2, was recently identified.27 SIRPβ2 transcripts are variably expressed in many human tissues, including the brain, lung, and placenta, and are particularly abundant in liver. The predicted SIRPβ2 protein is highly homologous to SIRPα and SIRPβ1 in the extracellular domain, but lacks both cytoplasmic ITIMs and the transmembrane lysine required for association with DAP12. Thus, it is unclear whether and how SIRPβ2 mediates signaling; the ligand for SIRPβ2 is also unknown.
We investigated expression, specificity, and function of the SIRPβ2 protein and found that SIRPβ2 is quite distinct from SIRPα and SIRPβ1. SIRPβ2 is the only member of the SIRP family that is expressed on T cells, CD56bright natural killer (NK) cells, and all activated NK cells. SIRPβ2 does bind CD47, albeit with less affinity than SIRPα. This interaction mediates cell-cell adhesion, rather than inhibitory signals. The adhesion mediated by contact of SIRPβ2 on T cells with CD47 on antigen-presenting cells (APCs) promotes antigen-specific T-cell proliferation and costimulates T-cell activation.
Materials and methods
Cells
Human peripheral blood mononuclear cells (PBMCs) were separated from peripheral blood of healthy donors by Ficoll gradient centrifugation. CD56+ CD3- NK cells were separated from PBMCs by cell sorting and cultured in medium containing recombinant interleukin 2 (IL-2), phytohemagglutinin (PHA), and irradiated feeder cells. CD47-deficient Jurkat T cells (Jurkat-CD470)28 were kindly provided by Bill Frazier (Washington University School of Medicine, Saint Louis, MO).
cDNAs and transfectants
Full-length SIRPα (NM_080792), SIRPβ1 (NM_006065), and SIRPβ2 (NM_018556) cDNAs were amplified by reverse transcriptase-polymerase chain reaction (RT-PCR), cloned into pCDNA3 (Invitrogen, Carlsbad, CA) or pMX,29 and transfected into the murine T-cell hybridoma BW (BW-SIRPα, BW-SIRPβ1, BW-SIRPβ2). Expression of SIRPs on stably transfected cells was assessed by flow cytometry using monoclonal antibody (mAb) 148.23
SIRP-IgG fusion proteins
We expressed the 2 membrane distal immunoglobulin domains (D1D2) of SIRPα, SIRPβ1, and SIRPβ2 as C-terminus fusion proteins with the Fc portion of human IgG. SIRP cDNA fragments were amplified by PCR with the primer pairs indicated in Table 1 and cloned into pFLAG-CMV1 (Sigma, St Louis, MO) in frame with a cDNA fragment encoding the Fc portion of human IgG fusion proteins.30 SIRP-D1D2-IgG chimeric cDNAs were transiently expressed in 293 cells using Lipofectamine (Invitrogen) and secreted SIRP-IgG fusion proteins were purified from culture supernatant on protein A (Pharmacia Amersham, Uppsala, Sweden).
Oligonucleotide primers for construction of SIRP-IgG fusion proteins
SIRPα-D1D2 forward | 5′-TAGTAGAAGCTTATGGAGCCCGCCGGCCCGGCC-3′ |
| SIRPα-D1D2 reverse | 5′-TAGTAGGTCGACAACTCGGATGGTCTCAGACAAGTTGG-3′ |
| SIRPβ1-D1D2 forward | 5′-TAGTAGAAGCTTAGACTCACAGGAGTGGCA-3′ |
| SIRPβ1-D1D2 reverse | 5′-TAGTAGGTCGACTGGAACTCGGATGGCCTCAG-3′ |
| SIRPβ2-D1D2 forward | 5′-TAGTAGAAGCTTCTCCAAAATGCCTGTCCCAGCCTCC-3′ |
| SIRPβ2-D1D2 reverse | 5′-TAGTAGGTCGACGGCCTCAGACAAGTTGGCAGTCCC-3′ |
| Human IgG-Fc forward | 5′-TAGTAGGTCGACAAAACTCACACATGCCC-3′ |
| Human IgG-Fc reverse | 5′-TAGTAGGGATCCTCATTTACCCGGAGACAGGG-3′ |
SIRPα-D1D2 forward | 5′-TAGTAGAAGCTTATGGAGCCCGCCGGCCCGGCC-3′ |
| SIRPα-D1D2 reverse | 5′-TAGTAGGTCGACAACTCGGATGGTCTCAGACAAGTTGG-3′ |
| SIRPβ1-D1D2 forward | 5′-TAGTAGAAGCTTAGACTCACAGGAGTGGCA-3′ |
| SIRPβ1-D1D2 reverse | 5′-TAGTAGGTCGACTGGAACTCGGATGGCCTCAG-3′ |
| SIRPβ2-D1D2 forward | 5′-TAGTAGAAGCTTCTCCAAAATGCCTGTCCCAGCCTCC-3′ |
| SIRPβ2-D1D2 reverse | 5′-TAGTAGGTCGACGGCCTCAGACAAGTTGGCAGTCCC-3′ |
| Human IgG-Fc forward | 5′-TAGTAGGTCGACAAAACTCACACATGCCC-3′ |
| Human IgG-Fc reverse | 5′-TAGTAGGGATCCTCATTTACCCGGAGACAGGG-3′ |
Restriction sites are underlined.
Antibodies
To obtain mAbs against SIRPβ1 (clone LSB1.50, mouse IgG1) and SIRPβ2 (clone LSB2.20, mouse IgG1), we immunized mice with SIRPβ1-D1D2-IgG and SIRPβ2-D1D2-IgG, respectively. We selected hybridomas that specifically stained BW-SIRPβ1 or BW-SIRPβ2. mAb 148 has been described.23 The mAbs against CD2, CD4, CD8, CD3, CD20, and CD56 are mouse IgG2a and IgG2b (Beckman-Coulter Immunotech, Fullerton, CA and BD Biosciences, Mountain View, CA). The mAbs against human CD47 included a mouse IgG1 (B6H12; BD Biosciences) and a mouse IgG2b (36-61.3) generated in our laboratory. Primary antibodies were detected with biotin- or phycoerythrin (PE)–labeled goat antimouse IgG1 or IgG2a/b (Southern Biotechnology, Birmingham, AL), followed by streptavidin conjugated with allophycocyanin (Molecular Probes, Eugene, OR).
Immunohistochemistry and immunofluorescence
Specimens from human tissues included reactive lymph nodes and thymuses removed for diagnostic purposes or during cardiac surgery. Cryostat sections of frozen specimens were air dried overnight at room temperature and fixed in acetone for 10 minutes before staining. SIRPβ2 was detected with mAb LSB2.20, followed by biotinylated anti-immunoglobulin multilinks secondary antibody (Biogenex, San Ramon, CA) and streptavidin-immunoperoxidase. In 2-color immunofluorescence, LSB2.20 was detected with fluorescein isothiocyanate (FITC)–conjugated isotype-specific antibody (Southern Biotechnology); CD3 (rabbit polyclonal; Dako, Glostrup, Denmark) and CD11c (LeuM5; BD Biosciences) were revealed with biotinylated secondary antibodies (Dako) followed by Texas red–conjugated streptavidin (Southern Biotechnology). Sections were examined with a fluorescence microscope Olympus BX60, equipped with a DP-70 Olympus digital camera (Olympus, Melville, NY). Images were acquired using analySIS Image Processing software (Soft Imaging System GmbH, Münster, Germany).
Immunoprecipitations
Cells were surface labeled with 1 mCi (37 MBq) 125I using the sulfosuccinimidyl-3-(4-hydroxyphenyl)propionate method. Labeled cells were lysed in 1% Triton X-100, 100 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, and 10 μg/mL leupeptin. After overnight preclearing with protein G-Sepharose, lysates were incubated with mAb LSB2.20, mAb148, or isotype-matched control mAb at 4°C for 4 hours, and immune complexes were precipitated by addition of protein G-Sepharose for 1.5 hours. Precipitates were washed 3 times with lysis buffer, followed by a final wash with 10 mM Tris-HCl, pH 7.4, 15 mM NaCl, and then resuspended in reducing sample buffer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed according to a standard procedure. Gels were dried and exposed to autoradiography film (Amersham Pharmacia Biotech, Piscataway, NJ) for 2 to 5 days.
Binding assay
SIRP-IgGs (100 μg/mL) were incubated with various cells for 10 minutes at 37°C and 30 minutes on ice in the presence or absence of antibodies against SIRPs and CD47. After one wash, binding of fusion proteins to cells was detected by flow cytometry using a biotinylated mouse antihuman IgG-Fc (BD Biosciences) followed by streptavidin conjugated with allophycocyanin (Molecular Probes).
Cell conjugations
BW-SIRPβ2 was labeled with carboxy-fluoresceindiacetate-succinimidylester (CFSE) (Molecular Probes). Jurkat and Jurkat-CD470 cells were stained with anti-CD45-allophycocyanin (Beckman-Coulter Immunotech) when conjugated with BW transfectants. Alternatively, Jurkat and Jurkat-CD470 were stained with Vibrant (Molecular Probes) and CFSE before conjugation. Various combinations of 2 of these cell types (2 × 105 of each) were mixed, spun down, and incubated at 37°C for 30 minutes in the presence or absence of antibodies against SIRPs, CD47, or CD18 (HB203, mouse IgG1; American Type Culture Collection [ATCC], Manassas, VA). Conjugates were gently resuspended in a small volume of medium for flow cytometric analysis on a FACSCalibur (BD Biosciences).
T-cell assays
The CD4+ SIRPβ2+ T-cell clone Vβ3Φ was generated from the peripheral blood of a healthy donor and selected for expression of T-cell receptor (TCR)–Vβ3. This clone (5 × 104) was incubated with irradiated B-lymphoblastoid cells RPMI 8866 (105; kindly provided by Bice Perussia, Philadelphia, PA) that had been pulsed for 2 hours with serial dilutions of Staphylococcus enterotoxin E (SEE). Anti-CD47 mAb (B6H12), anti-SIRPβ2 (LSB2.20), or control mouse IgG was added to T/B-cell cocultures as indicated. After 48 hours, T-cell proliferation was measured by standard 3H-thymidine incorporation assay. Mixed lymphocyte cultures (MLCs) were performed by incubating 105 PBMCs from a healthy donor (responder) with graded numbers of allogeneic immature DCs (stimulators). Culture supernatants were collected after 4 days and interferon γ (IFN-γ) was measured by cytometric bead array (BD Biosciences). T-cell proliferation was measured by standard 3H-thymidine incorporation assay. For costimulation assays, CD4+ T cells were purified from human peripheral blood by anti-CD4 magnetic microbeads (Miltenyi Biotec, Auburn, CA) and plated on serial dilution of mAb anti-CD3 (OKT3; ATCC) and 50 μg/mL mAbs against SIRPβ2, CD28 (Beckman-Coulter Immunotech), or control IgG1 (anti-CD19; Beckman-Coulter Immunotech). T-cell proliferation was measured after 72 hours by standard 3H-thymidine incorporation assay.
Results
SIRPβ2 is expressed on CD4+ T cells, CD8+ T cells, CD56bright NK cells, and all activated NK cells
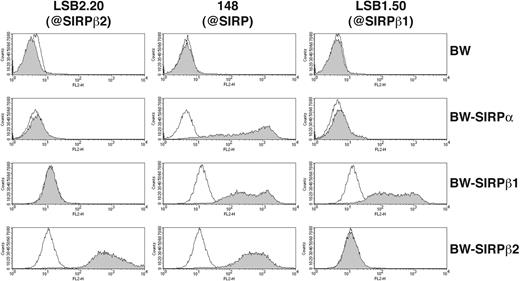
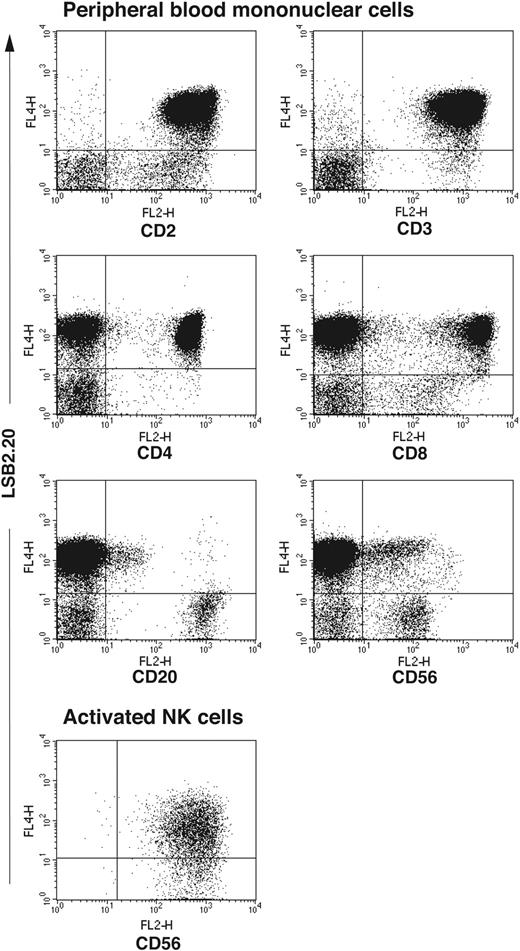
To obtain a SIRPβ2-specific mAb we immunized mice with a recombinant protein consisting of the 2 membrane-distal IgG domains of SIRPβ2 fused to the Fc portion of human IgG (SIRPβ2-D1D2-IgG). Hybridoma supernatants were selected for their ability to stain BW transfectants expressing full-length SIRPβ2 (BW-SIRPβ2). The mAb LSB2.20 stained BW-SIRPβ2, but not BW-SIRPβ1 or BW-SIRPα, demonstrating absolute specificity for SIRPβ2 (Figure 1). mAb 148, which recognizes SIRPα and SIRPβ1,23 also stained BW-SIRPβ2 and hence has a broad specificity for all SIRPs (Figure 1). Using mAb LSB2.20, we evaluated the cellular distribution of SIRPβ2 in PBMCs. SIRPβ2 was detected on all T cells, including CD4+ and CD8+ T cells, as well as a few CD20+ cells, which may correspond to a B-cell subset. SIRPβ2 was not expressed on NK cells directly isolated from blood, with the exception of CD56bright NK cells in most donors. However, it was up-regulated on all NK cells upon activation in vitro with IL-2, feeder cells, and PHA (Figure 2). SIRPβ2 was also expressed on several T and NK cell lines, including Jurkat and NK92 (data not shown). In contrast, monocytes, DCs, and granulocytes did not express SIRPβ2 (data not shown).
Specificity of anti-SIRP mAbs. Staining of BW and BW-SIRPβ2, BW-SIRPα, BW-SIRPβ1 transfectants (gray profiles) with mAbs LSB2.20 (anti-SIRPβ2, left column), 148 (anti-pan SIRP, middle column), and LSB1.50 (anti-SIRPβ1, right column). Primary antibodies were detected with a PE-conjugated goat antimouse antibody. Thin solid lines represent background staining with mouse control IgG and secondary antibody.
Specificity of anti-SIRP mAbs. Staining of BW and BW-SIRPβ2, BW-SIRPα, BW-SIRPβ1 transfectants (gray profiles) with mAbs LSB2.20 (anti-SIRPβ2, left column), 148 (anti-pan SIRP, middle column), and LSB1.50 (anti-SIRPβ1, right column). Primary antibodies were detected with a PE-conjugated goat antimouse antibody. Thin solid lines represent background staining with mouse control IgG and secondary antibody.
Expression of SIRPβ2 in peripheral blood and activated NK cells. SIRPβ2 is expressed on CD4+ T cells, CD8+ T cells, and a few CD20+ B cells. Notably, SIRPβ2 is expressed on a subset of CD56dim cells, which express CD3 and correspond to NKT cells (data not shown), but not on CD56dim resting NK cells. SIRPβ2 is also expressed on CD56bright cells, which do not express CD3 and correspond to an NK cell subset. PBMCs and activated NK cells were stained with antibodies against CD2, CD3, CD8, CD4, CD20, CD56 (all mouse IgG2a or IgG2b), and SIRPβ2 (mouse IgG1) followed by PE- and biotin-labeled goat antimouse IgG2a/b and IgG1 antibodies, respectively, and streptavidin-allophycocyanin. NK cells were activated in vitro with IL-2, PHA, and feeder cells and stained with anti-SIRPβ2 mAb.
Expression of SIRPβ2 in peripheral blood and activated NK cells. SIRPβ2 is expressed on CD4+ T cells, CD8+ T cells, and a few CD20+ B cells. Notably, SIRPβ2 is expressed on a subset of CD56dim cells, which express CD3 and correspond to NKT cells (data not shown), but not on CD56dim resting NK cells. SIRPβ2 is also expressed on CD56bright cells, which do not express CD3 and correspond to an NK cell subset. PBMCs and activated NK cells were stained with antibodies against CD2, CD3, CD8, CD4, CD20, CD56 (all mouse IgG2a or IgG2b), and SIRPβ2 (mouse IgG1) followed by PE- and biotin-labeled goat antimouse IgG2a/b and IgG1 antibodies, respectively, and streptavidin-allophycocyanin. NK cells were activated in vitro with IL-2, PHA, and feeder cells and stained with anti-SIRPβ2 mAb.
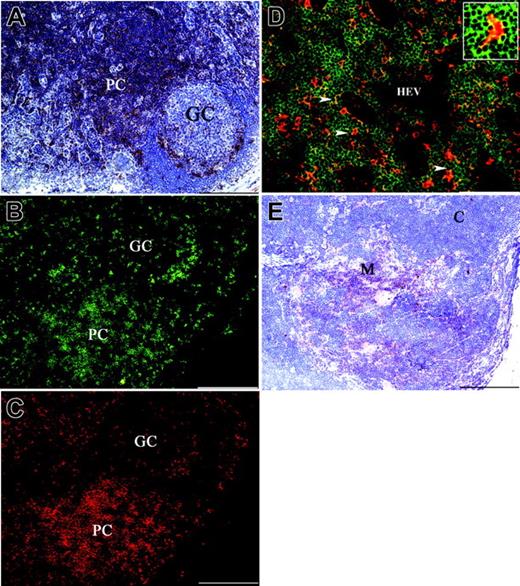
Analysis of SIRPβ2 expression in human lymph nodes revealed that SIRPβ2 is mainly present in the paracortical T-cell area (Figure 3A), whereas only a few SIRPβ2+ cells were observed in the mantle and germinal center of B-cell follicles (Figure 3A). Two-color immunofluorescence analysis showed that these SIRPβ2+ cells coexpress CD3 and therefore correspond to T cells (Figure 3B-C). Examination of the paracortical area at high magnification revealed clustering of SIRPβ2+ T cells around interdigitating DCs, which did not express SIRPβ2 (Figure 3D). In the human thymus SIRPβ2+ lymphocytes were primarily located in the medulla, whereas no expression was detected on the majority of cortical thymocytes (Figure 3E). Thus, SIRPβ2 is selectively expressed on mature T lymphocytes that have undergone thymic selection.
Expression of SIRPβ2 in lymph nodes and thymus. (A) SIRPβ2 is mainly expressed in the paracortical area (PC) of lymph nodes with only sparse positive cells in the mantle and in the germinal center (GC) of B-cell follicles. (B-C) Two-color immunofluorescence of lymph nodes with mAbs anti-SIRPβ2 (B) (green) and anti-CD3 (red) (C). The large majority of lymph node SIRPβ2+ cells, including those found in the germinal center, coexpress CD3. (D) Two-color immunofluorescence of a lymph node shows diffuse expression of SIRPβ2 in lymphocytes of the T-cell area (green), clustered around scattered CD11c+ DCs (red). Cell-cell interactions between SIRPβ2+ lymphocytes and CD11c+ DCs appear as yellow dots (arrowheads and insert). HEV indicates high endothelial venule. (E) SIRPβ2+ lymphocytes are numerous in the thymic medulla (M) and scattered in the cortex (C). SIRPβ2 was detected by indirect immunoperoxidase technique and counterstaining with Meyer hematoxylin (A,E). Scale bars are 200 μm (A-C,E) and 100 μm (D). Magnification is 100 × (A-C,E) and 200 × (D).
Expression of SIRPβ2 in lymph nodes and thymus. (A) SIRPβ2 is mainly expressed in the paracortical area (PC) of lymph nodes with only sparse positive cells in the mantle and in the germinal center (GC) of B-cell follicles. (B-C) Two-color immunofluorescence of lymph nodes with mAbs anti-SIRPβ2 (B) (green) and anti-CD3 (red) (C). The large majority of lymph node SIRPβ2+ cells, including those found in the germinal center, coexpress CD3. (D) Two-color immunofluorescence of a lymph node shows diffuse expression of SIRPβ2 in lymphocytes of the T-cell area (green), clustered around scattered CD11c+ DCs (red). Cell-cell interactions between SIRPβ2+ lymphocytes and CD11c+ DCs appear as yellow dots (arrowheads and insert). HEV indicates high endothelial venule. (E) SIRPβ2+ lymphocytes are numerous in the thymic medulla (M) and scattered in the cortex (C). SIRPβ2 was detected by indirect immunoperoxidase technique and counterstaining with Meyer hematoxylin (A,E). Scale bars are 200 μm (A-C,E) and 100 μm (D). Magnification is 100 × (A-C,E) and 200 × (D).
We have previously shown that mAb 148 detects SIRPα and SIRPβ1 on monocytes, granulocytes, and DCs.23 Because mAb 148 recognizes SIRPβ2 on transfected cells (Figure 1), one would expect this mAb to stain peripheral T cells, as does mAb LSB2.20. However, we found that mAb 148 does stain monocytes, granulocytes, and DCs, but not T cells or Jurkat cells.23 This unexpected discrepancy between the staining patterns of mAbs LSB2.20 and 148 suggests that the SIRPβ2 protein expressed on T cells and activated NK cells may differ significantly from that expressed on BW transfectants, possibly due to cell-specific posttranslational modifications. On the other hand, SIRPβ2 may be expressed as an alternatively spliced form that lacks one of the predicted domains of the protein. Accordingly, analysis of SIRPβ2 transcripts by RT-PCR revealed that T-cell mRNA includes not only a SIRPβ2 full-length transcript, but also 2 alternatively spliced forms that lack either one or 2 membrane-proximal Ig domains (GenBank accession nos. AY748247, AY748248, and NM_080816).
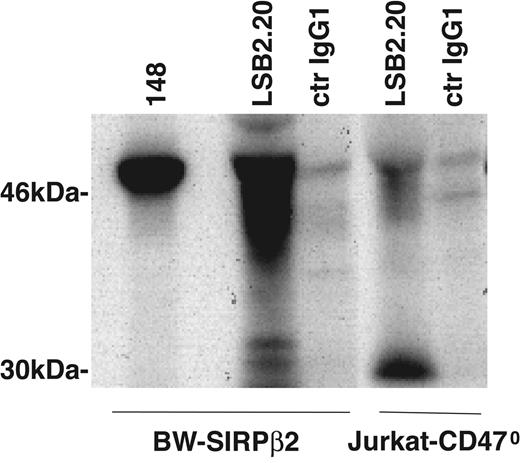
To investigate if mAbs 148 and LSB2.20 detect different isoforms of SIRPβ2, we compared 148 and LSB2.20 immunoprecipitates from BW cells transfected with SIRPβ2. Moreover, we analyzed the biochemical characteristics of SIRPβ2 in a mutated Jurkat cell line, which lacks CD47 (Jurkat-CD470).28 This T-cell line expresses high levels of SIRPβ2, which are detected by LSB2.20 but not 148. In LSB2.20 immunoprecipitates, SIRPβ2 appeared as a broad cluster of approximately 45- to 50-kDa proteins, most likely reflecting heterogeneous glycosylation (Figure 4). Moreover, LSB2.20 immunoprecipitates included a sharp approximately 30-kDa protein, which may correspond to the alternatively spliced form of SIRPβ2 that lacks the membrane-proximal Ig domain and contains only one site for N-linked glycosylation (AY748247 and AY748248). In contrast, the mAb 148 only detected a major about 50-kDa protein (Figure 4). Thus, T cells express isoforms of SIRPβ2 that are preferentially recognized by the SIRPβ2-specific mAb LSB2.20 rather than the anti-SIRP mAb 148, which may explain why SIRPs were not previously detected on T cells and NK cells.
Differential biochemical features of SIRPβ2 detected by mAbs LSB2.20 and 148. LSB2.20 immunoprecipitates from BW-SIRPβ2 transfectants and Jurkat-CD470 reveal a broad cluster of approximately 45- to 50-kDa proteins, which may correspond to differentially glycosylated isoforms of SIRPβ2. Moreover, LSB2.20 immunoprecipitates include a 30-kDa protein, which may correspond to the alternatively spliced form of SIRPβ2, which lacks the membrane-proximal immunoglobulin domain and contains only one site for N-linked glycosylation (NCBI accession nos. AY748247 and AY748248). In contrast, the 148 immunoprecipitate from BW-SIRPβ2 cells reveals only a 50-kDa protein.
Differential biochemical features of SIRPβ2 detected by mAbs LSB2.20 and 148. LSB2.20 immunoprecipitates from BW-SIRPβ2 transfectants and Jurkat-CD470 reveal a broad cluster of approximately 45- to 50-kDa proteins, which may correspond to differentially glycosylated isoforms of SIRPβ2. Moreover, LSB2.20 immunoprecipitates include a 30-kDa protein, which may correspond to the alternatively spliced form of SIRPβ2, which lacks the membrane-proximal immunoglobulin domain and contains only one site for N-linked glycosylation (NCBI accession nos. AY748247 and AY748248). In contrast, the 148 immunoprecipitate from BW-SIRPβ2 cells reveals only a 50-kDa protein.
SIRPβ2 is a receptor for CD47
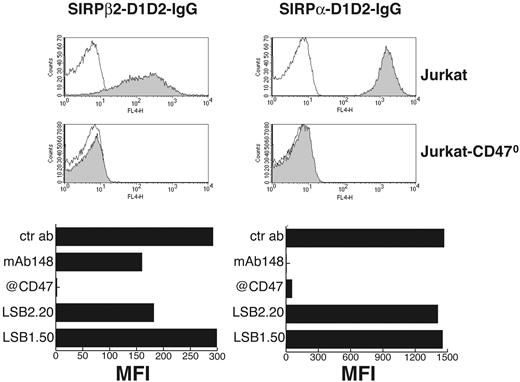
To investigate whether SIRPβ2 expressed on T cells and activated NK cells recognizes CD47 we tested the ability of a SIRPβ2-D1D2-IgG fusion protein to bind the T-cell line Jurkat, which expresses CD47, and Jurkat-CD470 by flow cytometry. SIRPβ2-D1D2-IgG bound Jurkat but not Jurkat-CD470; the binding was totally blocked by mAb B6H12 and 36-61.3, which recognize CD47, and partially inhibited by mAbs 148 and LSB2.20, which recognize SIRPβ2, corroborating the specificity of binding (Figure 5). Importantly, binding of SIRPβ2-D1D2-IgG to Jurkat consistently yielded a lower median fluorescence intensity than did binding of SIRPα-D1D2-IgG in flow cytometry, suggesting that the affinity of SIRPβ2 for CD47 is lower that that of SIRPα (Figure 5). Of note, mAb 148 completely abrogated binding of SIRPα-D1D2-IgG to Jurkat but only partially reduced that of SIRPβ2-D1D2-IgG, confirming its preferential recognition of SIRPα versus SIRPβ2. These results demonstrate that SIRPβ2 is a receptor for CD47, although it probably binds with lower affinity than SIRPα.
Soluble SIRPβ2 specifically binds cells expressing CD47. Gray profiles represent binding of SIRPβ2-D1D2-IgG (left column) or SIRPα-D1D2-IgG (right column) to Jurkat (top row) and Jurkat-CD470 (middle row). Thin solid lines indicate background staining of Jurkat and Jurkat-CD470. (Bottom row) Bar graphs represent binding of SIRPβ2-D1D2-IgG (left panel) or SIRPα-D1D2-IgG (right panel) to Jurkat in the presence of mAbs against CD47 (maximal inhibition), SIRPα (148), SIRPβ2 (LSB2.20; partial inhibition), SIRPβ1 (LSB1.50), and control mAb (no inhibition). Bar graphs presented here represent one of 4 independent experiments with similar results. MFI indicates mean fluorescence intensity.
Soluble SIRPβ2 specifically binds cells expressing CD47. Gray profiles represent binding of SIRPβ2-D1D2-IgG (left column) or SIRPα-D1D2-IgG (right column) to Jurkat (top row) and Jurkat-CD470 (middle row). Thin solid lines indicate background staining of Jurkat and Jurkat-CD470. (Bottom row) Bar graphs represent binding of SIRPβ2-D1D2-IgG (left panel) or SIRPα-D1D2-IgG (right panel) to Jurkat in the presence of mAbs against CD47 (maximal inhibition), SIRPα (148), SIRPβ2 (LSB2.20; partial inhibition), SIRPβ1 (LSB1.50), and control mAb (no inhibition). Bar graphs presented here represent one of 4 independent experiments with similar results. MFI indicates mean fluorescence intensity.
SIRPβ2-CD47 interaction mediates cell-cell adhesion
Because SIRPβ2 lacks a cytoplasmic domain with known signaling motifs or a transmembrane residue allowing association with DNAX activation protein 12 (DAP12)/killer cell activating receptor-associated protein (KARAP), its involvement in inhibitory or activating signaling is unlikely. Accordingly, we observed that antibodies against SIRPβ2 alone do not activate or inhibit NK cell–mediated lysis of Fc receptor–positive target cells in redirected cytotoxicity assays (data not shown). Given this, we hypothesized that SIRPβ2 may be involved in cell-cell adhesion rather than inhibitory or activating signaling.
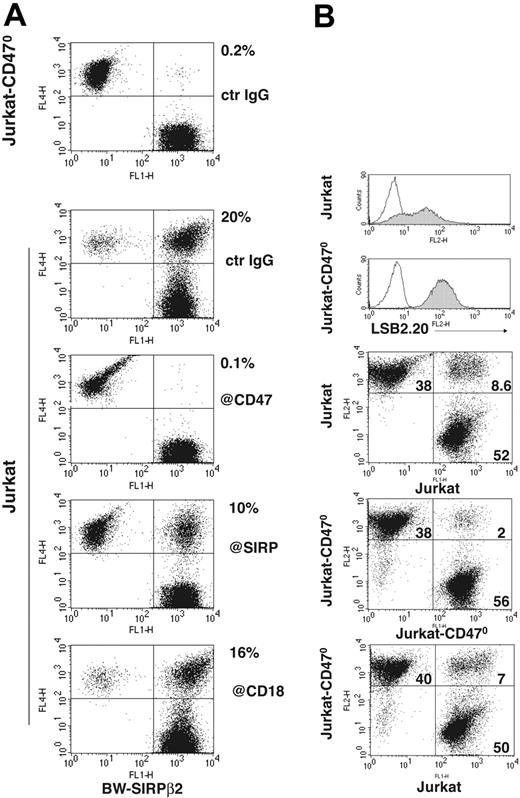
To test this, we mixed BW cells transfected with SIRPβ2 (BW-SIRPβ2) with Jurkat or Jurkat-CD470. After 30 minutes of incubation at 37°C we measured formation of conjugates by 2-color flow cytometry. Under these conditions, BW-SIRPβ2 made abundant conjugates with Jurkat but not with Jurkat-CD470 (Figure 6A). Conjugate formation was partially blocked by anti-SIRP and anti-CD47 antibodies, confirming the specificity of the interaction. In contrast, antibodies against CD18 (Figure 6A) or CD11a (data not shown) did not significantly block cell conjugation, suggesting that SIRPβ2-CD47 interaction mediates cell conjugation by a mechanism that is independent of leukocyte function–associated molecule-1 (LFA-1). To corroborate that SIRPβ2-CD47 interaction mediates cell-cell adhesion, we compared the ability of Jurkat, which expresses both CD47 and SIRPβ2, and JurkatCD470, which expresses only SIRPβ2, to form conjugates either alone or in combination with each other. Jurkat-CD470 formed fewer conjugates with itself than when mixed with Jurkat, and fewer than Jurkat formed with itself (Figure 6B). We conclude that SIRPβ2-CD47 interactions significantly contribute to adhesion of T cells to cells expressing CD47.
Conjugate formation between cells expressing SIRPβ2 and CD47. (A) BW-SIRPβ2 transfectants make conjugates with Jurkat but not with Jurkat-CD470. Conjugation is partially blocked by anti-CD47 and anti-SIRP antibodies, whereas no significant inhibition is observed with an antibody against CD18 (β2 integrin). Percentages of conjugates are indicated next to the upper right quadrants. BW-SIRPβ2 was labeled with CFSE. Jurkat and Jurkat-CD470 were stained with anti-CD45-allophycocyanin. (B) The frequency of conjugation between Jurkat alone (top panel), Jurkat and JurkatCD470 (bottom panel), and Jurkat-CD470 alone (middle panel). Jurkat-CD470 forms fewer conjugates with itself than when mixed with Jurkat, and fewer than Jurkat forms with itself. Jurkat and Jurkat-CD470 were stained with Vibrant and CFSE before conjugation. Notably, Jurkat-CD470 cells express higher levels of SIRPβ2 than Jurkat cells (top histograms, gray profiles). This high expression of SIRPβ2 may allow Jurkat-CD470 cells to form as many conjugates with Jurkat cells as Jurkat forms with itself.
Conjugate formation between cells expressing SIRPβ2 and CD47. (A) BW-SIRPβ2 transfectants make conjugates with Jurkat but not with Jurkat-CD470. Conjugation is partially blocked by anti-CD47 and anti-SIRP antibodies, whereas no significant inhibition is observed with an antibody against CD18 (β2 integrin). Percentages of conjugates are indicated next to the upper right quadrants. BW-SIRPβ2 was labeled with CFSE. Jurkat and Jurkat-CD470 were stained with anti-CD45-allophycocyanin. (B) The frequency of conjugation between Jurkat alone (top panel), Jurkat and JurkatCD470 (bottom panel), and Jurkat-CD470 alone (middle panel). Jurkat-CD470 forms fewer conjugates with itself than when mixed with Jurkat, and fewer than Jurkat forms with itself. Jurkat and Jurkat-CD470 were stained with Vibrant and CFSE before conjugation. Notably, Jurkat-CD470 cells express higher levels of SIRPβ2 than Jurkat cells (top histograms, gray profiles). This high expression of SIRPβ2 may allow Jurkat-CD470 cells to form as many conjugates with Jurkat cells as Jurkat forms with itself.
SIRPβ2-CD47 interaction enhances superantigen-dependent T cell-mediated proliferation and costimulates T-cell activation
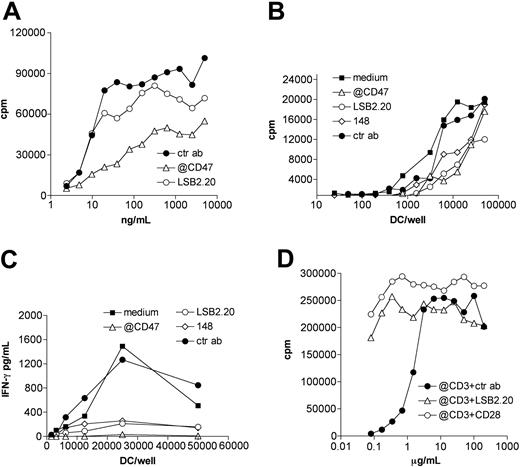
To determine whether adhesion mediated by SIRPβ2-CD47 binding supports functional activation of T cells, we investigated its impact on superantigen-dependent T-cell proliferation. CD4+ T cells that express Vβ3 actively proliferate in the presence of APCs pulsed with SEE. SEE binds major histocompatibility complex (MHC) class II on APCs and Vβ3 on T cells. Thus, we selected a T-cell clone that expresses Vβ3 and SIRPβ2 (Vβ3Φ). To distinguish the function of SIRPβ2 on T cells, we purposely chose APCs that express MHC class II and CD47 but not SIRPβ2 or other SIRPs, specifically the B-cell line RPMI 8866. In this experimental system, SIRBβ2-CD47 interaction is unidirectional, not bidirectional. Vβ3Φ cells efficiently proliferated in the presence of RPMI 8866 pulsed with different concentrations of SEE (Figure 7A). T-cell proliferation was strongly inhibited by the anti-CD47 antibody and partially inhibited by the anti-SIRPβ2 mAb, consistent with the established abilities of anti-CD47 and anti-SIRPβ2 antibodies to block SIRPβ2-CD47 interactions (Figures 5 and 6A). Similarly, mAbs against SIRPβ2 and CD47 inhibited T-cell proliferation and T-cell secretion of IFN-γ triggered by allogeneic immature DCs in mixed lymphocyte reactions (Figure 7B-C), indicating that SIRPβ2-CD47 interaction is important in promoting not only T-cell proliferation but also cytokine secretion.
SIRPβ2-CD47 interaction enhances superantigen-mediated T-cell proliferation and costimulates T-cell activation. (A) The CD4+ SIRPβ2+ Vβ3+ T-cell clone Vβ3Φ was incubated with irradiated B lymphoblastoid cells RPMI 8866 that had been pulsed with serial dilutions of SEE (ng/mL). Anti-CD47 (▵), anti-SIRPβ2 (○), or control mouse antibodies (•) were added to T/B-cell cocultures as indicated. T-cell proliferation was measured by 3H-thymidine incorporation assay. (B-C) Blockade of SIRPβ2-CD47 interaction with mAbs partially inhibits T-cell proliferation and IFN-γ production triggered by allogeneic immature DCs in MLCs. Symbols represent same as in panel A; in addition, ▪ indicates medium, and ⋄, 148. (D) CD4+ T cells purified from peripheral blood were plated on serial dilution of anti-CD3 antibody (μg/mL) in the presence of fixed amounts of mAbs against SIRPβ2 (▵), CD28 (○), or control IgG (•). T-cell proliferation was measured after 72 hours as described.
SIRPβ2-CD47 interaction enhances superantigen-mediated T-cell proliferation and costimulates T-cell activation. (A) The CD4+ SIRPβ2+ Vβ3+ T-cell clone Vβ3Φ was incubated with irradiated B lymphoblastoid cells RPMI 8866 that had been pulsed with serial dilutions of SEE (ng/mL). Anti-CD47 (▵), anti-SIRPβ2 (○), or control mouse antibodies (•) were added to T/B-cell cocultures as indicated. T-cell proliferation was measured by 3H-thymidine incorporation assay. (B-C) Blockade of SIRPβ2-CD47 interaction with mAbs partially inhibits T-cell proliferation and IFN-γ production triggered by allogeneic immature DCs in MLCs. Symbols represent same as in panel A; in addition, ▪ indicates medium, and ⋄, 148. (D) CD4+ T cells purified from peripheral blood were plated on serial dilution of anti-CD3 antibody (μg/mL) in the presence of fixed amounts of mAbs against SIRPβ2 (▵), CD28 (○), or control IgG (•). T-cell proliferation was measured after 72 hours as described.
To further investigate the T-cell stimulatory function of SIRPβ2, we determined whether engagement of SIRPβ2 can enhance activation of CD4+ T cells in the presence of serial dilution of a TCR ligand. The anti-SIRPβ2 mAb enhanced the proliferation of peripheral blood CD4+ T cells in the presence of suboptimal concentration of anti-CD3 (Figure 7D). Remarkably, ligation of SIRPβ2 was almost as effective as the engagement of CD28 in costimulating T-cell proliferation. Thus, we conclude that SIRPβ2-CD47 interaction enhances superantigen-dependent T-cell proliferation and has a critical role as an accessory costimulatory molecule on T cells.
Discussion
Here we demonstrate that SIRPβ2 is a unique member of the SIRP receptor family; it is the only SIRP that has been detected on T cells and activated NK cells. Despite considerable homology among the SIRPs, previously established anti-SIRP antibodies failed to detect SIRPβ2 on T cells and NK cells. Accordingly, the newly generated mAb LSB2.20 specific for SIRPβ2 preferentially detected posttranslational modifications or alternative spliced forms of SIRPβ2 that may occur in T cells and NK cells, creating unique epitopes undetected by previously established anti-SIRP antibodies.
Remarkably, SIRPβ2 can bind CD47, providing T cells and NK cells with a cell surface molecule capable of interacting with CD47. Because SIRPβ2 lacks a cytoplasmic domain with known signaling motifs or a transmembrane residue allowing association with DAP12/KARAP, SIRPβ2 does not deliver activating or inhibitory signals on its own. SIRPβ2-CD47 interaction mediates strong cell-cell adhesion and supports T cell-APC contact, enhancing antigen presentation and consequent T-cell proliferation and cytokine secretion. In contrast, we did not detect a significant effect of SIRPβ2-CD47 interaction on CD8 T cell– or NK cell–mediated cytotoxicity (data not shown). This discrepancy may reflect the differential impact of SIRPβ2 on these disparate functions. T-cell proliferation requires sustained activation of T cells,31 and therefore SIRPβ2-CD47 interactions may significantly contribute to this process by stabilizing T cell-APC binding. In contrast, SIRPβ2-CD47 adhesion may be dispensable for the more transient interactions that mediate T cell– and NK cell–mediated cytotoxicity.32 Further insight might be provided by investigating the behavior of SIRPβ2 in the formation and stabilization of T-cell synapsis.33
SIRPβ2 enhanced T-cell proliferation induced by suboptimal concentration of T-cell receptor ligand. Whether SIRPβ2 acts as a costimulator similar to CD28 or synergizes with TCR signaling by other mechanisms is presently unknown. Interestingly, it has been shown that engagement of CD47 with some antibodies also results in augmentation of T-cell activation34,35 and that the costimulatory function of CD47 depends on its capacity to induce cell spreading.28 Thus, SIRPβ2 may facilitate T-cell activation by a similar mechanism. It is also possible that SIRPβ2-CD47 interaction promotes other functions of T cells and NK cells dependent on cell-cell adhesion, such as attachment to endothelial cells and transmigration into lymph nodes or peripheral tissues, as previously reported for SIRPα-CD47.20-22
The characterization of SIRPβ2 in this study provides strong evidence for structural and functional diversity of the SIRP receptors. To date, 3 SIRPs have been characterized, each with a different affinity for CD47 and distinct signaling properties. Whereas SIRPα4-7 and SIRPβ2 (this study) bind CD47, a soluble form of SIRPβ1 encompassing the 2-membrane distal IgG domains does not (data not shown). We showed that the binding of SIRPα to CD47 is stronger than that of SIRPβ2. These differences in specificity may depend on the diversity of SIRP extracellular domains. Moreover, 15 distinct SIRP cDNAs have been reported in the literature2 and 2 additional SIRP loci, called protein tyrosine phosphatase nonreceptor type substrate 1-like 2 (PTPNS1L2) and PTPNS1L3, have been annotated in the National Center for Biotechnology Information (NCBI) database.39 Thus, SIRP family diversity may be even broader than presently known, due to the additional SIRP genes and, possibly, polymorphisms of the SIRPα, SIRPβ1, and SIRPβ2 genes as well. Similar mechanisms of diversification have been observed for other immune gene loci, particularly those encoding KIRs, LILRs, and Ly49s.36,37 What selective pressure is responsible for evolution and diversification of SIRP molecules? One clue to this question is provided by the observation that poxviruses encode homologues of CD47.38 Thus, it is possible that the SIRP diversity reflects a sort of arms race between the host and poxviruses, in which viruses try to exploit or disrupt endogenous SIRP-CD47 interactions to elude immune responses, whereas the host counteracts this viral strategy by changing the specificity and function of endogenous SIRPs.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-07-2823.
Supported by the National Institutes of Health grant U54AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE). L.P. was partly supported by IRCCS Ospedale Maggiore Policlinico di Milano, Milan, Italy.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Susan Gilfillan and Bill Frazier for critically reading the manuscript; Francesca Gentili (supported by Fondazione Beretta, Brescia, Italy) for performing immunohistochemical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal