Comment on Tanaka et al, page 2324
Signal transduction pathways involving KitWT have been extensively studied, whereas there is limited information regarding mutant Kit signaling. In this issue of Blood, Tanaka and colleagues describe the constitutive activation of NF-κBin this regard.
Human Kit is a type III receptor tyrosine kinase, the protein product of the protooncogene c-kit, which is localized to chromosome 4q12. Stem cell factor (SCF) and wild-type Kit (KitWT) are considered the key ligand-receptor pair for the growth and survival of normal mast cells. Kit is normally activated by autophosphorylation brought about by ligand-induced dimerization of Kit monomers. Activation of Kit in neoplastic mast cells bypasses SCF through gain-of-function mutations that are usually clustered around the kinase domain (eg, KitAsp816Val) but which also occur at the juxtamembrane region (eg, KitVal560Gly) of the receptor. Post-Kit signal transmission in the imatinib mesylate (STI571)–resistant mutant receptor (KitAsp816Val) has been shown to require activation of phosphatidylinositol 3 (PI3) kinase and, further downstream, that of the serine/threonine kinases Jnk1 and Jnk2 and signal transducer and activator of transcription 3 (STAT3) but not the mitogen-activated protein (MAP) kinase family members Erk1 and Erk2.1,2 PI3 kinase and STAT3 are also constitutively activated in the murine homologue of the human catalytic domain mutant receptor (KitAsp814Tyr/Val) where an ubiquitin-mediated degradation of the tyrosine phosphatase SHP-1 as well as activation of the δ isoform of protein kinase C (PKCδ) have also been reported.3-6
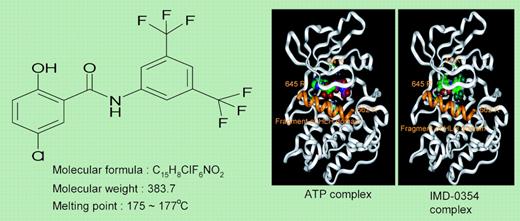
In this issue of Blood, Tanaka and colleagues provide additional information regarding mutant Kit signaling by demonstrating a cell-cycle progression–relevant (ie, associated with cyclin D3 expression) constitutive activation of nuclear factor kappa B (NF-κB), which was accompanied by phosphorylation of its inhibitor (IκB), in the context of both KitAsp816Val and KitVal560Gly. Furthermore, consistent with the aforementioned observations, inhibition of either the PI3 kinase or PKC but not the MAP kinase pathway impaired NF-κB activation as well as cell proliferation by mutant Kit. Taken together, this suggested IκB phosphorylation as the essential link between upstream signaling events and NF-κB activation associated with mutant Kit. Consistent with this possibility, NF-κB activation as well as cell proliferation, in the context of both juxtamembrane and catalytic domain Kit mutants, were inhibited by an IκB kinase inhibitor (IMD-0354; N-(3,5-Bis-trifluoromethyl-phenyl)-5-chloro-2-hydroxy-benzamide; see figure). It was interesting to note that the drug inhibited NF-κB activation but not cell proliferation in normal human mast cells, which suggested a limited degree of selectivity.
Inhibitory effect of IMD-0354 on cell proliferation of HMC-1 cells. See the complete figure in the article beginning on page 2324.
Inhibitory effect of IMD-0354 on cell proliferation of HMC-1 cells. See the complete figure in the article beginning on page 2324.
The above set of observations allows one to envision different molecular levels of drug intervention in systemic mastocytosis (SM). At the receptor level, novel dual kinase inhibitors currently being evaluated in clinical trials have already been shown to inhibit KitAsp816Val in vitro. Alternatively, proteasomal degradation of mutant Kit might be enhanced by derivatives of geldanamycin, which bind heat shock protein 90 and thus deprive mutant Kit of its chaperone. Similarly, drugs that target the PKC and PI3 kinase pathways (eg, PKC412, mammalian target of rapamycin inhibitors) are also being considered for pilot treatment studies in SM. Finally, as suggested by the paper from Tanaka and colleagues, IκB kinase provides yet another target for the development of drugs against Kit-driven malignancies. Furthermore, combination treatment approaches that include drugs directed at downstream effectors of mutant Kit might overcome resistance emerging from monotherapy with kinase inhibitors. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal