Abstract
Cyclopentenone prostaglandins are potent inhibitors of nuclear factor-κB (NF-κB), a transcription factor with a critical role in promoting inflammation and connected with multiple aspects of oncogenesis and cancer cell survival. In the present report, we investigated the role of NF-κB in the antineoplastic activity of the cyclopentenone prostaglandin 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) in multiple myeloma (MM) and Burkitt lymphoma (BL) cells expressing constitutively active NF-κB. 15d-PGJ2 was found to suppress constitutive NF-κB activity and potently induce apoptosis in both types of B-cell malignancies. 15d-PGJ2-induced apoptosis occurs through multiple caspase activation pathways involving caspase-8 and caspase-9, and is prevented by pretreatment with the pan-caspase inhibitor ZVAD (z-Val-Ala-Asp). NF-κB inhibition is accompanied by rapid down-regulation of NF-κB-dependent antiapoptotic gene products, including cellular inhibitor-of-apoptosis protein 1 (cIAP-1), cIAP-2, X-chromosome-linked inhibitor-of-apoptosis protein (XIAP), and FLICE-inhibitory protein (cFLIP). These effects were mimicked by the proteasome inhibitor MG-132, but not by the peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist troglitazone, suggesting that 15d-PGJ2-induced apoptosis is independent of PPAR-γ. Knockdown of the NF-κB p65-subunit by lentiviral-mediated shRNA interference also resulted in apoptosis induction in malignant B cells with constitutively active NF-κB. The results indicate that inhibition of NF-κB plays a major role in the proapoptotic activity of 15d-PGJ2 in aggressive B-cell malignancies characterized by aberrant regulation of NF-κB. (Blood. 2005;105:1750-1758)
Introduction
Prostaglandins (PGs) are a family of naturally occurring cyclic 20-carbon fatty acids that are synthesized mainly from arachidonate or other fatty acids released from membrane phospholipids by the action of phospholipases.1 Arachidonate is first transformed to an unstable endoperoxide intermediate by cyclooxygenase (COX) and subsequently converted into one of the several related products, including PGD2, PGE2, and PGF2α, through the action of specific PG synthases. Prostaglandins of the A and J series originate from dehydration within the cyclopentane ring of PGE and PGD, respectively, which produce a cyclopentenone structure characterized by the presence of a reactive α,β-unsaturated carbonyl.
Cyclopentenone prostaglandins (cyPGs) are potent bioactive molecules that possess anti-inflammatory2 and antiviral activity.3 In addition to these effects, cyPGs have been shown to induce cell growth arrest4,5 and apoptosis in a number of cancer cell types.1 In particular, the terminal derivative of prostaglandin J2 (PGJ2) metabolism, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), is emerging as the most potent antineoplastic agent of this class of prostaglandins. Anticancer activity of 15d-PGJ2 has been reported both in vitro and in vivo in a multiplicity of tissues including breast,6 prostate,7 colon,8 lung,9 brain,10 gastric,11 skin,12 hematopoietic,13 and lymphoid.14,15 In most types of cancer, 15d-PGJ2 inhibits tumor cell proliferation and induces apoptosis. However, the mechanism of 15d-PGJ2 antineoplastic activity has not been fully elucidated as yet. 15d-PGJ2 was shown to exert some of its effects by binding to the peroxisome proliferator-activated receptor γ (PPAR-γ).16 More recent evidence, however, indicates that 15d-PGJ2 can act independently from PPAR-γ activation.13,15,17,18 In particular, we have shown that 15d-PGJ2 is a potent inhibitor of the IκB kinase IKK,19 preventing the activation of nuclear factor-κB (NF-κB) through PPAR-γ-independent means.
NF-κB is a critical regulator of the immediate early pathogen response, playing an important role in promoting inflammation, in the control of cell proliferation and survival,20,21 as well as in the regulation of virus replication.22 NF-κB normally exists as an inactive cytoplasmic complex, whose predominant form is a heterodimer composed of p50 and p65 (RelA) subunits bound to inhibitory proteins of the IκB family (IκBs); NF-κB is induced in response to a variety of pathogenic stimuli (including UV radiation and exposure to proinflammatory cytokines or mitogens) and to bacterial or viral infection.20,21 In the case of proinflammatory cytokines, induction requires the activation of the IκB kinase complex, containing 2 catalytic subunits (IKKα and IKKβ) and the IKKγ regulatory subunit, which phosphorylates IκBs triggering their ubiquitination and proteasome-mediated degradation.23 Release of IκBs results in nuclear translocation of NF-κB and its binding to DNA at specific κB sites, rapidly inducing a variety of genes encoding, among others, cell adhesion molecules, inflammatory and chemotactic cytokines, cytokine receptors, and enzymes that produce inflammatory mediators.21 More recently, NF-κB activation has been connected with multiple aspects of oncogenesis, including the control of apoptosis, cell migration, cell cycle progression, and cell differentiation.24 NF-κB can promote cell proliferation either by up-regulating transcription of cyclin D25 and c-Myc,26 which activate the G1 to S phase progression of the cell cycle, or via induction of target genes encoding growth factors and cytokines that stimulate tumor cell proliferation.21 Numerous studies have recently indicated that NF-κB activation also suppresses cell death pathways by switching on genes that dampen proapoptotic signals.24 Recently, it has been shown that NF-κB is constitutively activated in several types of neoplastic cells, and its activation has been associated with various hematologic malignancies.27 In addition, activation of NF-κB in cancer cells by chemotherapy or by radiation has been shown to induce the multidrug resistance response, which impinges on the ability of the therapy itself to induce cell death.28 In this perspective, inhibition of NF-κB is expected to be therapeutic in those tumors where NF-κB appears to play a unique survival role such as Hodgkin and non-Hodgkin B-cell lymphomas,29,30 diffuse large B-cell lymphomas,31 multiple myeloma,32 and Burkitt lymphoma.33
Starting from the observation that cyclopentenone prostanoids are potent inhibitors of NF-κB, in the present study we investigated the effect of the cyclopentenone prostaglandin 15d-PGJ2 on NF-κB activity and survival of B-cell neoplasms. We report that 15d-PGJ2 is able to induce apoptosis in different types of B-cell malignancies, which present constitutively elevated NF-κB levels. These include the multiple myeloma (MM) cell lines U266 and RPMI-8226, and the Burkitt lymphoma (BL) cell lines BL-41 and HS-Sultan. Induction of apoptosis by 15d-PGJ2 was associated with inhibition of constitutive NF-κB DNA-binding activity in all cell lines. Using as a model the HS-Sultan cell line, we show that inhibition of NF-κB by 15d-PGJ2 leads to down-regulation of the expression of several NF-κB-dependent antiapoptotic gene products and to the activation of caspase-8 and caspase-9.
Materials and methods
Cell culture and treatments
Human HS-Sultan, BL-41, and RPMI-8226 cell lines were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA). U266 and K562 were kindly provided by Dr Inghirami (New York University, New York, NY). All cell lines were grown in RPMI 1640 medium, supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and antibiotics at 37°C in a 5% CO2 humidified atmosphere. Treatments with prostaglandins were performed in cells (5 × 105/mL, unless differently specified) seeded in RPMI 1640 medium without serum. Fetal calf serum was added 30 minutes after prostaglandin treatment to a final concentration of 10%. Cell viability was determined at the designed intervals by vital-dye exclusion assay (trypan blue, 0.1%). 12-O-tetradecanoylphorbol 13-acetate (TPA) and dexamethasone were obtained from Sigma (St Louis, MO). Prostaglandins A1 (PGA1), A2 (PGA2), D2 (PGD2), E2 (PGE2), F2α (PGF2α), J2 (PGJ2), 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), and troglitazone were obtained from Cayman Chemical (Ann Arbor, MI). Z-VAD-FMK (z-Val-Ala-Asp-fluoromethylketone), Z-Ile-Glu-Asp-Thr-FMK (Z-IEDT-FMK), Z-Leu-Glu-His-Asp-FMK (Z-LEHD-FMK), Z-Asp-Glu-Val-Glu-FMK (Z-DEVD-FMK), and MG-132 were from Alexis Biochemicals (San Diego, CA).
Lentiviral vector construction, virus production, and in vitro transduction
Human p65-specific short hairpin RNA (shRNA) oligo adapted from the murine p65 shRNA described by Tiscornia et al34 was introduced into the pSUPER vector (OligoEngine). The fidelity of the inserts was verified by sequencing the region of interest. The sense strands of the shRNA (shp65) inserts were as follows: gatccccGAGTTTCAGCAGCTGCTGAACttcaagagaGTTCAGCAGCTGCTGAAACTCtttttggaaa. The 19-nucleotide p65 target sequences are indicated in uppercase in the oligonucleotide sequence. To this sequence, 4 point mutations were randomly introduced to generate the control shRNA (shC). LV-shp65 and LV-shC vectors were constructed by subcloning the H1 promoter-p65shRNA cassette into the EcoRV-XhoI sites of the vector pCCL.sin.PPT.hPGK.GFPWpre, kindly provided by Dr Luigi Naldini (San Raffaele Scientific Institute, Milan, Italy). High-titer lentiviral vector stock was produced in 293T cells by calcium phosphate-mediated transfection of the modified transfer vector and the packaging vectors pMDLg/pRRE, pRSV-Rev, and pMD2.VSVG. Virus was harvested over the following 36 to 60 hours and concentrated by ultracentrifugation (50 000g). Titers of virus preparations were determined by measuring the amount of HIV-1 p24 antigen by enzyme-linked immunosorbent assay (ELISA; NEN Life Sciences, Boston, MA). HS-Sultan, K562, and RPMI-8226 cells (1 × 105 cells/well in 24-well plates) were transduced with 300 ng lentiviral p24 in the presence of polybrene (8 μg/mL).
Western blot analysis
For Western blot analysis, whole-cell extracts were prepared after lysis in extraction buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, 5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM Na3VO4, and 1 mM phenylmethyl sulfonyl fluoride and protease inhibitors). Equal amounts of protein (20 μg/sample) from whole-cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The filters were first blocked (5% low-fat milk in phosphate-buffered saline [PBS] with 0.1% Tween 20; 1 hour at room temperature) and then incubated with the primary antibodies for 1 hour at room temperature. After 3 washes, filters were incubated with horseradish peroxidase-conjugated goat antimouse or antirabbit antibodies (1:10 000; Amersham, Arlington Heights, IL) for one hour at room temperature. Detection of immunocomplexes was performed with an enhanced chemiluminescence system (SuperSignal West; Pierce, Rockford, IL). Monoclonal antibodies (MAbs) to α-tubulin were purchased from Sigma; heat shock protein 70 (Hsp70), from Stressgen (Victoria, BC); and Bcl-2, from DAKO (Carpinteria, CA). Rabbit polyclonal antibodies to caspase-8, caspase-9, IκBα, poly(adenosine diphosphate-ribose) polymerase (PARP), Bcl-XL, p65, and goat polyclonal to caspase-3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to FLICE-inhibitory protein (cFLIP) were purchased from Upstate Biotechnology (Lake Placid, NY); rabbit polyclonal antibodies to X-chromosome-linked inhibitor-of-apoptosis protein (XIAP), cellular inhibitor-of-apoptosis protein 1 (cIAP-1), and cIAP-2, from R&D Systems (Minneapolis, MN); phospho-IκBα Ser32, from Cell Signaling Technology (Beverly, MA); and green fluorescent protein (GFP), from Molecular Probes (Eugene, OR).
Electrophoretic mobility-shift assay (EMSA)
Aliquots of total extracts (10 μg protein/sample) in 0.1% Triton X-100 lysis buffer were incubated with 32P-labeled κB DNA probe35 in binding buffer for 30 minutes at room temperature as described.36 DNA-protein complexes were analyzed by nondenaturing 4% polyacrylamide gel electrophoresis. Specificity of protein-DNA complexes was verified by supershift analysis with polyclonal antibodies for c-Rel, p65 (Rel A), p50, and p52 from Santa Cruz Biotechnology. Quantitative evaluation of NF-κB-κB complex formation was determined by Typhoon 8600 imager (Molecular Dynamics, Sunnyvale, CA) with the use of ImageQuant software from Amersham (MDP analysis). For control of equal loading, NF-κB values were normalized to the level of the nonspecific protein-DNA complex (ns) in the same lane.
Kinase assay
Cell lysates were immunoprecipitated with anti-IKKα antibodies (Cell Signaling Technology) in the presence of 20 μL protein-A-Sepharose (Sigma) at 4°C for 16 hours. After extensive washing, endogenous IKK activity was determined using glutathione-S-transferase (GST)-IκBα (1-54) as substrate.37 Western blot analysis of IKKα was performed as kinase loading control.
Flow cytometry
For DNA content determination, cells were fixed for one hour in 70% ethanol at 4°C. After washing, cells were treated with RNase (0.25 mg/mL) and stained with propidium iodide (PI, 50 μg/mL). Cell cycle profile was analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA). The sub-G1/G0 phase fraction was calculated using the CellQuest program (Becton Dickinson). For annexin V staining, cells were washed once with PBS and resuspended in staining buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl) with 5 mL annexin V-PI (annexin V-fluorescein isothiocyanate [FITC]; Becton Dickinson). After 15 minutes of incubation in the dark, cells were directly analyzed by FACScan and evaluated by the CellQuest program. Data were plotted using a histogram algorithm, in which the intensities of annexin V-positive cells were represented in a log 10 scale.
Results
15d-PGJ2 inhibits cell growth and induces apoptosis in human HS-Sultan Burkitt lymphoma cells
Cyclopentenone prostaglandins have been implicated in the inhibition of cell cycle progression and/or in the induction of apoptosis in different types of cancers. To investigate the effect of cyPGs on the proliferation and cell death of B-cell malignancies, human Burkitt lymphoma HS-Sultan cells were plated at a density of 3 × 105 cells/mL and treated with 10 μM 15d-PGJ2 or control diluent. In the same experiment, the effect of 15d-PGJ2 was compared with the effect of the noncyclopentenone prostaglandin PGF2α. The number of viable cells was determined for the next 72 hours at 24-hour intervals. As shown in Figure 1A, 15d-PGJ2 treatment caused a complete block of HS-Sultan cell proliferation, whereas the same concentration of PGF2α had no effect.
15d-PGJ2inhibits cell growth and induces apoptosis in HS-Sultan cells. (A-B) HS-Sultan cells (3 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM, ▪), PGF2α (10 μM, ⋄), or control diluent (×) for the indicated time. The number of viable (A) and dead (B) cells was determined at 24 (□), 48 (▦), and 72 hours (▪) after treatment. The results are representative of 3 independent experiments. (C) HS-Sultan cells (5 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM). Cell cycle profile was evaluated at 0 (Control), 24, 48, or 72 hours after treatment by PI staining using flow cytometry. The percentage of sub-G0/G1 cells is indicated on each panel. (D) HS-Sultan cells (5 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM). Apoptosis was evaluated at 0 (control), 4, 8, and 24 hours after treatment by annexin V-PI staining using flow cytometry. The percentage of annexin V+-PI- cells is indicated on each panel. Early externalization of phosphatidylserine confirmed that 15d-PGJ2 induces apoptosis in HS-Sultan cells.
15d-PGJ2inhibits cell growth and induces apoptosis in HS-Sultan cells. (A-B) HS-Sultan cells (3 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM, ▪), PGF2α (10 μM, ⋄), or control diluent (×) for the indicated time. The number of viable (A) and dead (B) cells was determined at 24 (□), 48 (▦), and 72 hours (▪) after treatment. The results are representative of 3 independent experiments. (C) HS-Sultan cells (5 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM). Cell cycle profile was evaluated at 0 (Control), 24, 48, or 72 hours after treatment by PI staining using flow cytometry. The percentage of sub-G0/G1 cells is indicated on each panel. (D) HS-Sultan cells (5 × 105 cells/mL) were treated with 15d-PGJ2 (10 μM). Apoptosis was evaluated at 0 (control), 4, 8, and 24 hours after treatment by annexin V-PI staining using flow cytometry. The percentage of annexin V+-PI- cells is indicated on each panel. Early externalization of phosphatidylserine confirmed that 15d-PGJ2 induces apoptosis in HS-Sultan cells.
To investigate whether treatment with 15d-PGJ2 would lead to cell death, cell survival was measured by trypan blue dye exclusion and by determination of the DNA content through fluorescence-activated cell sorter (FACS) analysis on HS-Sultan cells treated with 10 μM 15d-PGJ2 or PGF2α for 24, 48, and 72 hours. 15d-PGJ2 induced a progressive increase in cell death, as determined by trypan blue (Figure 1B) and by FACS analysis of hypodiploid cells (sub-G0/G1 phase) (Figure 1C). The time-dependent increase in cell death after 15d-PGJ2 treatment reached a ratio higher than 70% at 72 hours, while PGF2α had no effect on cell viability. Similar results were shown in a different type of B-cell malignancy, the human multiple myeloma RPMI-8226 cells (data not shown).
To further analyze the mechanism of 15d-PGJ2-induced cell death, induction of apoptosis was measured by annexin V-PI staining, which detects the externalization of phosphatidylserine, a characteristic feature of cells entering apoptosis. 15d-PGJ2 (10 μM) was found to induce the appearance of annexin V+-PI- cells, early after treatment. As shown in Figure 1D, 30% of treated cells were annexin V+-PI- already at 4 hours, and 79% at 8 hours after addition of 15d-PGJ2. The number of annexin V+ cells was higher than 80% at 24 hours after treatment, confirming that 15d-PGJ2 is a potent inducer of apoptosis in this type of malignancy.
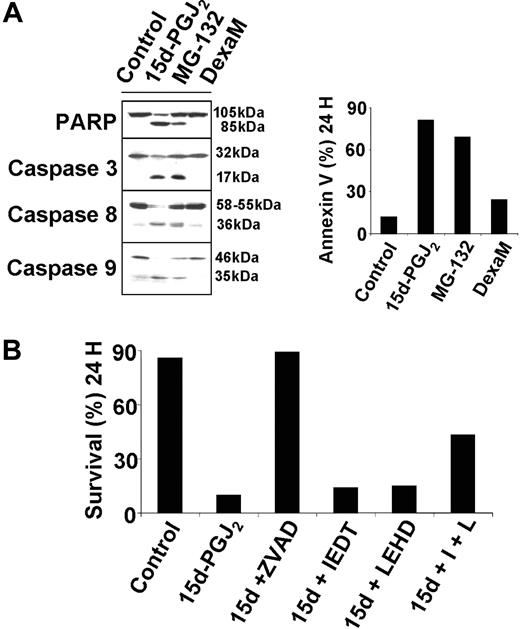
15d-PGJ2-induced apoptosis requires activation of multiple caspases
The results described in the previous section prompted us to focus on the apoptotic signaling induced by 15d-PGJ2 in these cells, and specifically on the cascade of caspase activation. The effect of 15d-PGJ2 on HS-Sultan cells was compared with caspase activation triggered by the proteasome inhibitor MG-132, which is known to block NF-κB activation by preventing IκBα degradation, or by the conventional drug dexamethasone. Cells were treated with 15d-PGJ2, MG-132, dexamethasone, or control diluent, and after 8 hours whole-cell lysates were processed for analysis of caspase activation by immunoblotting. In the same experiment, apoptosis was evaluated after 24 hours by annexin V-PI staining using flow cytometry. Activation of caspases by 15d-PGJ2 was suggested by the cleavage of the caspase-3 substrate, poly(ADP-ribose) polymerase (PARP), and confirmed by the cleavage of caspase-3, caspase-8, and caspase-9 (Figure 2A, left panels). The activation of caspases induced by both MG-132 and 15d-PGJ2 was parallel to high levels of apoptosis at 24 hours (Figure 2A, right panel), whereas dexamethasone was only a weak inducer of apoptosis and did not cause caspase activation, as detected by Western blotting. To evaluate the functional involvement of caspases in 15d-PGJ2-induced cell death, we used the pan-caspase inhibitor ZVAD-FMK and specific inhibitors of caspase-3, caspase-8, or caspase-9. Pretreatment with the pan-caspase inhibitor ZVAD-FMK completely abolished 15d-PGJ2-induced apoptosis (Figure 2B). On the contrary, the caspase-3 (DEVD-FMK) (not shown), the caspase-8 (IETD-FMK), or the caspase-9 (LEHD-FMK) inhibitors had no protective effect if provided singularly; however, the combined treatment of caspase-8 and caspase-9 inhibitors partially protected HS-Sultan cells from 15d-PGJ2-induced apoptosis (Figure 2B). These studies confirm the requirement for caspase activation during 15d-PGJ2-triggered apoptosis and suggest the activation of multiple apoptotic signaling cascades involving both caspase-8 and caspase-9.
Functional involvement of caspases in 15d-PGJ2-induced apoptosis. (A) HS-Sultan cells were treated with 10 μM 15d-PGJ2, 0.25 μM proteasome inhibitor MG-132, 10 μM dexamethasone (DexaM), or control diluent for 8 or 24 hours. Whole-cell lysates at 8 hours after treatment were immunoblotted with the indicated antibodies (left panel). Apoptosis was evaluated at 24 hours by annexin V-PI staining using flow cytometry (right panel). (B) HS-Sultan cells were pretreated with 20 μM of the pancaspase inhibitor ZVAD-FMK (ZVAD), the caspase-8 inhibitor IETD-FMK (IEDT), the caspase-9 inhibitor LEHD-FMK (LEHD), or with a combination of IETD-FMK and LEHD-FMK (I + L), one hour before exposure to 15d-PGJ2 (10 μM). Cell survival, evaluated at 24 hours after 15d-PGJ2 treatment, is expressed as the percentage of annexin V--PI- cells measured by FACS analysis. The results are representative of 3 independent experiments.
Functional involvement of caspases in 15d-PGJ2-induced apoptosis. (A) HS-Sultan cells were treated with 10 μM 15d-PGJ2, 0.25 μM proteasome inhibitor MG-132, 10 μM dexamethasone (DexaM), or control diluent for 8 or 24 hours. Whole-cell lysates at 8 hours after treatment were immunoblotted with the indicated antibodies (left panel). Apoptosis was evaluated at 24 hours by annexin V-PI staining using flow cytometry (right panel). (B) HS-Sultan cells were pretreated with 20 μM of the pancaspase inhibitor ZVAD-FMK (ZVAD), the caspase-8 inhibitor IETD-FMK (IEDT), the caspase-9 inhibitor LEHD-FMK (LEHD), or with a combination of IETD-FMK and LEHD-FMK (I + L), one hour before exposure to 15d-PGJ2 (10 μM). Cell survival, evaluated at 24 hours after 15d-PGJ2 treatment, is expressed as the percentage of annexin V--PI- cells measured by FACS analysis. The results are representative of 3 independent experiments.
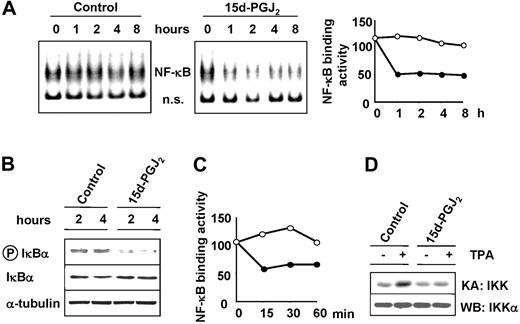
15d-PGJ2 inhibits constitutive NF-κB activity in HS-Sultan cells
NF-κB, a transcription factor involved in the control of apoptosis, cell cycle progression, and cell differentiation,38 has been shown to be constitutively activated in several types of neoplastic cells, and this event has been associated with various hematologic malignancies.27 We have shown that cyclopentenone prostanoids, including 15d-PGJ2, are potent inhibitors of NF-κB activation by inflammatory cytokines, mitogens, and viral infection in different types of cells.19,39-42 To investigate the effect of 15d-PGJ2 on NF-κB activity in B malignant cells, HS-Sultan cells were treated with 10 μM 15d-PGJ2 or control diluent. In a parallel experiment, HS-Sultan cells were treated with 10 μM 15d-PGJ2 or control diluent for 2 hours and then stimulated with the phorbol ester TPA (100 ng/mL). At different times after treatment, whole-cell extracts were analyzed for NF-κB activity by EMSA, and the levels of NF-κB DNA-binding activity were quantified by MDP analysis. HS-Sultan cells were found to display a constitutively high NF-κB DNA-binding activity (Figure 3A). The major NF-κB band in HS-Sultan cells consisted of the p65/RelA subunit, as determined by supershift analysis (data not shown). 15d-PGJ2 caused a marked inhibition of constitutive NF-κB activity in HS-Sultan cells already at one hour after treatment (Figure 3A). Inhibition of NF-κB DNA-binding activity persisted for at least 8 hours. CyPGs have been shown to inhibit tumor necrosis factor α (TNF-α) or TPA-induced NF-κB activation by blocking the activity of the IκB kinase IKK in Jurkat cells.19 In HS-Sultan cells, inhibition of constitutive IKK activity was suggested by the decreased levels of phosphorylated IκBα in 15d-PGJ2-treated cells as detected by Western blot analysis (Figure 3B). Under the conditions used, treatment with TPA only slightly increased the level of NF-κB activity, and this effect was prevented by 15d-PGJ2 treatment (Figure 3C). As determined by kinase assay, TPA treatment caused an increase in IKK activity at 15 minutes after stimulation (Figure 3D). 15d-PGJ2 treatment prevented TPA-induced IKK stimulation, indicating that, as previously shown in other cell types, the prostanoid is able to inhibit the IκB kinase in HS-Sultan cells.
15d-PGJ2 inhibits NF-κB activity in HS-Sultan cells. HS-Sultan cells were treated with 15d-PGJ2 (10 μM) or control diluent. (A) At the indicated times, whole-cell extracts were analyzed for NF-κB activation by EMSA (left panel). A section of the fluorogram is shown. Positions of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. The levels of NF-κB DNA-binding activity in control (○) and 15d-PGJ2-treated (•) cells were quantified by MDP analysis and expressed as arbitrary units (right panel). (B) At 2 and 4 hours after 15d-PGJ2 treatment, levels of total (IκBα) and phosphorylated (P-IκBα) IκBα were determined by Western blot analysis. Levels of α-tubulin are shown as control. (C) In a parallel experiment, HS-Sultan cells were treated with 10 μM 15d-PGJ2 (•) or control diluent (○) for 2 hours and then stimulated with the phorbol ester TPA (100 ng/mL). At different times after treatment, whole-cell extracts were analyzed for NF-κB DNA-binding activity by EMSA, and the levels of NF-κB DNA-binding activity were quantitated by MDP analysis and expressed as arbitrary units. (D) HS-Sultan cells were treated with 15d-PGJ2 (10 μM) or control diluent for 2 hours prior to stimulation with TPA (100 ng/mL). At 15 minutes after TPA addition, whole-cell extracts were analyzed for IKK activity by kinase assay (KA: IKK). Endogenous IKK recovery was determined in the same samples by immunoblot analysis for IKKα (WB: IKKα).
15d-PGJ2 inhibits NF-κB activity in HS-Sultan cells. HS-Sultan cells were treated with 15d-PGJ2 (10 μM) or control diluent. (A) At the indicated times, whole-cell extracts were analyzed for NF-κB activation by EMSA (left panel). A section of the fluorogram is shown. Positions of NF-κB-DNA (NF-κB) and nonspecific protein-DNA (ns) complexes are indicated. The levels of NF-κB DNA-binding activity in control (○) and 15d-PGJ2-treated (•) cells were quantified by MDP analysis and expressed as arbitrary units (right panel). (B) At 2 and 4 hours after 15d-PGJ2 treatment, levels of total (IκBα) and phosphorylated (P-IκBα) IκBα were determined by Western blot analysis. Levels of α-tubulin are shown as control. (C) In a parallel experiment, HS-Sultan cells were treated with 10 μM 15d-PGJ2 (•) or control diluent (○) for 2 hours and then stimulated with the phorbol ester TPA (100 ng/mL). At different times after treatment, whole-cell extracts were analyzed for NF-κB DNA-binding activity by EMSA, and the levels of NF-κB DNA-binding activity were quantitated by MDP analysis and expressed as arbitrary units. (D) HS-Sultan cells were treated with 15d-PGJ2 (10 μM) or control diluent for 2 hours prior to stimulation with TPA (100 ng/mL). At 15 minutes after TPA addition, whole-cell extracts were analyzed for IKK activity by kinase assay (KA: IKK). Endogenous IKK recovery was determined in the same samples by immunoblot analysis for IKKα (WB: IKKα).
15d-PGJ2 activity has been associated with both NF-κB inhibition and PPARγ activation.16,39 In order to investigate the role of NF-κB in 15d-PGJ2-induced apoptosis, the effect of different arachidonic acid metabolites, including noncyclopentenone PGs (PGE2, PGF2α) and reactive cyclopentenone PGs (PGA2, 15d-PGJ2), on inhibition of NF-κB and induction of apoptosis was examined in HS-Sultan cells. The effect of the prostanoids was compared with that of the proteasome inhibitor MG-132 and of the PPARγ agonist troglitazone. NF-κB DNA-binding activity was assayed by EMSA 3 hours after treatment. As shown in Figure 4A, NF-κB activity was strongly inhibited in cells treated with the proteasome inhibitor. 15d-PGJ2 was found to be as potent as MG-132 in inhibiting NF-κB in these cells, while PGA2 was less active. The noncyclopentenone prostaglandins PGE2 and PGF2α and troglitazone had no effect on NF-κB activity.
Effects of arachidonic acid metabolites, MG-132, and troglitazone on cell death and NF-κB activity. HS-Sultan cells were treated with 10 μM PGA2, PGE2, PGF2α, 15d-PGJ2, 0.25 μM proteasome inhibitor MG-132, 10 μM PPARγ agonist troglitazone (Troglit), or control diluent. (A) After 3 hours, whole-cell lysates were assayed for NF-κB DNA-binding activity by EMSA (top panel). The levels of NF-κB DNA-binding activity were quantified by MDP analysis and expressed as percent of control (bottom panel). (B) At 8 (□), 24 (▦), and 48 hours (▪), apoptosis was evaluated by FACS analysis of annexin V+ cells.
Effects of arachidonic acid metabolites, MG-132, and troglitazone on cell death and NF-κB activity. HS-Sultan cells were treated with 10 μM PGA2, PGE2, PGF2α, 15d-PGJ2, 0.25 μM proteasome inhibitor MG-132, 10 μM PPARγ agonist troglitazone (Troglit), or control diluent. (A) After 3 hours, whole-cell lysates were assayed for NF-κB DNA-binding activity by EMSA (top panel). The levels of NF-κB DNA-binding activity were quantified by MDP analysis and expressed as percent of control (bottom panel). (B) At 8 (□), 24 (▦), and 48 hours (▪), apoptosis was evaluated by FACS analysis of annexin V+ cells.
In the same experiment, apoptosis was measured by FACS analysis of annexin V+-PI- cells at 8, 24, and 48 hours. Among the different arachidonic acid derivatives, the cyclopentenone prostanoids induced apoptosis, with 15d-PGJ2 being the most active compound and inducing apoptosis in more than 80% of the cells at 24 hours, while PGE2 and PGF2α did not induce any significant increase in annexin V-positive cells (Figure 4B). The proteasome inhibitor MG-132 was able to stimulate apoptosis at an extent comparable with 15d-PGJ2. Despite the fact that 15d-PGJ2 is a PPARγ activator, apoptosis was not mimicked by the synthetic PPARγ ligand troglitazone (10 μM). These data suggest that 15d-PGJ2-induced apoptosis is mediated via a mechanism independent of PPARγ ligation, and most likely dependent on NF-κB inhibition.
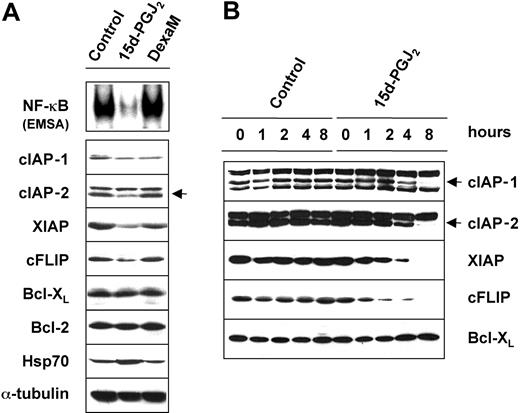
15d-PGJ2 down-regulates the expression of antiapoptotic proteins
Since induction of apoptosis by 15d-PGJ2 was found to be associated with NF-κB inhibition in HS-Sultan cells, we evaluated the effect of this prostanoid on the level of several survival-regulating proteins, whose expression is known to be modulated by the transcriptional activity of NF-κB. The effect of 15d-PGJ2 was compared with that of the chemotherapeutic drug dexamethasone. HS-Sultan cells were treated with 10 μM 15d-PGJ2 or dexamethasone for 8 hours. At this time, whole-cell extracts were analyzed for the level of NF-κB DNA-binding activity by EMSA, whereas expression of NF-κB-dependent gene products was determined by Western blot analysis. As a control of 15d-PGJ2 activity, we determined the level of the heat shock protein Hsp70, whose expression is known to be induced by cyclopentenone prostanoids via the activation of the heat shock transcription factor type 1.43 As expected, the high level of NF-κB constitutive activity in HS-Sultan cells was dramatically decreased by treatment with 15d-PGJ2, whereas dexamethasone had no effect (Figure 5A, top panel). The analysis of NF-κB-modulated genes showed that treatment with 15d-PGJ2 decreased the expression of proteins that inhibit apoptosis, such as cellular inhibitor-of-apoptosis protein 1 (cIAP-1) and cIAP-2, X-chromosome-linked inhibitor-of-apoptosis protein (XIAP), and FLICE-inhibitory protein (cFLIP) (Figure 5A, lower panels). In contrast, Bcl-XL and Bcl-2 protein levels were not altered, while the level of Hsp70 was increased as expected. Dexamethasone treatment had no effect on any of the proteins analyzed.
15d-PGJ2down-regulates the expression of cellular inhibitor-of-apoptosis proteins (cIAPs) and cFLIP. (A) HS-Sultan cells were treated with 10 μM 15d-PGJ2,10 μM dexamethasone (DexaM), or control diluent for 8 hours. Whole-cell lysates were assayed for NF-κB DNA-binding activity by EMSA (top panel) or immunoblotted with the indicated antibodies (lower panels). Inhibition of NF-κB by 15d-PGJ2 is associated with a decrease in the expression of cIAP-1, cIAP-2, cFLIP, and XIAP. (B) Time-course modulation of IAP protein expression in HS-Sultan cells treated with 15d-PGJ2 (10 μM) or control diluent. At the indicated times, the levels of cIAP-1, cIAP-2, XIAP, cFLIP, and Bcl-XL were determined by Western blot analysis. cIAP-1 and cIAP-2 bands are indicated by arrows.
15d-PGJ2down-regulates the expression of cellular inhibitor-of-apoptosis proteins (cIAPs) and cFLIP. (A) HS-Sultan cells were treated with 10 μM 15d-PGJ2,10 μM dexamethasone (DexaM), or control diluent for 8 hours. Whole-cell lysates were assayed for NF-κB DNA-binding activity by EMSA (top panel) or immunoblotted with the indicated antibodies (lower panels). Inhibition of NF-κB by 15d-PGJ2 is associated with a decrease in the expression of cIAP-1, cIAP-2, cFLIP, and XIAP. (B) Time-course modulation of IAP protein expression in HS-Sultan cells treated with 15d-PGJ2 (10 μM) or control diluent. At the indicated times, the levels of cIAP-1, cIAP-2, XIAP, cFLIP, and Bcl-XL were determined by Western blot analysis. cIAP-1 and cIAP-2 bands are indicated by arrows.
In a separate experiment, the levels of the antiapoptotic proteins cIAP-1, cIAP-2, XIAP, cFLIP, and Bcl-XL were determined at different times after 15d-PGJ2 treatment. Levels of cFLIP and XIAP were found to be decreased as early as 2 hours after exposure to the prostanoid, while levels of cIAP-1 and cIAP-2 proteins started to decrease at later times (4 hours) after treatment (Figure 5B). Finally, among the NF-κB targets that positively regulate cell cycle and proliferation, we found that c-Myc and cyclin D3 expression were also dramatically decreased by 15d-PGJ2 treatment (data not shown).
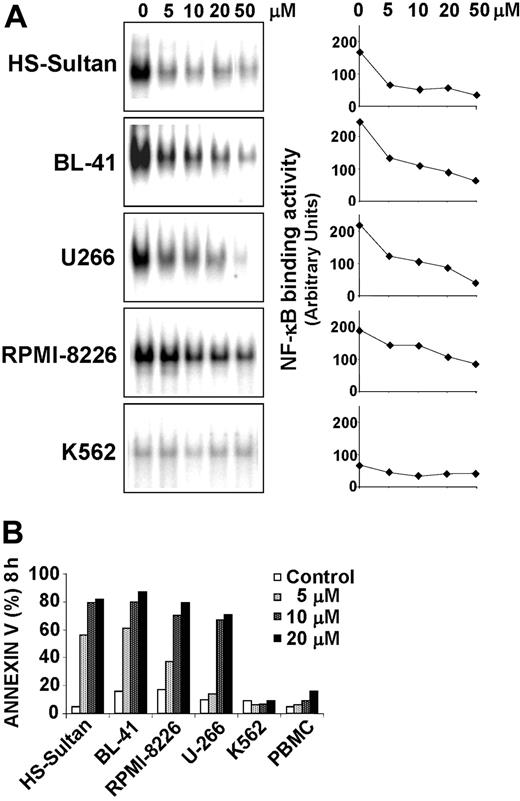
15d-PGJ2 induces apoptosis in different types of MM and BL cells with constitutively high levels of NF-κB DNA-binding activity
In order to investigate whether 15d-PGJ2 was able to induce apoptosis in other types of B malignant cells and whether this effect was associated with inhibition of NF-κB, we determined the status of constitutive NF-κB DNA-binding activity in a different Burkitt lymphoma cell line (BL-41), 2 multiple myeloma cell lines (RPMI-8226 and U-266), and a human acute leukemia cell line (K-562). HS-Sultan, BL-41, U266, RPMI-8226, and K562 cells (0.5 × 106 cell/mL) were treated with different concentrations of 15d-PGJ2 or control diluent for 3 hours. At this time, whole-cell extracts were analyzed for NF-κB activity by EMSA, and the levels of NF-κB DNA-binding activity were quantified by MDP analysis. The results shown in Figure 6A indicate that all MM and BL cell lines tested expressed constitutively high levels of NF-κB DNA-binding activity, while K562 cells showed very low levels of active NF-κB. 15d-PGJ2 treatment caused a significant inhibition of NF-κB activity in all MM and BL cell lines tested with an average median inhibitory concentration (IC50) lower than 10 μM. In a parallel experiment, the effect of 15d-PGJ2 on induction of apoptosis was determined on the same cell lines after 8 hours of treatment. As shown in Figure 6B, treatment with 15d-PGJ2 at concentrations ranging from 5 to 10 μM caused a dramatic increase in the number of annexin V-positive cells (>70%) in HS-Sultan, BL-41, RPMI-8226, and U-266 cells. On the contrary, K562 cells, which presented very low levels of NF-κB DNA-binding activity not altered by 15d-PGJ2 treatment, were resistant to 15d-PGJ2-induced apoptosis even at high concentrations (20 μM). No evidence of apoptosis induction was found in K562 cells also at later times, at up to 24 hours of exposure to 15d-PGJ2 (data not shown).
15d-PGJ2inhibits constitutive NF-κB binding activity and induces apoptosis in different types of B-cell malignancies. (A) Burkitt lymphoma HS-Sultan and BL-41 cells, multiple myeloma U266 and RPMI-8226 cells, and acute leukemia K562 cells (0.5 × 106 cell/mL) were treated with the indicated concentration of 15d-PGJ2 for 3 hours and tested for NF-κB DNA-binding activity by EMSA (left column). The levels of NF-κB DNA-binding activity were quantified by MDP analysis and expressed as arbitrary units (right column). (B) HS-Sultan, BL-41, RPMI-8226, U266, and K562 cells, and healthy donor peripheral blood mononucleated cells (PBMC) were treated with 15d-PGJ2 at the indicated concentrations (control represented by open bars; 5 μM, light gray bars; 10 μM, dark gray bars; 20 μM, filled bars) for 8 hours. Apoptosis was evaluated by FACS analysis of annexin V+ cells.
15d-PGJ2inhibits constitutive NF-κB binding activity and induces apoptosis in different types of B-cell malignancies. (A) Burkitt lymphoma HS-Sultan and BL-41 cells, multiple myeloma U266 and RPMI-8226 cells, and acute leukemia K562 cells (0.5 × 106 cell/mL) were treated with the indicated concentration of 15d-PGJ2 for 3 hours and tested for NF-κB DNA-binding activity by EMSA (left column). The levels of NF-κB DNA-binding activity were quantified by MDP analysis and expressed as arbitrary units (right column). (B) HS-Sultan, BL-41, RPMI-8226, U266, and K562 cells, and healthy donor peripheral blood mononucleated cells (PBMC) were treated with 15d-PGJ2 at the indicated concentrations (control represented by open bars; 5 μM, light gray bars; 10 μM, dark gray bars; 20 μM, filled bars) for 8 hours. Apoptosis was evaluated by FACS analysis of annexin V+ cells.
In the same experiment, peripheral blood mononucleated cells (PBMCs) from healthy volunteers were also examined for their susceptibility to 15d-PGJ2. As shown in Figure 6B, 15d-PGJ2 treatment did not induce a significant increase in the number of annexin V-positive cells in PBMCs from a healthy donor even at highest concentrations (20 μM). These findings are consistent with the reported increased sensitivity to NF-κB inhibitors of malignant B cells relative to normal lymphocytes.44
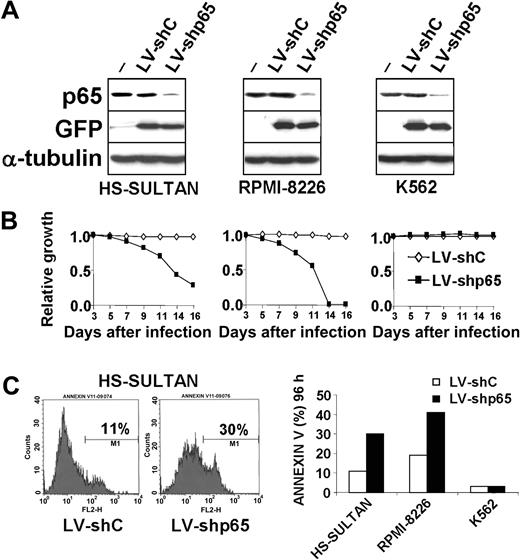
Down-regulation of endogenous p65 expression results in induction of apoptosis in HS-Sultan and RPMI-8226 cells
In order to provide a functional demonstration that the induction of apoptosis observed in malignant B cells is mediated by inactivation of NF-κB, we used lentiviral-mediated RNA interference to induce a knockdown of the NF-κB p65 subunit, which, as indicated previously, was found to represent the major constituent of the NF-κB complex in HS-Sultan cells. Lentiviral vectors containing shRNA for the p65 gene (LV-shp65) and a GFP reporter were generated as described in “Material and methods,” and used to knockdown p65 expression in HS-Sultan and RPMI-8226 cells, which presented constitutively elevated NF-κB activity and were sensitive to the proapoptotic effect of 15d-PGJ2. In parallel, the effect of the LV-shp65 vector was determined in human acute leukemia K562 cells, which presented very low levels of NF-κB DNA-binding activity and were resistant to 15d-PGJ2-induced apoptosis.
In order to determine whether interfering with p65 expression would lead to cell growth inhibition, HS-Sultan, RPMI-8226, and K562 cells were transduced with the LV-shp65 vector or the mutated LV-shC vector as a control. Starting 3 days after infection, the percentage of GFP-positive cells was determined by flow cytometry and monitored for a period of 2 weeks, as a measure of the relative growth disadvantage that is conferred by the knockdown of p65 compared with the mock vector-infected population growing under identical conditions. In each experiment, the initial percentage of GFP-positive cells, determined 3 days after infection, varied between 85% and 99% (data not shown). At different times after transduction, p65 protein levels were analyzed by immunoblotting. Partial knockdown of p65 expression was evident at 72 hours after infection in all cell lines (Figure 7A). Measurement of GFP-positive cells evidenced a growth disadvantage in HS-Sultan and RPMI-8226 cell lines transduced with the LV-shp65 construct compared with cells transfected with the control LV-shC vector (Figure 7B). On the other hand, LV-shp65 transduction did not induce any significant variation in the GFP-positive population of K562 cells (Figure 7B).
Inhibition of p65 expression induces apoptosis in HS-Sultan and RPMI-8226 cells. (A) HS-Sultan, RPMI-8226, and K562 cells mock-transduced (-) or transduced with the LV-shp65 or the mutated control LV-shC vectors were lysed 72 hours after infection, and whole-cell lysates were immunoblotted with antibodies to p65, GFP, and α-tubulin, as a loading control. (B) Growth-inhibiting function of p65 RNA interference in HS-Sultan and RPMI-8226 cells. HS-Sultan (left), RPMI-8226 (middle), and K562 cells (right) were transduced with the PGK-GFP LV-shC (♦) or LV-shp65 (▪) lentiviral vectors. The percentage of GFP-positive cells was determined by flow cytometry and monitored every 48 hours beginning at day 3 after infection for a period of 2 weeks. Decrease of GFP-positive population over time indicates a relative growth disadvantage. (C) p65 RNA interference induces apoptosis in HS-Sultan and RPMI-8226 cells. (Left) Histograms of FACS analysis of annexin V staining in HS-Sultan cells 5 days after infection with LV-shp65 or control LV-shC vectors. (Right) Apoptosis in HS-Sultan, RPMI-8226, and K562 cells transduced with either LV-shp65 (▪) or control LV-shC (□) vectors was evaluated by FACS analysis of annexin V+ cells 4 days after transduction. The results are representative of 3 independent experiments.
Inhibition of p65 expression induces apoptosis in HS-Sultan and RPMI-8226 cells. (A) HS-Sultan, RPMI-8226, and K562 cells mock-transduced (-) or transduced with the LV-shp65 or the mutated control LV-shC vectors were lysed 72 hours after infection, and whole-cell lysates were immunoblotted with antibodies to p65, GFP, and α-tubulin, as a loading control. (B) Growth-inhibiting function of p65 RNA interference in HS-Sultan and RPMI-8226 cells. HS-Sultan (left), RPMI-8226 (middle), and K562 cells (right) were transduced with the PGK-GFP LV-shC (♦) or LV-shp65 (▪) lentiviral vectors. The percentage of GFP-positive cells was determined by flow cytometry and monitored every 48 hours beginning at day 3 after infection for a period of 2 weeks. Decrease of GFP-positive population over time indicates a relative growth disadvantage. (C) p65 RNA interference induces apoptosis in HS-Sultan and RPMI-8226 cells. (Left) Histograms of FACS analysis of annexin V staining in HS-Sultan cells 5 days after infection with LV-shp65 or control LV-shC vectors. (Right) Apoptosis in HS-Sultan, RPMI-8226, and K562 cells transduced with either LV-shp65 (▪) or control LV-shC (□) vectors was evaluated by FACS analysis of annexin V+ cells 4 days after transduction. The results are representative of 3 independent experiments.
To determine whether the growth disadvantage induced by p65 knockdown in HS-Sultan and RPMI-8226 cells was a consequence of reduced proliferation or stimulation of cell death, the percentage of apoptotic cells was measured by FACS analysis of annexin V+ cells at different times after LV-shp65 and LV-shC transduction. As shown in Figure 7C, HS-Sultan and RPMI-8226 cells transduced with LV-shp65 displayed a consistent increase in the percentage of apoptotic cells between 4 and 7 days after infection compared with cells transduced with the control vector. LV-shp65 transduction, instead, did not result in induction of apoptosis in the 15d-PGJ2-resistant K562 cell line (Figure 7C).
Discussion
Cyclopentenone prostaglandins have been known to possess potent antiproliferative and antitumor activity for decades4,5 ; however, the molecular mechanisms responsible for these effects have not been elucidated as yet. We have previously shown that cyPGs are potent NF-κB inhibitors, and block phorbol ester- and TNFα-induced NF-κB activation by inhibition and direct modification of the IKK complex, via binding of the prostanoid to cysteine 179 in the activation loop of the β subunit.19,39 The presence of an α,β-unsaturated carbonyl group in the cyclopentane ring allows, in fact, cyclopentenone prostaglandins to covalently bind to thiol groups of target cysteine residues of specific proteins via the formation of Michael adducts.45,46
NF-κB has been known for several years to play a pivotal role in the control of the inflammatory and innate immune responses. As anticipated in “Introduction,” recent evidence indicates that NF-κB is also involved in the regulation of cell proliferation and survival, and that aberrant regulation of this factor may underlie different types of cancer.24,27,28 Starting from the first observation that RelA-/- mice died at embryonic day 15 as a result of extensive liver apoptosis,47 a large body of evidence has described the antiapoptotic function of NF-κB. Collectively, these findings show that NF-κB induces the expression of a number of genes whose products can inhibit apoptosis, including cellular inhibitors of apoptosis (cIAP-1, cIAP-2, and X chromosome-linked IAP [XIAP]), the FLICE-inhibitory protein (cFLIP), members of the Bcl-2 family (Bcl-XL and A1/Bfl-1), as well as TNF receptor (TNFR)-associated factors 1 and 2 (TRAF1 and TRAF2). NF-κB can also attenuate the apoptotic response to anticancer drugs and ionizing radiation.28 The fact that many tumors show constitutively activated NF-κB has suggested that the antiapoptotic function of this factor may represent a major obstacle to cancer therapy. Tumors with constitutively active NF-κB include various types of hematologic malignancies, including Burkitt lymphoma.27
Burkitt lymphoma (BL) is an aggressive B-cell tumor whose hallmark is the activation of the c-myc oncogene through a reciprocal translocation that juxtaposes the c-myc gene on chromosome 8 to one of the immunoglobulin's loci. It has been shown that NF-κB is an important regulatory factor for the c-myc promoter48,49 and that NF-κB activity is required for the expression of c-myc under the control of the immunoglobulin heavy chain enhancer.50 Interestingly, NF-κB/Rel proteins are expressed at high levels in the nuclei of Burkitt lymphoma cells,50 suggesting that interference with NF-κB function may represent a successful approach to Burkitt lymphoma treatment.
In the present study, we investigated the effect of the cyclopentenone prostaglandin 15d-PGJ2 on NF-κB activity and survival in BL HS-Sultan cells. Differently from the noncyclopentenone prostaglandin PGF2α that did not affect cell proliferation, 15d-PGJ2 at the concentration of 10 μM caused a complete growth arrest, and progressively induced cell death, which reached 70% of HS-Sultan cells at 72 hours after treatment. Analysis by annexin V staining revealed that the predominant effect of 15d-PGJ2 was induction of apoptosis, and more than 70% of cells were annexin V-positive already at 8 hours after 15d-PGJ2 treatment. At the same time, 15d-PGJ2 was as effective as the potent NF-κB inhibitor MG-132 in inducing the cleavage of the well-known target of caspase activity, poly (ADP-ribose) polymerase. An insight on the molecular mechanisms of cell death induced by 15d-PGJ2 came by the observation of the activation of multiple caspases demonstrated by the cleavage of caspase-3, caspase-8, and caspase-9. The biologic relevance of multiple caspase activation was supported by the fact that only the pretreatment with the pan-caspase inhibitor (ZVAD) was able to completely rescue cells from death, while treatment with each single caspase inhibitor was ineffective.
The effect of 15d-PGJ2 on NF-κB activation and function was then analyzed. In agreement with other studies in B-cell malignancies,32,50,51 our results indicate that HS-Sultan cells expressed constitutively active NF-κB. We had previously shown that 15d-PGJ2 is able to inhibit TNFα- and TPA-induced NF-κB activation in different types of cells.19,39,41 The analysis of NF-κB DNA-binding activity in HS-Sultan cells revealed that 15d-PGJ2 is also able to suppress constitutive NF-κB activity in cancer cells. A marked inhibition of NF-κB DNA-binding activity was detected already at one hour after treatment with 15d-PGJ2, and persisted for at least 8 hours. This effect was accompanied by a decrease in the level of phosphorylated IκBα. 15d-PGJ2 treatment was also able to prevent TPA-induced IKK activity.
Numerous reports have evidenced that interfering with NF-κB activity via proteasome inhibitors, IKK inhibitors, peptides, or antibodies arrested proliferation and induced cell death of multiple myeloma cells.32,44,52-54 We speculate that inhibition of NF-κB is responsible also for 15d-PGJ2 proapoptotic activity in BL HS-Sultan cells. This is supported by the fact that apoptosis induction was dose-dependently related to inhibition of NF-κB by 15d-PGJ2; moreover, in a study in which the activity of the noncyclopentenone prostanoids PGE2 and PGF2α was compared with the effect of the cyclopentenone prostanoids PGA2 and 15d-PGJ2, only the cyclopentenone arachidonic acid derivatives, which can inhibit NF-κB, were able to induce apoptosis of HS-Sultan cells, with 15d-PGJ2 being at the same time the most active inhibitor of NF-κB and the most effective proapoptotic prostanoid. The implication of NF-κB inhibition in the apoptotic process is also supported by the fact that the proteasome inhibitor MG-132 is a potent inducer of apoptosis in these cells. Finally, the fact that knockdown of the NF-κB p65-subunit by lentiviral-mediated shRNA interference also resulted in apoptosis induction in HS-Sultan cells provides direct evidence that inhibition of NF-κB is required for apoptosis in these cells.
It has been recently shown that 15d-PGJ2 antiproliferative and apoptotic effects on human B cells could be mimicked by PPAR-γ agonists (ie, thiazolidinediones), suggesting that the mechanism by which 15d-PGJ2 negatively affects B-lineage cells might involve PPAR-γ.14,55 However, PPARγ-independent proapoptotic activity of 15d-PGJ2 has been recently shown in Jurkat T lymphoblastoid cells via activation of the mitochondrial apoptosis pathway independent of external death receptor signaling.15 Moreover, several other studies have clearly excluded the involvement of PPAR-γ in 15d-PGJ2-induced apoptosis of breast cancer cells,56 human oral squamous cell carcinomas,57 dendritic cells,58 and granulocytes,18 suggesting that apoptosis is indeed mediated by a PPAR-γ-independent inhibition of NF-κB. The fact that the PPAR-γ agonist troglitazone was unable to inhibit NF-κB and induce apoptosis in HS-Sultan cells indicates that 15d-PGJ2-induced cell death is independent of PPAR-γ in these cells.
We next examined the effect of 15d-PGJ2 on the level of several proteins whose expression is regulated by NF-κB. NF-κB inhibition by 15d-PGJ2 was found to be accompanied by a rapid decrease in protein level of several gene products regulated by NF-κB, including c-myc, cyclin D, and the antiapoptotic proteins cIAP-1, cIAP-2, XIAP, and cFLIP. Levels of cFLIP and XIAP were found to be decreased as early as 2 hours after exposure to the prostanoid, while levels of cIAP-1 and cIAP-2 proteins started to decrease at later times (4 hours) after treatment. Levels of Bcl-2 and Bcl-XL were instead not affected up to 8 hours after exposure to the prostanoid. IAP proteins are known to inhibit caspase-8 and caspase-9 activity. In particular, apart of the effect on the down-stream caspases, the down-regulation of cFLIP by 15d-PGJ2 may contribute to the induction of caspase-8 activity, while the decreased levels of XIAP may unleash caspase-9 action.59,60
Finally, in order to investigate whether 15d-PGJ2 was able to induce apoptosis in other types of B malignant cells and whether this effect was associated with inhibition of NF-κB, we determined the status of constitutive NF-κB DNA-binding activity in a different Burkitt lymphoma cell line (BL-41) and in 2 MM cell lines (RPMI-8226 and U-266). Multiple myeloma, a malignant tumor that affects terminally differentiated B cells, is the second most common hematologic malignancy, affecting 15 000 patients in the United States annually. Even though combination chemotherapy offers initial response rates of 40% to 70%, refractoriness usually develops, and the disease is presently incurable.61 Several studies have suggested that the constitutive activation of NF-κB, detected both in MM cell lines or primary MM samples, plays a central role in plasma cell survival.32,50,51 In agreement with other studies,32,51 MM RPMI-8226 and U266 were found to express constitutively active NF-κB. Treatment with 15d-PGJ2 caused a dose-dependent inhibition of constitutive NF-κB DNA-binding activity in all cell lines. In all cases, NF-κB inhibition was associated with induction of apoptosis evidenced by analysis of DNA content and by annexin V staining. Notably, apoptosis of malignant B cells took place in cell lines carrying either wild-type (HS-Sultan) or mutant p53 (BL-41, RPMI-8226 and U-266),62,63 confirming prior reports that 15d-PGJ2 can induce apoptosis in both settings.64,65 On the contrary, 15d-PGJ2 at the same concentrations did not induce apoptosis in acute leukemia K562 cells that present low levels of constitutive NF-κB DNA-binding activity and whose survival depends on the activation of the Bcr/Abl-Stat5-Bax pathway, which is independent of NF-κB.66 Under the conditions used, 15d-PGJ2 also did not induce apoptosis in peripheral blood mononucleated cells from healthy donors.
Moreover, using lentiviral-mediated shRNA interference, we have shown that down-regulation of NF-κB function via knockdown of the p65 subunit also resulted in apoptosis induction in both multiple myeloma (RPMI-8226) and Burkitt lymphoma (HS-Sultan) cells with constitutively active NF-κB, while it had no effect on apoptosis of K562 cells. These results altogether indicate that inhibition of NF-κB activity and of NF-κB-dependent expression of cell survival proteins plays a major role in the proapoptotic activity of 15d-PGJ2 in B-cell malignancy. However, it has to be pointed out that additional pathways regulating cell survival appear to be modified by 15d-PGJ2 treatment; in particular, 15d-PGJ2 was found to stimulate c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) kinase activities, while it down-regulates protein kinase B (PKB)/Akt kinase in HS-Sultan cells (R.P., G.B., and M.G.S., unpublished observations, February 2, 2004). Activation of the JNK and p38 signaling pathways have been mechanistically implicated in regulation of apoptosis.67 The serine/threonine kinase PKB/Akt also appears to provide a cell survival/proliferation signal for many cell types.68 The fact that 15d-PGJ2 induces JNK activity, while it causes a rapid decline in Akt phosphorylation followed by the loss of total Akt protein, indicates that the potent antitumor activity of 15d-PGJ2 may derive from the simultaneous down-regulation of proliferative and survival pathways, up-regulation of apoptotic signaling, and the activation of stress kinases.
Prostaglandins are used clinically in the treatment of congenital heart disease, gastroduodenal ulcers, erectile dysfunction, and to facilitate labor, and are generally effective and well tolerated.69,70 The results described in the present report indicate that cyclopentenone prostanoids are potent inducers of apoptosis in aggressive B-cell malignancies that are often resistant to chemotherapy, and encourage the search for novel prostanoids or prostanoid-derived molecules for therapeutic intervention in the treatment of cancers characterized by aberrant regulation of NF-κB.
Prepublished online as Blood First Edition Paper, October 21, 2004; DOI 10.1182/blood-2004-04-1360.
Supported by the Italian Ministry of University and Scientific Research (MIUR), by the Italian Ministry of Public Health (ISS project), and by Charterhouse Therapeutics, Oxford, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal