Abstract
Constitutive phosphorylation of c-kit tyrosine kinase is the major cause of factor-independent proliferation of mast cells. Recently available tyrosine kinase inhibitors have shown marked activity against mast cell lines that carry wild-type c-kit, and some, but not others, carry mutant c-kit. Here we clearly demonstrated that a novel NF-κB inhibitor, IMD-0354, restrained factor-independent proliferation of mast cells with c-kit mutations but not of normal mast cells. In HMC-1 cells with the Asp816Val and Val560Gly mutations, we found that NF-κB was constitutively activated without exogenous stimulation. When the DNA-binding activity of NF-κB was inhibited by treatment with IMD-0354, cell proliferation was completely suppressed. We detected the expression of cyclin D2, D3, and E in HMC-1 cells and observed that cyclin D3 expression was dramatically decreased by treatment with IMD-0354. Abolishing protein kinase C or phosphatidylinositol 3 kinase pathways also inhibited NF-κB translocation to the nucleus, indicating the involvement of these signaling cascades in NF-κB activation in HMC-1 cells. Our findings indicated that autophosphorylated c-kit receptors induced NF-κB activation, resulting in the up-regulation of cyclin D3 expression and cell cycle progression. The observations from the current study suggest a therapeutic potential, in systemic mastocytosis, for compounds that interfere with NF-κB signaling.
Introduction
Gain-of-function mutations in the c-kit receptor induce factor-independent proliferation of mast cells, thereby resulting in neoplastic transformation.1 Various c-kit receptor mutations have been identified in different mast cell lines that have an ability to proliferate in the absence of any growth factor.2-6 HMC-1 cells, which were derived from human mast cell leukemia, have 2 point mutations in the intracellular juxtamembrane domain (Val560Gly) and in the catalytic domain (Asp816Val) of the c-kit receptor.2,6 The former mutation was also found in the c-kit receptor in cells from gastrointestinal stromal tumor (GIST), and the latter is known to exist most commonly in cells from human mastocytosis.7,8 Because of these mutations, the tyrosine kinase of the c-kit receptor is automatically phosphorylated, and the related growth signal is activated. Several signaling molecules have been identified as candidates for transducing c-kit receptor signals, including mitogen-activated protein (MAP) kinase, phosphatidylinositol 3 (PI3) kinase, protein kinase C (PKC), and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways.9-11 Recently available tyrosine kinase inhibitors have been applied to c-kit-dependent diseases, including GIST and mastocytosis; however, therapeutic effects have been limited in the treatment of mastocytosis in contrast to the great success in the treatment of GIST.12-14 Mutational analysis has revealed some variants of the c-kit gene in patients with mastocytosis. Imatinib mesylate (STI571) was shown to be effective against mast cell diseases involving wild-type c-kit and mutants with Val560Gly or Phe522Cys substitutions.14,15 However, STI571 was not effective against aggressive systemic mastocytosis involving an Asp816Val mutation in the c-kit receptor.15
Homeostatic or nonhomeostatic cell renewal is controlled through cell cycle progression. Entry into the S phase normally relies on mitogenic stimulation, involving growth factors, and is regulated by cell cycle regulatory molecules, such as cyclins, cyclin-dependent kinases (CDKs), and a retinoblastoma protein (pRb).16,17 D-type cyclins, namely cyclin D1,D2, and D3, are growth factor sensitive, and their expression is facilitated in response to growth factor stimulation. Byl binding with CDK4 and CDK6, D-type cyclins control a kinase activity of CDK and phosphorylate substrates that are required for G1 progression and S phase entry.18-20 One of their important targets is pRb.18-20 We have already reported that the expression of cyclin D3 and the phosphorylation of pRb are accelerated in mouse bone marrow-derived cultured mast cells (BMCMCs) incubated with stem cell factor (SCF), indicating that these regulatory molecules are essential for cell cycle progression in mast cell proliferation.21
NF-κB, a dimeric transcription factor of the rel family, exists as an inactive form in the cytoplasm by binding its endogenous inhibitory molecule IκB.22-24 Exposure of cells to bacterial lipopolysaccharide (LPS), phorbol esters, or certain inflammatory cytokines triggers the degradation of IκB by activating IκB kinases (IKKs), such as IKKα and IKKβ, that are responsible for IκB phosphorylation.22-24 Phosphorylated IκB rapidly undergoes ubiquitination and proteolysis by the 26S proteasome.22-24 Released NF-κB subsequently translocates to the nucleus, where NF-κB expresses its function. Recent studies have indicated that NF-κB participates not only in immune and inflammatory processes but also in cell proliferation by controlling D-type cyclins.25-29 Although NF-κB directly up-regulates the transcription of D-type cyclins, with or without mitogenic stimulation of growth factors in some cell types,25-29 the exact role of NF-κB in mast cell proliferation is unknown. Here we clearly demonstrated that a selective IKKβ inhibitor, IMD-0354, inhibited NF-κB activity in HMC-1 cells, resulting in complete repression of growth factor-independent proliferation of mast cells. Inhibition of NF-κB activity decreased the expression of cyclin D3 and the phosphorylation of pRb, leading to cell cycle arrest and apoptosis. Interruption of c-kit receptor signals abolished the DNA-binding activity of NF-κB, indicating that NF-κB may play a critical role in the neoplastic proliferation of mast cells. In contrast, cord blood-derived human cultured mast cells (CBhCMCs) were resistant to IMD-0354. Thus, suppressing NF-κB activity may control neoplastic mast cell proliferation with no or few effects on normal mast cells.
Materials and methods
Cell culture
HMC-1 cells used in this study have the 2 point mutations in the intracellular juxtamembrane domain (Val560Gly) and in the catalytic domain (Asp816Val) of the c-kit receptor (a generous gift from Dr Kitamura, Department of Pathology, Osaka University School of Medicine, Japan).2,6 HMC-1 cells were cultured in α-minimum essential medium (α-MEM; Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Filtron, Brooklyn, Australia), 100 U/mL penicillin, and 100 ng/mL streptomycin.30 IC-2 cells with the wild-type c-kit receptor, with the Asp814Val (V814) mutation in the phosphotransferase domain of the c-kit receptor, or with the Val559Gly (G559) mutation in the juxtamembrane domain of the c-kit receptor were prepared as described previously.30-32 IC-2V814 cells and IC-2G559 cells were cultured without any factors in G418 containing α-MEM with 10% FBS and antibiotics. IC-2WT cells were cultured in G418 containing α-MEM with 10% FBS, antibiotics, and 100 ng/mL recombinant rat SCF (kindly provided by Amgen, Thousand Oaks, CA) as a growth factor. CBhCMCs were derived from cord blood collected from normal full-term deliveries. Mononuclear cells were isolated on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and were cultured in AIM-V medium (Invitrogen, Carlsbad, CA) with 5% fetal calf serum (FCS), 100 ng/mL recombinant human SCF (kindly provided by Amgen), and 50 ng/mL interleukin-6 (IL-6; kindly provided by Kirin Brewery, Maebashi, Japan), as described previously.33 All human subjects in this study provided written informed consent, and the Human Studies Internal Review Board at Kyoto University (no. 322) granted approval for the study.
Molecular design and synthesis of IMD-0354
A synthetic IKKβ inhibitor, IMD-0354 (molecular weight, 384.1), was molecular designed, synthesized, and provided by Institute of Medicinal Molecular Design Inc (Tokyo, Japan). Briefly, the 3-dimensional structure of a kinase domain of IKKβ was constructed by homology modeling with protein kinase A (PKA) as a template. The structure of active IKKβ was estimated by referring to a model of IKK regulation indicated by Delhase et al.34 The molecular structure of IMD-0354 was designed by analyzing a binding mode of aspirin to IKKβ.
Reagents
Signal inhibitors K-252a and LY294002 were purchased from Calbiochem-Boehring (La Jolla, CA), and PD98059 was from Cell Signaling Technology (Beverly, MA). Ro31-7549 was provided by Eisai (Ibaraki, Japan). STI571 was purchased from Novartis (Basel, Switzerland). Rabbit antihuman phospho-IκBα antibody, rabbit antihuman IκBα antibody, and horse-radish peroxidase (HRP)-conjugated antirabbit immunoglobulin G (IgG) antibody were obtained from Cell Signaling Technology. Mouse antihuman pRb mAb (clone G3-245) and rabbit antihuman cyclin D1/D2 antibody were purchased from PharMingen (San Diego, CA) and Upstate Biotechnology (Lake Placid, NY), respectively. Rabbit antihuman cyclin D3 antibody and rabbit antirat cyclin E antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated goat antimouse IgG antibody was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Unless otherwise indicated, all chemicals used in this study were obtained from Sigma Chemical (St Louis, MO).
Electrophoretic mobility shift assay
After they were incubated with indicated concentrations of IMD-0354 or with each signal inhibitor for 24 hours, nuclear extractions were prepared from 107 cells with the use of NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL) according to manufacturer's instructions. A biotin-labeled double-strand DNA probe containing the consensus DNA-binding sequence for NF-κB was synthesized by incubating sense and antisense oligonucleotides (sense, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; antisense, 5′-GCCTGGGAAAGTCCCCTCAACT-3′; Amersham Pharmacia) in Tris-EDTA (Tris-ethylenediaminetetraacetic acid) buffer for 2 minutes at 85°C, for 15 minutes at 65°C, for 15 minutes at 37°C, for 15 minutes at room temperature, and for 15 minutes on ice. With a LightShift Chemiluminescence electrophoretic mobility shift assay (EMSA) kit (Pierce), 0.02 pmol biotin-labeled DNA probe was incubated with 5 μg nuclear extraction for 20 minutes at room temperature. The conjugate, mixed with 5 × loading buffer and 20 μL mixture containing 4 μg nuclear protein, was applied to each lane of 6% DNA-polyacrylamide gel electrophoresis (DNA-PAGE) mini-gel (Tefco, Tokyo, Japan). Electrophoresis was performed in Tris-boric acid-EDTA buffer, and the separated proteins were transferred to Hybond-N+ membrane (Amersham Pharmacia Biotech). After ultraviolet (UV) cross-linking, the membrane was blocked and was incubated with LightShift stabilized streptavidin-HRP conjugate (Pierce) for 60 minutes. Positive reactions were visualized by incubating the membrane in LightShift Luminol/Enhancer solution (Pierce). All procedures were performed according to the manufacturer's instructions except as indicated. For competition assays, unlabeled NF-κB consensus oligonucleotides and mutant oligonucleotides with a single-base substitution (Santa Cruz Biotechnology) were used. For supershift assays, 4 μg anti-p65, anti-cRel, or anti-p50 subunit antibodies (Santa Cruz Biotechnology) in each reaction was added.
Chemiluminescence transcription factor assay
CBhCMCs (106 cells per each condition) were incubated with or without various concentrations of IMD-0354 for 24 hours at 37°C. After centrifugation, nuclear extractions were prepared with the use of NE-per nuclear and cytoplasmic extraction reagents (Pierce), as described in “Electrophoretic mobility shift assay.” Reactivity of the p65 subunit of NF-κB in nucleus was analyzed with the use of an EZ-Detect NF-κB p65 transcription factor kit (Pierce) according to manufacturer's instructions. Chemiluminescence signals were detected with the use of a luminometer (Mithras LB940; Berthold Technologies, Bad Wildbad, Germany).
Luciferase assay
With the use of an Effectene transfection reagent kit (Qiagen, Hilden, Germany), 200 ng pNF-κB-TA-Luc plasmid (BD Bioscience Clontech, Palo Alto, CA) was introduced into 2 × 106 HMC-1 cells according to manufacturer's instructions. Forty-eight hours later, cells were treated with various concentrations of IMD-0354 in α-MEM containing 10% FCS and were further incubated for 24, 48, and 72 hours. A luciferase activity in supernatants of cell lysates was measured with a Bright-Glo Luciferase Assay system (Promega, Madison, WI) as a substrate.
Western blot analysis
After they were washed in phosphate-buffered saline (PBS), 2 × 106 cells were lysed in 100 μL of a CelLytic-M reagent supplemented with a protease inhibitor cocktail (Sigma Chemical). Supernatants were collected by centrifugation, mixed with the same volume of 2 × sample buffer (20% glycerol, 10% 2-mercaptoethanol [2-ME], 4% sodium dodecyl sulfate [SDS], 100 mM Tris-HCl, pH 6.8), and boiled for 5 minutes. Samples were applied to SDS-PAGE with the use of 7.5% gels (Bio-Rad Laboratories, Hercules, CA) for pRb and 12.5% gels (Bio-Rad Laboratories) for IκBα, cyclin D1/D2, D3, and cyclin E. Separated proteins were transferred onto Immobilon-P membrane (Millipore, Bedford, MA). The membrane was blocked in 5% nonfat dry milk and was blotted with primary antibodies diluted in a blocking solution. After washing, the membrane was incubated with HRP-conjugated secondary antibodies. Positive reactions were visualized with the use of a Phototope-HRP Western Detection kit (Cell Signaling Technology). As positive controls, we used 3T3 cell lysate (Upstate Biotechnology) for cyclins D1/D2,D3, and E, MOLT-4 cell lysate (PharMingen) for pRb, and lysate from HeLa cells stimulated with or without tumor necrosis factor-α (TNF-α) for phospho- or total IκBα detection.
To detect the spontaneous expression of STATs, HMC-1 cells were incubated with various concentrations of IMD-0354 for 24 hours. Cells were washed, lysed, and boiled in sample buffer. Proteins from 105 cells were applied to each lane of 12.5% gels (Bio-Rad Laboratories). Immunoblots were performed with anti-STAT1, anti-STAT3, anti-STAT5, or anti-STAT6 antibodies and HRP-conjugated secondary antibodies (Cell Signaling Technology). To examine the phosphorylation of those STATs, HMC-1 cell lysates were immunoprecipitated with immobilized antiphosphotyrosine antibody (Cell Signaling Technology) at 4°C overnight. Immunoprecipitates were washed and boiled in sample buffer. Phosphotyrosine proteins obtained from 105 cells were applied to each lane of 12.5% gels (Bio-Rad Laboratories). Immunoblots were performed with anti-STAT1, anti-STAT3, anti-STAT5, or anti-STAT6 antibodies and HRP-conjugated secondary antibodies (Cell Signaling Technology). Positive reactions were visualized with the use of the ECL plus Western Blotting Detection System (Amersham Bioscience, Buckinghamshire, England).
Cell proliferation assay
HMC-1 cells (2 × 105 cells/mL) were incubated with various concentrations of IMD-0354, STI571, or pyrrolidine dithiocarbamate (PDTC)35 for the indicated hours, and viable cell numbers were calculated with the use of the trypan blue dye exclusion test at each time point.
MTT assay
Cells (2 × 105 cells/mL) were incubated in phenol red free α-MEM containing 10% FCS (for HMC-1 and IC-2 cells) or 5% FCS (for CBhCMCs), and antibiotics with or without various concentrations of IMD-0354, STI571, or PDTC. IC-2WT cells and CBhCMCs were incubated in the presence of 100 ng/mL recombinant rat or recombinant human SCF. One hundred microliters of cell suspension was applied to each well of 96-well culture plates and were incubated for 24, 48, and 72 hours. Before 4 hours from the end of the culture, 10 μL of 5 mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) dissolved in PBS was added to each well. The reaction was stopped with the addition of 100 μLof 10% SDS in 0.01 N HCl. Absorbance was measured at 577 nm with ImmunoMini NJ-2300 (Nalge Nunc International K.K., Tokyo, Japan).
DNA fragmentation assay
For DNA isolation, HMC-1 cells (2 × 105 cells/mL) were incubated with IMD-0354 or STI571 for 24 hours. After 1 wash in PBS, DNA was isolated from 2 × 106 cells with the use of an Apoptotic DNA Ladder kit (Roche Diagnostics, Mannheim, Germany). After we treated samples with 2 μg/mL RNase A for 10 minutes at room temperature, DNA was separated by a 1% agarose gel containing 0.5 μg/mL ethidium bromide in Tris-boric acid- EDTA buffer. A DNA ladder was visualized by placing the gel onto a UV light source.
Cell-cycle analysis
For cell-cycle analysis, HMC-1 cells (2 × 105 cells/mL) were incubated in the presence or absence of various doses of IMD-0354 or STI571. After 24 hours, cells were washed in ice-cold PBS, resuspended in 100 μL PBS, and fixed in 70% ethanol for 30 minutes on ice. Cells were recovered by centrifugation and treated with 0.5 mg/mL RNase A for 20 minutes at 37°C to avoid nonspecific propidium iodide (PI) binding. DNA was stained by incubating cells in 50 μg/mL PI dissolved in 0.1% sodium citrate for 10 minutes on ice. To remove clots, cell suspensions were passed through nylon mesh. DNA contents were analyzed with the use of an EPICS XL flow cytometer (Coulter, Hialeah, FL) with MacCycle AV software (Phoenix, San Diego, CA). We analyzed DNA histograms in the G0/G1, S, and G2/M phases in HMC-1 cells after incubation with IMD-0354 or STI571 and calculated the ratio of a 4N cell population in all cells with the use of the following formula: proliferation index = no. cells in S, G2, and M phases/no. cells in all phases × 100%.36
Annexin V analysis
Apoptotic status was analyzed with the use of a TACS annexin V-fluorescein isothiocyanate (FITC) kit (R&D Systems, Minneapolis, MN). Briefly, HMC-1 cells (2 × 105 cells/mL) were incubated for 24 hours in the presence or absence of indicated doses of IMD-0354 or STI571. After they were washed in ice-cold PBS, cells were incubated with annexin V-FITC and PI for 15 minutes at room temperature according to the manufacturer's instructions. Early apoptotic (only annexin V-positive) cells were distinguished from late apoptotic or necrotic (annexin V- and PI-double-positive) cells with the use of an EPICS XL flow cytometer.
Statistical analysis
Two-tailed Student t test was performed for statistical analysis of the data, and P < .01 was taken as the level of significance.
Results
Spontaneous activation of NF-κB in HMC-1 cells
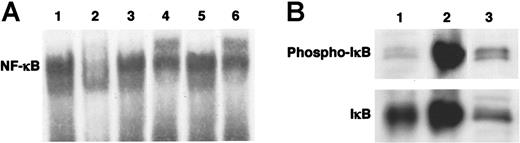
To examine NF-κB activation in mast cells, we performed EMSA for NF-κB translocation into the nucleus and Western blot analysis for IκBα phosphorylation. As shown in Figure 1A, when a nuclear extract was incubated with biotin-labeled DNA probes corresponding to a DNA-binding motif of NF-κB, binding activity of NF-κB was detected. The positive band disappeared during competition assay with unlabeled consensus oligonucleotides, whereas mutant oligonucleotides did not affect the reaction. When the conjugate of nuclear extract with DNA probes was preincubated with antibody against the p65 subunit of NF-κB, electrophoretic mobility decreased and the band shifted to a higher position (Figure 1A). The conjugate also reacted with anti-p50 antibody. On the other hand, it did not react with anti-cRel antibody. Next, we determined the phosphorylation of IκBα, a cytoplasmic inhibitory molecule of NF-κB. As shown in Figure 1B, Western blot analysis revealed the presence of phosphorylated IκBα in HMC-1 cells.
Spontaneous activation of NF-κB in HMC-1 cells. (A) NF-κB binding activity in nuclear extract obtained from HMC-1 cells was detected through EMSA (lane 1). Unlabeled consensus oligonucleotides (lane 2), but not mutant oligonucleotides (lane 3), competed the positive reaction. Antibodies against the p65 subunit (lane 4) and the p50 subunit (lane 6) of NF-κB shifted an electric mobility of the band to the upper position. On the other hand, anti-cRel antibody did not alter the mobility (lane 5). (B) Phosphorylation of IκBα in HMC-1 cells was detected by Western blotting (lane 1, HeLa cells; lane 2, HeLa cells stimulated with TNF-α; lane 3, HMC-1 cells).
Spontaneous activation of NF-κB in HMC-1 cells. (A) NF-κB binding activity in nuclear extract obtained from HMC-1 cells was detected through EMSA (lane 1). Unlabeled consensus oligonucleotides (lane 2), but not mutant oligonucleotides (lane 3), competed the positive reaction. Antibodies against the p65 subunit (lane 4) and the p50 subunit (lane 6) of NF-κB shifted an electric mobility of the band to the upper position. On the other hand, anti-cRel antibody did not alter the mobility (lane 5). (B) Phosphorylation of IκBα in HMC-1 cells was detected by Western blotting (lane 1, HeLa cells; lane 2, HeLa cells stimulated with TNF-α; lane 3, HMC-1 cells).
Constitutive activation of NF-κB in HMC-1 cells inhibited by IMD-0354
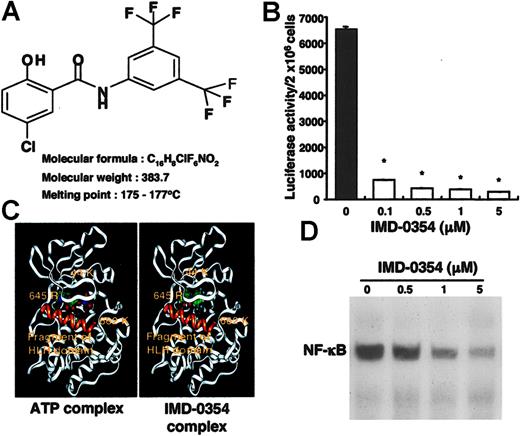
The molecular formula of IMD-0354 is shown in Figure 2A. IMD-0354 and the modeled IKKβ were expected to form a docking structure, as shown in Figure 2B. The inhibitory effect of IMD-0354 on NF-κB activity was examined by performing a reporter assay with the use of HMC-1 cells transfected with pNF-κB-TA-Luc plasmid. Luciferase activity, which represents transcriptional activation of NF-κB, was reduced dose dependently by treatment with IMD-0354 (Figure 2C). When HMC-1 cells were incubated with IMD-0354 for 24 hours, the translocation of NF-κB to the nucleus was completely suppressed (Figure 2D).
Inhibitory effect of IMD-0354 on cell proliferation of HMC-1 cells. (A) Molecular formula of IMD-0354. (B) Estimated docking structures of ATP or IMD-0354 with IKKβ. (C) Inhibitory effect of IMD-0354 on NF-κB activity was examined by a reporter assay with the use of HMC-1 cells transfected with pNF-κB-TA-Luc plasmid. Luciferase activity was reduced in a dose-dependent manner by treatment with IMD-0354 for 24 hours. Each value represents the mean ± SE at each time point of 3 different experiments with duplication. *P < .01 compared to medium alone. (D) EMSA revealed that more than 1 μM IMD-0354 inhibited the translocation of NF-κB to the nucleus after treatment for 24 hours. Results shown are representative of those of 5 different experiments.
Inhibitory effect of IMD-0354 on cell proliferation of HMC-1 cells. (A) Molecular formula of IMD-0354. (B) Estimated docking structures of ATP or IMD-0354 with IKKβ. (C) Inhibitory effect of IMD-0354 on NF-κB activity was examined by a reporter assay with the use of HMC-1 cells transfected with pNF-κB-TA-Luc plasmid. Luciferase activity was reduced in a dose-dependent manner by treatment with IMD-0354 for 24 hours. Each value represents the mean ± SE at each time point of 3 different experiments with duplication. *P < .01 compared to medium alone. (D) EMSA revealed that more than 1 μM IMD-0354 inhibited the translocation of NF-κB to the nucleus after treatment for 24 hours. Results shown are representative of those of 5 different experiments.
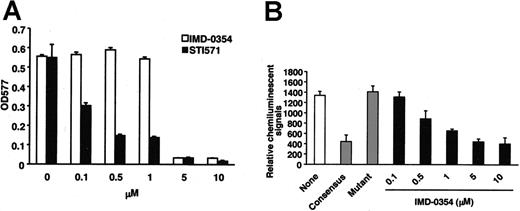
Factor-independent proliferation of neoplastic mast cells inhibited by IMD-0354
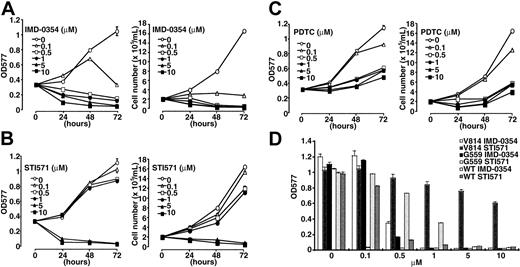
Next, we evaluated the effect of IMD-0354 on the neoplastic proliferation of mast cells. HMC-1 cells were incubated with increasing concentrations of IMD-0354 or STI571 for 24, 48, and 72 hours, and numbers and viability of cells were determined by a dye exclusion test and an MTT assay. As shown in Figure 3A, IMD-0354 suppressed cell proliferation in a time- and dose-dependent manner. The inhibitory effect of IMD-0354 was remarkable, even at lower concentrations, when compared with that of STI571 (Figure 3B). We used PDTC, which interferes with the DNA binding ability of NF-κB,35 as an alternative inhibitor of NF-κB. PDTC inhibited the proliferation of HMC-1 cells in a dose-dependent manner, but the effect of PDTC was weaker than that of IMD-0354 (Figure 3C). Recently, Ma et al37 reported that STI571 was effective for 1 subclone of HMC-1 cells, which expresses only the Val560Gly mutation in the c-kit intracellular juxtamembrane regions. However, another subclone of HMC-1 cells, which expresses both the Val560Gly mutation and the Asp816Val mutation in the kinase active site, was resistant to STI571. Therefore, we compared the effects of IMD-0354 and STI571 on IC-2G559 cells with the Val560Gly mutation in the juxtamembrane regions and on IC-2V814 cells with the Asp816Val mutation in the tyrosine kinase regions. As shown in Figure 3D and Table 1, STI571 completely inhibited the proliferation of IC-2G559 cells at concentrations lower than 0.1 μM, whereas IC-2V814 cells were resistant to treatment with STI571. On the other hand, IMD-0354 almost inhibited the proliferation of IC-2G559 cells and IC-2V814 cells at 0.5 μM. Thus, IMD0354 manifested inhibitory effects on the proliferation of HMC-1 and IC-2 cells with mutations in the intracellular juxtamembrane region, the kinase active site, or both (Table 1).
Inhibitory effect of IMD-0354 on proliferation of neoplastic mast cells. HMC-1 cells were incubated in the presence of increasing concentrations of IMD-0354 (A), STI571 (B), and PDTC (C) for 24, 48, and 72 hours, and the numbers and viability of cells were determined by a dye exclusion test and an MTT assay. Each point represents the mean ± SE of 6 different experiments. (D) IC-2WT cells, IC-2G559 cells, and IC-2V814 cells were incubated with various concentrations of IMD-0354 or STI571 in the presence (IC-2WT) or the absence (IC-2G559 and IC-2V814) of 100 ng/mL SCF. After 48 hours, proliferative activity of these cells was evaluated through MTT assay. Data represent the mean ± SE of 6 different experiments.
Inhibitory effect of IMD-0354 on proliferation of neoplastic mast cells. HMC-1 cells were incubated in the presence of increasing concentrations of IMD-0354 (A), STI571 (B), and PDTC (C) for 24, 48, and 72 hours, and the numbers and viability of cells were determined by a dye exclusion test and an MTT assay. Each point represents the mean ± SE of 6 different experiments. (D) IC-2WT cells, IC-2G559 cells, and IC-2V814 cells were incubated with various concentrations of IMD-0354 or STI571 in the presence (IC-2WT) or the absence (IC-2G559 and IC-2V814) of 100 ng/mL SCF. After 48 hours, proliferative activity of these cells was evaluated through MTT assay. Data represent the mean ± SE of 6 different experiments.
Inhibitory effect of IMD-0354 and ST1571 on cell proliferation
. | IC50, μM . | . | |
|---|---|---|---|
| Cells . | IMD-0354 . | ST1571 . | |
| HMC-1 | 0.28 | 3.0 | |
| IC-2G559 | 0.25 | 0.03 | |
| IC-2V814 | 0.35 | > 10 | |
| IC-2WT | 0.77 | 0.25 | |
| CBhCMC | 3.0 | 0.15 | |
. | IC50, μM . | . | |
|---|---|---|---|
| Cells . | IMD-0354 . | ST1571 . | |
| HMC-1 | 0.28 | 3.0 | |
| IC-2G559 | 0.25 | 0.03 | |
| IC-2V814 | 0.35 | > 10 | |
| IC-2WT | 0.77 | 0.25 | |
| CBhCMC | 3.0 | 0.15 | |
Cells were incubated for 48 hours at the concentration of 2 × 105 cells/mL in phenol red free α-MEM containing 10% FCS (for HMC-1 and IC-2 cells) or 5% FCS (for CBhCMCs) with or without various concentrations of IMD-0354 or ST1571. IC50 was calculated from results of an MTT assay.
Effects of IMD-0354 on cell cycle of HMC-1 cells
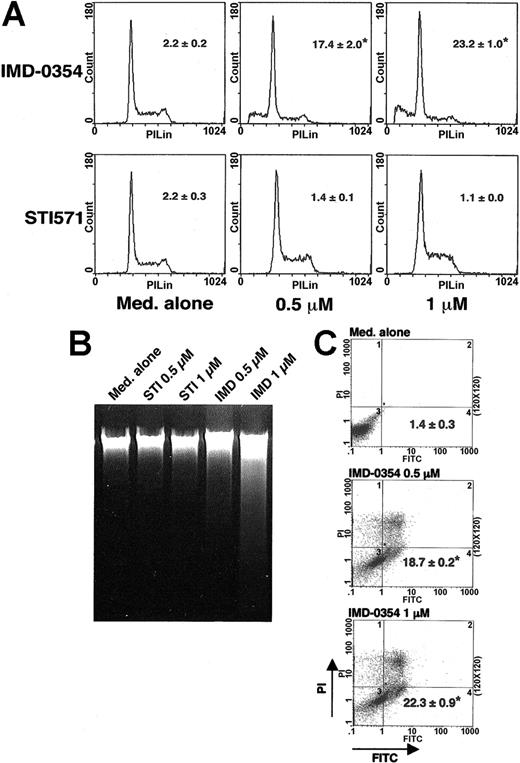
Given the spontaneous activation of NF-κB in HMC-1 cells, we attempted to determine its role in mast cell proliferation. HMC-1 cells were incubated with IMD-0354 for 24 hours, and cell cycle analysis was performed by PI uptake. As shown in Figure 4A, there was an obvious arrest of the cell cycle at the G0/G1 phase in cells treated with 0.5 μM IMD-0354 for 24 hours, whereas the same concentration of STI571 was ineffective. The number of cells with hypodiploid DNA content increased in 24 hours after the addition of 1 μM IMD-0354, but not after the addition of STI571 (Figure 4A). The proliferation index showed that the ratio of cells in S and G2/M phases was significantly decreased when HMC-1 cells were treated with IMD-0354 for 24 hours (Table 2). To demonstrate whether the inhibition of NF-κB activity resulted in apoptosis or necrosis, we performed agarose gel electrophoresis with the use of DNA collected from cells treated with IMD-0354 or STI571. DNA fragmentation indicating apoptosis was clearly observed in HMC-1 cells treated with 1 μM IMD-0354 for 24 hours, but not with STI571 (Figure 4B). To further clarify the apoptotic status of HMC-1 cells, cell surface changes that occur at the early stage of apoptosis were examined with the use of annexin V-FITC conjugates. After 24 hours, the proportion of annexin V-FITC positive-PI negative cells exhibiting apoptotic status increased in cells incubated with IMD-0354 (Figure 4C).
Suppressive effect of IMD-0354 on cell cycle progression of HMC-1 cells. (A) HMC-1 cells were incubated with IMD-0354 or STI571 for 24 hours, and the cell cycle was analyzed by PI staining. The percentage of cells with hypodiploid DNA contents was measured. Data represent the mean ± SE of 3 different experiments with duplication. (B) HMC-1 cells were treated with IMD-0354 or STI571 for 24 hours, and DNA was collected as described in “Materials and methods.” A ladder is marked in cells treated with 1 μM IMD-0354 for 24 hours. Results shown are representative of those of 3 different experiments. (C) HMC-1 cells were treated with indicated doses of IMD-0354 for 24 hours. Early apoptotic cells were detected by annexin V-FITC binding and flow cytometric analysis. Dots in the right under compartment were only annexin V-FITC-positive cells. Data represent the mean ± SE of 4 different experiments. *P < .01 compared with medium alone.
Suppressive effect of IMD-0354 on cell cycle progression of HMC-1 cells. (A) HMC-1 cells were incubated with IMD-0354 or STI571 for 24 hours, and the cell cycle was analyzed by PI staining. The percentage of cells with hypodiploid DNA contents was measured. Data represent the mean ± SE of 3 different experiments with duplication. (B) HMC-1 cells were treated with IMD-0354 or STI571 for 24 hours, and DNA was collected as described in “Materials and methods.” A ladder is marked in cells treated with 1 μM IMD-0354 for 24 hours. Results shown are representative of those of 3 different experiments. (C) HMC-1 cells were treated with indicated doses of IMD-0354 for 24 hours. Early apoptotic cells were detected by annexin V-FITC binding and flow cytometric analysis. Dots in the right under compartment were only annexin V-FITC-positive cells. Data represent the mean ± SE of 4 different experiments. *P < .01 compared with medium alone.
Inhibitory effect of IMD-0354 on cell cycle progression
Reagent, μM . | Proliferation index, % . |
|---|---|
| None | 43.0 ± 1.2 |
| IMD-0354, 0.5 | 21.6 ± 0.6* |
| IMD-0354, 1 | 18.3 ± 1.3* |
| ST1571, 0.5 | 44.2 ± 0.5 |
| ST1571, 1 | 42.7 ± 0.8 |
Reagent, μM . | Proliferation index, % . |
|---|---|
| None | 43.0 ± 1.2 |
| IMD-0354, 0.5 | 21.6 ± 0.6* |
| IMD-0354, 1 | 18.3 ± 1.3* |
| ST1571, 0.5 | 44.2 ± 0.5 |
| ST1571, 1 | 42.7 ± 0.8 |
Cells were incubated at the concentration of 106 cells/mL in α-MEM containing 10% FCS and antibiotics with or without indicated concentrations of IMD-0354 or ST1571. We analyzed DNA histograms in the G0/G1, S, and G2/M phases in HMC-1 cells stained with PI with the use of a flow cytometer and calculated the ratio of a 4N cell population in all cells with the use of the following formula: proliferation index = (no. cells in S, G2, and M phases)/no. cells in all phases × 100 (%).
P < .01 compared with None
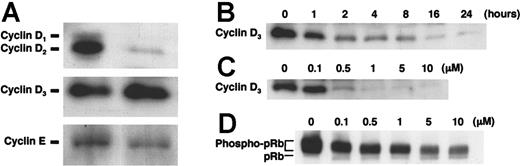
Effect of IMD-0354 on the expression of cyclins in HMC-1 cells
Recent studies have revealed that NF-κB contributes to cell growth and differentiation in various cell types through the transcriptional regulation of cyclins. Therefore, we examined whether NF-κB inhibition modified the expression of several cyclins. As shown in Figure 5A, we detected the spontaneous expression of cyclins D2, D3, and E in HMC-1 cells. The expression of cyclin D2 and cyclin E was weak in HMC-1 cells, and neither expression was affected by treatment with IMD-0354 (data not shown). Cyclin D3 expression was dramatically decreased in a time-dependent manner by the addition of 1 μM IMD-0354 (Figure 5B). IMD-0354 reduced cyclin D3 expression in a dose-dependent manner after 16 hours (Figure 5C). The D-type cyclins bound to CDK phosphorylate pRb, thereby resulting in the positive regulation of G1 progression and S-phase entry. Compared with untreated cells, pRb phosphorylation was suppressed dose dependently in cells incubated with IMD-0354 for 16 hours (Figure 5D). Thus, we concluded that pRb was spontaneously phosphorylated in HMC-1 cells and was dephosphorylated on inhibition of NF-κB activity by IMD-0354.
Cyclin D3 expression in HMC-1 cells down-regulated by treatment with IMD-0354. (A) Spontaneous expression of cyclins D2, D3, and E was detected in HMC-1 cells (right) by Western blotting. We used 3T3 cell lysate for positive control (left). (B) Cyclin D3 expression in HMC-1 cells incubated with 1 μM IMD-0354 for various hours. (C) Cyclin D3 expression in HMC-1 cells treated with various concentrations of IMD-0354 for 16 hours. (D) Spontaneously phosphorylated pRb was detected in HMC-1 cells with IMD-0354 for 16 hours. Results shown are representative of those of 3 different experiments.
Cyclin D3 expression in HMC-1 cells down-regulated by treatment with IMD-0354. (A) Spontaneous expression of cyclins D2, D3, and E was detected in HMC-1 cells (right) by Western blotting. We used 3T3 cell lysate for positive control (left). (B) Cyclin D3 expression in HMC-1 cells incubated with 1 μM IMD-0354 for various hours. (C) Cyclin D3 expression in HMC-1 cells treated with various concentrations of IMD-0354 for 16 hours. (D) Spontaneously phosphorylated pRb was detected in HMC-1 cells with IMD-0354 for 16 hours. Results shown are representative of those of 3 different experiments.
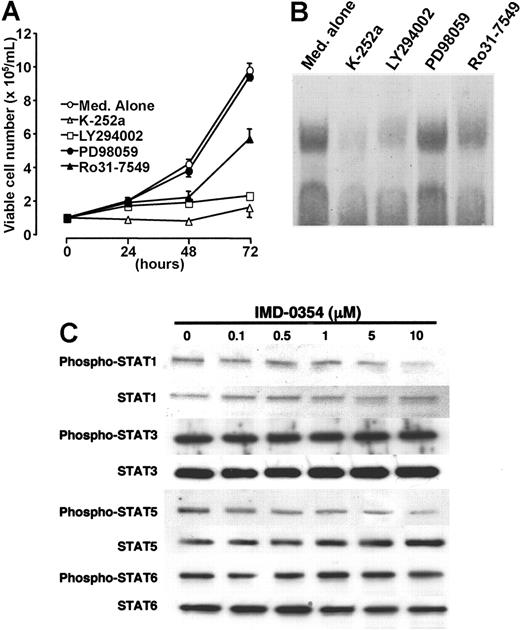
Signaling pathways involved in NF-κB activation in HMC-1 cells
HMC-1 cells proliferated without any growth factor because of the autophosphorylation of c-kit receptor tyrosine kinase by gain-of-function mutations. As indicated in Figure 6A, a protein kinase inhibitor (K-252a) and a PI3 kinase inhibitor (LY294002) completely blocked the proliferation of HMC-1 cells. A selective PKC inhibitor (Ro31-7549) mostly suppressed cell proliferation; however, a MAP kinase inhibitor (PD98059) had minimal or no effect (Figure 6A). To determine the possible relationship between c-kit signaling and NF-κB activation, we performed EMSA with the use of a nuclear extract obtained from cells incubated with different types of signal inhibitors for 24 hours. The addition of K-252a, LY294002, or Ro31-7549 suppressed the DNA-binding activity of NF-κB in HMC-1 cells, whereas the addition of PD98059 did not suppress the activity (Figure 6B). These results were supported by a dye exclusion test.
Signaling pathways involved in NF-κB activation in HMC-1 cells. (A) Cells were incubated with K-252a (50 ng/mL; ▵), LY294002 (20 μM; □), Ro31-7549 (10 μM; ▴), or PD98059 (20 μM; •) for 24 hours, and cell proliferation was assessed through the trypan blue dye exclusion test. Each point represents the mean ± SE of 6 different experiments. (B) To determine the possible interaction between c-kit signaling and NF-κB activation, we performed EMSA with the use of nuclear extract obtained from cells incubated for 24 hours with different types of signal inhibitors. (C) HMC-1 cells were incubated with various concentrations of IMD-0354 for 24 hours. Spontaneous expression of STAT1, STAT3, STAT5, and STAT6 in HMC-1 cells was detected by Western blotting. Phospho-STAT1, -STAT3, -STAT5, and -STAT6 were determined through immunoprecipitation and Western blotting. Results shown are representative of those of 2 different experiments.
Signaling pathways involved in NF-κB activation in HMC-1 cells. (A) Cells were incubated with K-252a (50 ng/mL; ▵), LY294002 (20 μM; □), Ro31-7549 (10 μM; ▴), or PD98059 (20 μM; •) for 24 hours, and cell proliferation was assessed through the trypan blue dye exclusion test. Each point represents the mean ± SE of 6 different experiments. (B) To determine the possible interaction between c-kit signaling and NF-κB activation, we performed EMSA with the use of nuclear extract obtained from cells incubated for 24 hours with different types of signal inhibitors. (C) HMC-1 cells were incubated with various concentrations of IMD-0354 for 24 hours. Spontaneous expression of STAT1, STAT3, STAT5, and STAT6 in HMC-1 cells was detected by Western blotting. Phospho-STAT1, -STAT3, -STAT5, and -STAT6 were determined through immunoprecipitation and Western blotting. Results shown are representative of those of 2 different experiments.
Given that a JAK/STAT signaling pathway is one of the most important cascades that may transduce c-kit signals to the nucleus in mast cells,11 we examined the effect of IMD-0354 on the expression and phosphorylation of STATs in HMC-1 cells. As shown in Figure 6C, the spontaneous phosphorylation of STAT1, STAT3, STAT5, and STAT6 was detected in HMC-1 cells. The expression and phosphorylation of STAT3 and STAT6 were much stronger than those of STAT1 and STAT5. IMD-0354 had no influence on the signals of STAT3 and STAT6, whereas the phosphorylation of STAT1 and STAT5 was very slightly suppressed at high concentrations. The signaling pathways affected in the drug inhibition of HMC-1 cell growth included the PI3 kinase and PKC, but not MAP kinase or JAK/STAT.
Lower suppressive effect of IMD-0354 on SCF-dependent proliferation of CBhCMCs and IC-2WT cells
Effective signal inhibitors sometimes suppress the proliferation or survival not only of neoplastic cells but also of normal cells. There is a possibility that the wide range of actions of inhibitors may cause serious side effects in vivo. Therefore, we examined whether IMD-0354 also suppressed SCF-dependent proliferation of normal human mast cells (CBhCMCs) and IC-2WT cells. CBhCMCs and IC-2WT cells were incubated with increasing concentrations of IMD-0354 or STI571 for 48 hours in the presence of SCF, and cell proliferation activity was evaluated with the use of an MTT assay. Adding STI571 to the culture induced a dose-dependent reduction in the proliferation of CBhCMCs and IC-2WT cells incubated with 100 ng/mL SCF (Figures 3 and 7); IC50 of STI571 for the inhibition of proliferation was lower than 0.5 μM (Table 1). On the other hand, CBhCMCs and IC-2WT cells were more resistant to treatment with IMD-0354, which had no influence on CBhCMC proliferation even at 1 μM (Figure 7A; Table 1). We detected NF-κB activity in nuclear extracts obtained from CBhCMCs with the use of a chemiluminescence transcription factor assay. The positive reaction disappeared in a competition assay with consensus oligonucleotides, whereas mutant oligonucleotides did not affect the reaction. IMD-0354 suppressed the translocation of NF-κB to the nucleus in CBhCMCs after 24 hours in a dose-dependent manner (Figure 7B).
Effect of IMD-0354 on SCF-dependent proliferation of CBhCMCs. (A) CBhCMCs (105 cells/mL) were incubated in the presence of increasing concentrations of IMD-0354 (□) or STI571 (▪) for 48 hours with 100 ng/mL SCF, and proliferative activity of cells was evaluated through MTT assay. Each value represents the mean ± SE of 6 different experiments. (B) After incubation with various concentrations of IMD-0354, nuclear extracts were collected from CBhCMCs. NF-κB activity in nuclear extracts was analyzed with the use of a chemiluminescence transcription factor assay. Positive reactions were detected using a luminometer. None indicates medium alone; Consensus, consensus oligonucleotides; Mutant, mutant oligonucleotides. Each value represents the mean ± SE of 3 different experiments.
Effect of IMD-0354 on SCF-dependent proliferation of CBhCMCs. (A) CBhCMCs (105 cells/mL) were incubated in the presence of increasing concentrations of IMD-0354 (□) or STI571 (▪) for 48 hours with 100 ng/mL SCF, and proliferative activity of cells was evaluated through MTT assay. Each value represents the mean ± SE of 6 different experiments. (B) After incubation with various concentrations of IMD-0354, nuclear extracts were collected from CBhCMCs. NF-κB activity in nuclear extracts was analyzed with the use of a chemiluminescence transcription factor assay. Positive reactions were detected using a luminometer. None indicates medium alone; Consensus, consensus oligonucleotides; Mutant, mutant oligonucleotides. Each value represents the mean ± SE of 3 different experiments.
Discussion
Much is known regarding how gain-of-function mutations in c-kit receptors are involved in neoplastic proliferation of mast cells1-6 ; however, the exact mechanism of regulation of mast cell proliferation has not been completely understood. Finding molecules associated with factor-independent proliferation enables us to overcome neoplastic disorders of mast cells. NF-κB is a dimeric transcription factor that is autoactivated in various neoplastic cells and is involved seriously in their proliferation.24 NF-κB has been detected in human lung and intestinal mast cells, and the importance of this transcription factor on cell functions has been described.38,39 Although the involvement of NF-κB in cytokine production of mast cells stimulated by IgE aggregation or LPS has been reported,40,41 the role of NF-κB in mast cell proliferation has not yet been determined. In the present study, we clearly demonstrated the presence of spontaneously phosphorylated IκBα and activated NF-κB in HMC-1 cells. A shift in the electrophoretic mobility of NF-κB was observed in supershift assays with anti-p65 antibody and anti-p50 antibody, indicating that the NF-κB expressed in HMC-1 cells may consist of a heterodimeric conjugation of p65 and p50 subunits. When HMC-1 cells were treated with IMD-0354, cell proliferation was suppressed in a dose- and a time-dependent manner, indicating that activation of NF-κB may be essential for proliferation in mast cells. Through cell cycle analysis, we found that G1 progression and S-phase entry were prevented dose dependently in cells incubated with IMD-0354. Furthermore, HMC-1 cells underwent apoptosis because of the inhibition of NF-κB activity. These results suggest the possible involvement of NF-κB in mast cell proliferation by the up-regulation of cell cycle progression. The effective concentration of IMD-0354 was considerably lower than that of STI571, supporting the possibility that IMD-0354 may be an useful agent for the treatment of STI571-resistant neoplastic disorders of mast cells. STI571 has been reported to be effective in patients with mastocytosis with a Phe522Cys mutation14 but not in patients with mastocytosis with an Asp816Val mutation. In the present study, STI571 showed a suppressive effect on the proliferation of IC-2G559 cells but not on the proliferation of IC-2V814 cells. IC-2G559 cells have a point mutation in the juxtamembrane region of the c-kit receptor that represents the mutation often observed in GIST. IC-2V814 cells, which have a point mutation in the tyrosine kinase domain of the c-kit receptor that represents the mutation observed in most types of mastocytosis, were resistant to STI571. These results, obtained with the use of IC-2 mutant cells, were comparable to those reported on HMC-1 subclones.37 On the other hand, IMD-0354 inhibited the proliferation of both types of mutant IC-2 cells. As previously reported, STI571 also suppresses SCF-dependent proliferation of IC-2WT cells and CBhCMCs at lower concentrations.37,42 However, IMD-0354 did not inhibit the cell activity of normal human mast cells at lower concentrations than effective for the inhibition of neoplastic mast cell proliferation. Despite the inhibition of NF-κB translocation to the nucleus, IMD-0354 did not suppress the cell activity of CBhCMCs, suggesting that the proliferation of normal mast cells may be supported mainly by transcription factors other than NF-κB. Therefore, IMD-0354 may be effective for different types of mastocytosis with tyrosine kinase mutations, juxtamembrane mutations, or both, and neoplastic mast cells may be largely dependent on NF-κB for their proliferation in comparison with normal mast cells. NF-κB, in lung and intestine mast cells, has been shown to be activated after IgE-mediated stimulation and may contribute to inflammatory disorders by releasing proinflammatory cytokines such as TNF-α.38,39 Compounds that interfere with NF-κB activity may be useful in therapies for allergic diseases. PDTC, an alternative inhibitor of NF-κB, suppressed the proliferation of HMC-1 cells. The effect of PDTC was weaker than that of IMD-0354 at the same concentrations, possibly because the effect of PDTC was reversible.35
Growth factor stimulation triggers pRb phosphorylation, thereby resulting in the activation of the S phase, promoting transcription factor E2F and cell cycle transition.43,44 pRb plays an important role in cell cycle arrest; thus, mitogenic cell proliferation is suppressed in differentiated cells.45 D-type cyclins promote S-phase entry by inhibiting pRb. Cyclin E is another endogenous pRb inhibitor.46 Recently, we reported that cyclin D3 expression was facilitated and pRb was phosphorylated in BMCMCs stimulated with SCF.21 In HMC-1 cells, we detected the expression of cyclin D2, D3, and E without induction. Although the expression levels of cyclin D2 and E were very low, the expression of cyclin D3 was potent. We examined the sensitivity of those molecules to NF-κB and found that cyclin D3 expression was suppressed in cells treated with IMD-0354, whereas neither cyclin D2 nor cyclin E was affected. Because IMD-0354 completely inhibited the proliferation of HMC-1 cells, activation of the c-kit receptor may induce cyclin D3 expression through NF-κB activation, resulting in the subsequent phosphorylation of pRb- and S-phase promotion in mast cells. As we have already reported,21 mast cell proliferation induced by c-kit signals with or without SCF stimulation may contribute to the high expression of cyclin D3. Cell cycle progression in mast cells may largely depend on cyclin D3. On the other hand, dependency on cyclin D1, D2, and E may not be dominant.
When the c-kit receptor is stimulated, multiple signal transduction molecules are activated in the downstream process. Given that JAK/STAT pathways are one of the important signaling cascades,11 we examined the relationship between JAK/STAT signals and NF-κB activation. Although we detected the spontaneous phosphorylation of STAT1, STAT3, STAT5, and STAT6 in HMC-1 cells, treatment with IMD-0354 had minimal or no effect on the signals at the minimum effective dose for the inhibition of cell proliferation. These results indicate that IMD-0354 inhibited NF-κB activity without altering signal transductions of JAK/STAT pathways.
Next, we attempted to identify the intracellular signals responsible for NF-κB activation. The protein kinase inhibitor K-252a abolished the DNA-binding activity of NF-κB in HMC-1 cells. The PI3 kinase inhibitor and the PKC inhibitor also suppressed NF-κB activity, but the MAP kinase inhibitor exhibited no effect. Because the proposed pathways are involved in the proliferative effects of NF-κB induced by growth factors, our results revealed that signals from the c-kit receptor might be transmitted, at least partially, through PKC or PI3 kinase cascades, resulting in mast cell proliferation through NF-κB activation. Serve et al47 reported differential roles of PI3 kinase and MAP kinase in proliferation, survival, and adhesion in mast cells that were transfected with different types of substitution in the c-kit gene. Further investigation must take place, but the dependency on each signaling molecule may be different in the proliferation of neoplastic mast cells and normal mast cells.
Taken together, we conclude that the activation of NF-κB by c-kit receptor autophosphorylation is essential for neoplastic mast cell proliferation. This proliferation occurs because of the inhibition of cell cycle regulation by the up-regulation of cyclin D3 expression and pRb phosphorylation. Given the necessity of NF-κB in neoplastic mast cell proliferation, we propose that IMD-0354 may have therapeutic advantages in mast cell-mediated disorders, including mastocytosis and mast cell leukemia.
Prepublished online as Blood First Edition Paper, November 23, 2004; DOI 10.1182/blood-2004-08-3247.
IMD-0354, a novel NF-κB inhibitor, was generated and provided by the Institute of Medicinal Molecular Design Inc (Tokyo, Japan). IMD-0354 is not a commercial product. The Institute has acquired a patent for the use of IMD-0354.
S.M. and A.I. are employed by the Institute of Medicinal Molecular Design Inc.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We greatly appreciate Dr Yukihiko Kitamura (Osaka University School of Medicine) for valuable suggestions and discussions. We also thank Miss Yoko Wakamatsu (Institute of Medicinal Molecular Design Inc) for her technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal