Abstract
Chromosomal translocations juxtaposing immunoglobulin (Ig) and MYC genes are the hallmarks of human Burkitt lymphoma (BL), with deregulated MYC expression being a critical factor in pathogenesis. By inserting an intact mouse Myc gene into the mouse genome, proximal to the Ig enhancer Eμ, the effect of a precise mimic of the major t(8;14) translocation of human endemic BL (eBL) could be investigated. Knock-in mice developed IgM-positive B-cell tumors, with most being typical of eBL by histology and immunophenotype, including expression of the germinal center (GC)–associated protein, BCL6. Unlike eBL, however, analysis of Ig VH sequences revealed no significant level of somatic mutation. Thus, constitutive expression of Myc in the knock-in mice is apparently able to induce “Burkitt-like” lymphomas before antigen stimulation and formation of a GC. In contrast, human eBL development occurs in a GC or post-GC site with a likely contribution to pathogenesis from Epstein-Barr virus (EBV) and other epigenetic factors.
Introduction
Reciprocal chromosomal translocations that activate the cellular oncogene MYC are characteristic of human Burkitt lymphoma (BL).1 In endemic BL (eBL) the most common translocation is t(8;14)(q24;q32), which recombines MYC at 8q24 with the immunoglobulin heavy-chain gene locus, IGH, at 14q32.1,2 Breakpoints usually locate in the joining gene region of the IGH locus, JH, and in the 5′-flank of MYC. The resulting exchanges allocate the intact MYC to the intronic heavy-chain enhancer, Eμ, in opposite transcriptional orientation. Deregulated MYC expression as a result of the chromosomal rearrangements is widely accepted as an initiating step in the development of BL.
The mechanism by which the t(8;14) translocation deregulates the expression of MYC and promotes the malignant transformation of B lymphocytes is not well understood.3 We recently created a mouse model of eBL t(8;14) translocation, designated iMycEμ, by inserting a single copy of a histidine-tagged mouse Myc gene (MycHis) into the intervening region between JH4 and Eμ in opposite transcriptional orientation to Igh.21 The inserted Myc included the noncoding first exon with an active P1/P2 natural promoter, a 5′-flank containing the normal transcription-regulatory region, and a short stretch of 3′-untranslated region harboring the Myc major polyadenylation site. This is a precise reconstruction of the translocation breakpoint region on human der(14) and mimics the defining translocation in eBL more accurately than other models. The heterozygous iMycEμ mice showed high incidence of various forms of B-cell neoplasms, with 35% of mice developing Burkitt-like lymphomas (BLLs) with a typical histologic appearance. It was considered, therefore, that the iMycEμ transgene could provide a useful system for studying the promoting role of deregulated Myc expression in BLL tumor development. However, we show here that, unlike human eBLs that arise from somatically mutated, postgerminal center (post-GC) B cells,4 VH sequences in BLL showed insignificant levels of somatic mutation. This study defines more closely the cellular origin of mouse BLL and highlights the influence of other factors, likely to include Epstein-Barr virus (EBV) and other infections, in the pathogenesis of human eBL.
Study design
Generation and characterization of iMycEμ mice
The iMycEμ mice showed an increased Myc expression in B220lowIgM- pro-B/pre-B cells, transitional B220lowIgM+ B cells, and mature B220hiIgM+ bone marrow B cells. Constitutive Myc expression led to B-cell neoplasms with an overall incidence of 70% by 21 months of age, of which BLL formed the major group (50%). In contrast to other models,5 mice had normal B-cell numbers, normal levels of serum immunoglobulin M (IgM)/IgG, and were able to respond to T-cell–dependent antigens.4
Sequence analysis of expressed VH genes
Total RNA extraction, cDNA synthesis, and VH gene amplification and sequencing were performed as previously described.6 Sequence analysis was performed using the IgBLAST program (http://www.ncbi.nlm.nih.gov/igblast).
Results and discussion
Cellular features of BLL
The insertion in the iMycEμ mice includes the complete set of correctly spaced Igh enhancers, designed to create the same complex promoter and enhancer interactions that govern the expression of the translocated MYC in human eBL.7 Positioning within the Igh chromatin domain also presumably subjects the gene to the same higher-order regulatory influences, including those imposed by chromatin remodeling and positional effects in the interphase nucleus. Among 32 tumors, only 1 tandem mutation was found in the transgenic Myc cDNA.4
Tumors expressed MycHis protein as diffuse cytoplasmic and speckled nuclear staining by immunocytochemistry. Presentation was commonly in the mesenteric lymph node, closely followed by Peyer patches, sites reminiscent of human sporadic BL and some cases of eBL.7 Later stages exhibited generalized splenomegaly and lymphoadenopathy. Histologically, BLL cases were lymphoblastic B-cell lymphomas with a typical “starry sky” appearance. This is due to tingible body macrophages engulfing transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)–positive apoptotic tumor cells—again similar to human BL.7
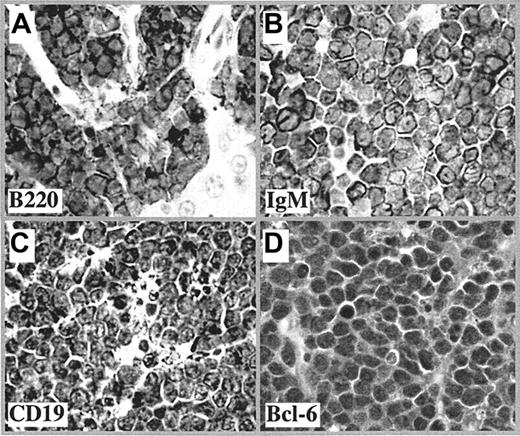
Tumor cells had a high proliferative index and expressed the B-cell markers B220, IgM, and CD19 (Figure 1A-C), consistent with mature B cells. However, most lacked the cytoplasmic lipid vacuoles characteristic of classic human BL. In parallel with human BL, mouse BLL also expressed the GC-associated protein, BCL6 (Figure 1D).
Immunophenotype of mouse BLL. Shown are representative tissue sections of lymphomas after immunostaining with antibody to B220 (A), IgM (B), CD19 (C), and Bcl-6 (D), followed by horseradish peroxidase (HRP)–conjugated secondary antibody (1:200 dilution). The images were captured by a Zeiss Axiophot microscope equipped with a DC330E CCD camera (Dage MTI, Michigan City, IN) and Scion Image software (Scion, Frederick, MD). The original magnification of the objective lens was 40 ×, and its numerical aperture was 0.75. Scion Image software (Scion, Frederick, MD) was used to capture images.
Immunophenotype of mouse BLL. Shown are representative tissue sections of lymphomas after immunostaining with antibody to B220 (A), IgM (B), CD19 (C), and Bcl-6 (D), followed by horseradish peroxidase (HRP)–conjugated secondary antibody (1:200 dilution). The images were captured by a Zeiss Axiophot microscope equipped with a DC330E CCD camera (Dage MTI, Michigan City, IN) and Scion Image software (Scion, Frederick, MD). The original magnification of the objective lens was 40 ×, and its numerical aperture was 0.75. Scion Image software (Scion, Frederick, MD) was used to capture images.
Analysis of Ig VH gene sequences
Southern blot analysis revealed clonal B-cell rearrangements in 7 of 7 of the BLL cases (not shown). The Ig VH genes were sequenced, with results summarized in Table 1. Strikingly, all were essentially unmutated. The VH sequence of one tumor perfectly matched a germ-line gene of the SM7 VH family. Five tumor sequences showed 100% identity to rearranged VH segments in the IgBLAST database. They were also considered as unmutated because it is unlikely that rearranged VH genes from different cells share exactly the same somatic mutations. The remaining tumor sequence (tumor “ARS”) contained one base difference in comparison with its best-matched VH gene. The status of 2 additional nucleotides in the tumor sequence could not be accurately established because of the ambiguity in the matched VH.
VH gene sequence analysis of BLL
. | Best-matched VH genes . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Tumor designation . | Family . | Accession no. . | Homology, % . | D segment . | JH segment . | ||
| 86 | S107 | AF045506 | 100 | DSP2.9 | JH4 | ||
| 97 | Q52 | U88686 | 100 | n.d. | JH1 | ||
| 99 | J558 | X03088 | 100 | DQ52 | JH2 | ||
| 103 | J558 | X03088 | 100 | DSP2.2 | JH1 | ||
| 180 | 36-60 | X63801 | 100 | DFL16.1 | JH4 | ||
| 193 | SM7 | AC073563 | 100 | DSP2.9 | JH3 | ||
| ARS* | J558 | J04547 | 99 | DSP2.13 | JH2 | ||
. | Best-matched VH genes . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Tumor designation . | Family . | Accession no. . | Homology, % . | D segment . | JH segment . | ||
| 86 | S107 | AF045506 | 100 | DSP2.9 | JH4 | ||
| 97 | Q52 | U88686 | 100 | n.d. | JH1 | ||
| 99 | J558 | X03088 | 100 | DQ52 | JH2 | ||
| 103 | J558 | X03088 | 100 | DSP2.2 | JH1 | ||
| 180 | 36-60 | X63801 | 100 | DFL16.1 | JH4 | ||
| 193 | SM7 | AC073563 | 100 | DSP2.9 | JH3 | ||
| ARS* | J558 | J04547 | 99 | DSP2.13 | JH2 | ||
n.d. indicates not determined.
Sequence contains 2 ambiguous bases
Cell of origin
These data provide conflicting results regarding the placement of the cell of origin in the normal scheme of B-cell development. The BCL6 protein is required for GC formation in normal B cells and expressed at high levels in that site.8 This is mirrored in human B-cell malignancies where BCL6 can be detected in GC tumors, particularly diffuse large cell lymphoma, and in eBL.9
By analogy, BCL6 protein in BLL of iMycEμ mice would point to a GC origin. One possible explanation is that deregulated Myc expression may impose GC features on B cells that have never seen a GC. This is analogous to the consequences of Myc overexpression in vitro that leads lymphoblastoid cells to adopt BL morphology and phenotype.10,11 Although Bcl6 is not known to be a direct target of Myc, it is possible that its expression could be up-regulated indirectly by aberrant Myc expression. The lack of somatic mutations in VH genes in the current model is also characteristic of B-cell lymphomas developing in the previous λ–Myc-transgenic model12 (our unpublished observations, June 2004), indicating a similar origin of these “Burkitt lymphomas” from pre-GC cells.
Our study emphasizes the value of VH-gene data in adding to morphologic and immunophenotypic description of B-cell malignancies.13 This designation can have significant clinical impact as is evident for chronic lymphocytic leukemia where tumors with unmutated or mutated Ig V genes have a completely different prognosis.14,15 Somatic mutational analysis of V genes therefore is now a part of the molecular description of B-cell tumors.13
It is interesting that the cell of origin of human eBL differs from that in the mouse model. In eBL, it had been assumed that the translocation and consequent up-regulation of MYC occurred during VDJ recombination,16 but it is also possible that it occurs in the GC via somatic mutation events.17 Most human eBL and sporadic BL (sBL) have somatically mutated V genes, often with ongoing mutational activity characteristic of a GC location.4 Somatic mutation may also contribute to pathogenesis by introducing sites in the V-gene sequences for addition of oligosaccharides.18 These are positively selected in eBL and could be important for maintenance and growth of tumor in the GC site.18,19 Intense antigen stimulation associated with malaria and other infections would provide continuous drive on the immune system, a factor difficult to mimic in mice but which could influence tumorigenesis. A further major factor likely to influence development of eBL is EBV, associated with virtually all cases. EBV persists in normal B memory cells with a pattern of viral gene expression mimicking that of BL.20 In B cells already bearing a translocation, the proliferative or antiapoptotic pressure from persisting EBV could render them vulnerable to tumor development at the memory stage. This multifactorial route to tumor development is difficult to model in mice, and correlations should be made cautiously.
Prepublished online as Blood First Edition Paper, November 2, 2004; DOI 10.1182/blood-2004-07-2573.
Supported in part by Cancer Research UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal