Abstract
The use of chemical modifiers as radiosensitizers in combination with low-dose irradiation may increase the therapeutic effect on cancer by overcoming a high apoptotic threshold. Here, we showed that phytosphingosine treatment in combination with γ-radiation enhanced apoptotic cell death of radiation-resistant human T-cell lymphoma in a caspase-independent manner. Combination treatment induced an increase in intracellular reactive oxygen species (ROS) level, mitochondrial relocalization of B-cell lymphoma-2(Bcl-2)-associated X protein (Bax), poly-adenosine diphosphate (ADP)-ribose polymerase 1 (PARP-1) activation, and nuclear translocation of apoptosis-inducing factor (AIF). siRNA targeting of AIF effectively protected cells from the combination treatment-induced cell death. An antioxidant, N-acetyl-L-cysteine (NAC), inhibited Bax relocalization and AIF translocation but not PARP-1 activation. Moreover, transfection of Bax-siRNA significantly inhibited AIF translocation. Pretreatment of PARP-1 inhibitor, DPQ (3,4-dihydro-5-[4-(1-piperidinyl)-butoxy]-1(2H)-isoquinolinone), or PARP-1-siRNA also partially attenuated AIF translocation, whereas the same treatment did not affect intracellular ROS level and Bax redistribution. Taken together, these results demonstrate that enhancement of cell death of radiation-resistant cancer cells by phytosphingosine treatment in combination with γ-radiation is mediated by nuclear translocation of AIF, which is in turn mediated both by ROS-dependent Bax relocalization and ROS-independent PARP-1 activation. The molecular signaling pathways that we elucidated in this study may provide potential drug targets for radiation sensitization of cancers refractive to radiation therapy. (Blood. 2005;105:1724-1733)
Introduction
Radiation resistance is a significant problem in the treatment of malignant tumors.1 Many factors affect susceptibility of tumor cells to radiation. Among them, intrinsic apoptosis sensitivity or resistance seems to play an important role.2 The use of chemical modifiers as radiosensitizers in combination with low-dose irradiation may increase the therapeutic efficacy by overcoming a high apoptotic threshold.3
Mitochondrial membrane permeabilization is considered to be one of the initial events of the apoptotic process induced by chemotherapeutic drugs.4-6 Opening of the mitochondrial permeability transition pore, which is under the control of members of the B-cell lymphoma-2 (Bcl-2) family, can result in the permeabilization of the outer mitochondrial membrane and subsequent release of potentially apoptogenic proteins such as cytochrome c and apoptosis-inducing factor (AIF) from the intermembrane space.7-9 Cytosolic cytochrome c binds to apoptotic protease-activating factor 1 (Apaf-1) in a ternary complex with caspase-9, leading to activation of caspase-9, which in turn activates caspase-3.7 Cleavage of inhibitor of the caspase-activated DNase (ICAD) by caspase-3 leads to activation of caspase-activated DNase (CAD) and cleavage of DNA into characteristic oligonucleosomal-length fragments.10 AIF was more recently cloned and identified as a mitochondrial intermembrane space protein with homology to bacterial nicotinamide adenine dinucleotide (NADH) oxidoreductases. In response to apoptotic stimuli, AIF is released from mitochondria and translocates to the nucleus and participates in the induction of chromatin condensation, the exposure of phosphatidylserine in the outer leaf of the plasma membrane, and the dissipation of the mitochondrial transmembrane potential. These effects seem to be caspase-independent, since none of them are prevented by the broad-spectrum caspase inhibitor z-VAD-fmk (benzyloxycarbonyl-Val-Ala-Asp(oME)-fluoromethylketone) and are independent of the apoptosome complex.8,11
Recent studies suggest that reactive oxygen species (ROSs) may play an important role during apoptosis induction.12 Many stimuli such as tumor necrosis factor α (TNF-α), anticancer drugs, and chemopreventive agents stimulate cells to produce ROSs.13-22 ROSs can directly activate the mitochondrial permeability transition and result in mitochondrial membrane potential (MMP) loss.23,24 Mitochondrial dysfunction such as loss of MMP results in cytochrome c release from mitochondria into cytoplasm. This release of cytochrome c leads to activation of Asp-Glu-Val-Asp-DEVD specific caspases and subsequent nuclear fragmentation in vitro.25
Poly-adenosine diphosphate (ADP)-ribose polymerase 1 (PARP-1), the most abundant protein of the poly (ADP-ribose) polymerase family members, is rapidly activated by DNA damage. PARP-1 uses NAD+ to form poly (ADP-ribose) (PAR) polymers on specific acceptor proteins. PARP-1 activation appears to facilitate DNA repair under moderate stress conditions.26,27 However, under conditions that cause extensive DNA damage such as excitotoxicity and ischemia, PARP-1 activation causes NAD+ depletion leading to adenosine triphosphate (ATP) depletion28,29 and subsequent cell death. Although PARP-1-mediated cell death has been thought to be necrotic,28,29 recent reports have demonstrated that PARP-1-mediated cell death also has many features in common with apoptotic forms of cell death,30,31 such as nuclear translocation of AIF from mitochondria.
Sphingolipid metabolites such as ceramides, sphingosines, sphingosine 1-phosphates, and phytosphingosine have emerged as key regulators of apoptosis.32 Sphingolipid metabolites have also been implicated as an important component of ionizing radiation-induced apoptosis of human cancer cells.33-36 This apoptotic pathway is initiated by hydrolysis of sphingomyelin, a membrane lipid, attributable to the activation of sphingomyelinases to generate ceramide. Ceramide, in turn, can activate several pathways important for the induction of apoptosis.37 Moreover, direct exposure of cells to the cell-permeable ceramide analogs has also been shown to sensitize cancer cells to ionizing radiation, which lends further support to the notion that ceramide generation might be an important step for radiation-induced apoptosis in human cancer cells.38 Although many reports emphasized the contributions of ceramide to enhancement of the radiation response, the role of other sphingolipid metabolites in the modulation of radiation sensitivity and their precise action mechanisms were largely unknown.
In the present study, we investigated the mechanisms underlying modulation of radiation response by phytosphingosine in radiation-resistant human T-cell lymphoma. We demonstrated that combination treatment of phytosphingosine with radiation synergistically enhances caspase-independent apoptotic cell death of radiation-resistant Jurkat T-cell clones. We showed that nuclear translocation of AIF from mitochondria is necessary for enhancement of cell death by combination treatment, and that ROS-mediated Bcl-2-associated X protein (Bax) translocation and ROS-independent PARP-1 activation are associated with AIF release from mitochondria. Our data provide a potential mechanism for radiosensitization effect of phytosphingosine and suggest a potential clinical application of combination treatment of radiation-resistant cancers with ionizing radiation and phytosphingosine.
Materials and methods
Materials
Phytosphingosine was purchased from Avanti (Alabaster, AL). Antibodies specific for polyclonal anti-AIF, heat shock protein 60 (HSP 60), and redox factor-1 (Ref-1) were from Santa Cruz Biotechnology (Santa Cruz, CA). β-Actin was from Sigma (St Louis, MO). Anticleaved caspase-3 antibody and PARP were from Cell Signaling Technology (Beverly, MA). Polyclonal anti-cytochrome c antibody was from Pharmingen (San Diego, CA). The specific PARP-1 inhibitor DPQ (3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone) was from Calbiochem (Cambridge, MA). The broad-spectrum caspase inhibitor z-VAD-fmk was from Calbiochem. Other chemicals were obtained from Calbiochem.
Cell culture
Jurkat human T-cell lymphoma (Type II) was obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), penicillin, and streptomycin at 37°C in a humidified incubator with 5% CO2.
Irradiation
Cells were plated in 10-cm dishes and incubated at 37°C under humidified 5% CO2, 95% air in culture medium until 70% to 80% confluent. Cells were then exposed to γ-rays from a 137Cs γ-ray source (Atomic Energy of Canada, Canada, located in Korea Institute of Radiological and Medical Sciences, Seoul, Korea) at a dose rate of 3.81 Gy/minute.
Establishment of ionizing radiation-resistant Jurkat clones
To establish ionizing radiation-resistant Jurkat clones, Jurkat T cells were first distributed into 96-well plates at a density of 0.2 cells per well. After 12 hours, individual wells were visually checked for the presence of a single cell. The clones were grown and tested for the radiation sensitivity, and those showing more than 95% survival rate at 48 hours after 10-Gy irradiation were chosen for further study as radiation-resistant clones. Among the 100 clones tested, clone nos. 1, 6, 11, and 16 were chosen as radiation-resistant clones for further study. The average survival rate of parental Jurkat T cells at 48 hours after 10-Gy irradiation was about 64%.
Clonogenic survival assay in soft agar
Cells were plated onto 60-mm dishes at a density of 2 × 106 cells/dish and exposed to a range of doses of γ-radiation at 0 to 3 Gy. After irradiation, cells were harvested, and 104 cells/dish were suspended in 2 mL of 0.3% Difco Noble agar (Difco, Detroit, MI) supplemented with complete culture medium. This suspension was layered over 2 mL of 0.8% agar-medium base layer in 60-mm dishes and followed by incubation for 14 days at 37°C in 5% CO2 incubator. Prior to counting colonies, the culture medium was decanted and the cells were fixed in 95% methanol and stained with 0.5% crystal violet, and the numbers of colonies (> 50 cells) from triplicate dishes were counted. Mean colony numbers relative to unirradiated colony numbers were plotted.
Small interfering RNA (siRNA) transfection
RNA interferences of AIF or Bax were performed using 21-base pair (including a 2-deoxynucleotide overhang) siRNA duplexes purchased from Ambion (Austin, TX). The sense strand nucleotide sequence for AIF siRNA was GGAAAUAUGGGAAAGAUCCdTdT. The sense strand nucleotide sequence for PARP-1 siRNA was GGCCAGGAUGGAAUUGGUAdTdT. The coding strand for Bax siRNA was AACATGGAGCTGCAGAGGATGAdTdT. A control siRNA specific to the green fluorescent protein DNA sequence CCACTACCTGAGCACCCAG was used as a negative control. For transfection, γ-radiation resistant Jurkat clones were seeded in 6-well plates at 30% confluency, and siRNA duplexes (200 nM) were introduced into the cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations.
Hoechst 33258 staining
Hoechst 33258 staining was performed as described previously.39 Briefly, cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature and then washed once with phosphate-buffered saline (PBS). Hoechst 33258 (50 ng/mL) was added to the fixed cells, incubated for 30 minutes at room temperature, and washed with PBS. Cells were mounted and examined by fluorescence microscopy. Apoptotic cells were identified by the condensation and fragmentation of their nuclei. The percentage of apoptotic cells was calculated from the ratio of apoptotic cells to total cells counted. At minimum, 500 cells were counted for each treatment.
Measurement of mitochondrial membrane potential and ROS generation
The measurement of mitochondrial transmembrane potential (Δψm) and reactive oxygen species (ROS) generation were performed as described by Marchetti et al.40 Briefly, cells (2 × 106/mL) were exposed to 10-Gy radiation and/or 5 μg/mL phytosphingosine for the indicated times. After exposure, cells were incubated in 30 nM DioC3(6) (3,3′-dihexyloxacarboxyanine iodide; Molecular Probes, Eugene, OR) or 10 μM 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes) at 37°C for 30 minutes to measure the mitochondrial membrane potential and ROS level, respectively. The cells were harvested and washed with cold PBS solution 3 times, and the ROS level and Δψm were determined by fluorescence-activated cell sorter (FACS) analysis.
Confocal microscopy
The γ-radiation-resistant Jurkat no. 6 clones treated with 10-Gy radiation and/or 5 μg/mL phytosphingosine were washed twice with ice-cold PBS and fixed with ice-cold methanol. After blocking with 2% bovine serum albumin in PBS containing 0.2% Triton X-100, cells were incubated with the primary antibodies against AIF or Bax for 1 hour. Cells were washed with blocking solution 3 times and incubated with the secondary antibodies conjugated with fluorescein isothiocyanate (FITC; Molecular Probes) for 1 hour. Nuclei were stained with 2.5 μg/mL propidium iodide (PI; Sigma) for 10 minutes, and the mitochondria were stained with 25 nM Mitotracker Red CMXRos (Molecular Probes) for 30 minutes at room temperature. After washing 3 times with PBS, coverslips were mounted onto microscopic slides using ProLong antifade mounting reagent (Molecular Probes). The slides were analyzed by a Leica TCS SP2 confocal laser-scanning microscope (Leica Microsystems, Heidelberg, Germany) equipped with an HCX PL APO objective lens with 63 × 8 original magnification and a numerical aperture of 1.4, and acquired with Leica confocal software version 2 (Leica Microsystems). All images were processed with Adobe Photoshop software version 7.0 (Adobe, San Jose, CA).
Western blot analysis
Western blot analysis was performed as described.41 Briefly, cell lysates were prepared by extracting proteins with lysis buffer (40 mM Tris-Cl, pH 8.0; 120 mM NaCl; 0.1% Nonidet-P40 [NP-40]) supplemented with protease inhibitors. Proteins were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and then incubated with primary antibodies for 1 hour at room temperature. Blots were developed by peroxidase-conjugated secondary antibody, and proteins were visualized by enhanced chemiluminescence (ECL) procedures (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's recommendations.
Preparation of nuclear and mitochondrial fractions
The cells were washed with ice-cold PBS, left on ice for 10 minutes, and then resuspended in isotonic homogenization buffer (250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM NaOH (Na)-ethylenediaminetetraacetic acid [EDTA], 1 mM Na-ethyleneglycotetraacetic acid [EGTA], 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonylfluoride, 10 mM Tris-HCl, pH 7.4) containing a proteinase inhibitor cocktail (Roche, Indianapolis, IN). After 80 strokes in a Dounce homogenizer, the unbroken cells were spun down at 30g for 5 minutes. The nuclei and heavy mitochondria fractions were fractionated at 750g for 10 minutes and 14 000g for 20 minutes, respectively, from the supernatant. The nuclei fraction was washed 3 times with homogenization buffer containing 0.01% NP-40.
Cellular enzyme-linked immunosorbent assay (ELISA) method for detection of PARP-1 activation
PARP enzyme activity was measured using a commercial kit (Oncogene, San Diego, CA). Briefly, cell extracts were incubated in reaction buffer (56 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 28 mM KCl, 28 mM NaCl, 2 mM MgCl2) containing 0.01% digitonin and 10 μM biotinylated NAD+. After incubating at 37°C for 30 minutes, reactions were developed with TACS-Saphire (Trevigen, Gaithersburg, MD) substrate (100 μL/well). The optical density was measured with a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Data were expressed as mean ± SD of quadruplicate samples.
Results
Phytosphingosine treatment in combination with ionizing radiation enhances apoptotic cell death of radiation-resistant cancer cells
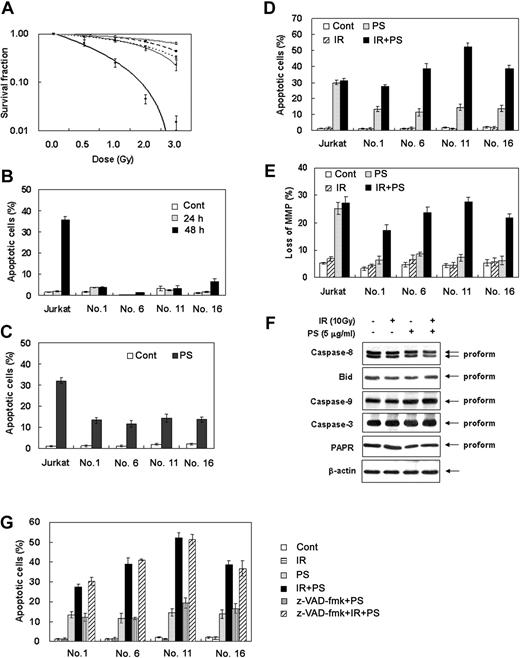
We first selected variant clones from Jurkat human T-cell lymphoma for resistance to ionizing radiation-induced apoptotic cell death. As shown in Figure 1A, clonogenic survival assay revealed that clone nos. 1, 6, 11, and 16 showed resistance to radiation compared with the parental Jurkat T cells. We next investigated whether the radiation-resistant cells that showed an increased clonogenic survival following radiation also had resistance to the radiation-induced apoptotic cell death. To examine radiation-induced apoptosis, the cells were treated with 10 Gy radiation and were stained with Hoechst 33258 at 24 or 48 hours. In Figure 1B, ionizing radiation at a single dose of 10 Gy effectively killed parental Jurkat T cells but failed to induce cell death in clone nos. 1, 6, 11, and 16 at 48 hours after γ-irradiation. Previously, we have reported that phytosphingosine can potently induce apoptotic cell death in Jurkat T cells.42,43 To examine whether there is a cross-resistance to the phytosphingosine in radiation-resistant Jurkat clones, we measured apoptotic cell death in parental Jurkat and radiation-resistant clones at 3 hours after phytosphingosine treatment. A dramatic decrease in the number of apoptotic cells was observed in radiation-resistant clones compared with the parental cells, suggesting that the radiation-resistant Jurkat clones have a cross-resistance to the phytosphingosine (Figure 1C).
Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells. (A) Selection of radiation-resistant Jurkat clones. Single cells in limiting dilution condition (0.2 cells/well) were incubated in 96-well plates for 2 months and then individual clones were irradiated with increasing doses of γ-radiation. Cells were allowed to grow on soft agar for 10 to 14 days and were stained with 0.5% crystal violet and scored for colony formation. Results are given as means ± SEM of 3 independent experiments. (B) Individual clones were treated with 10 Gy of γ-radiation and cultured for 24 or 48 hours. Cells were stained with Hoechst 33258, and apoptotic cells were analyzed by fluorescence microscopy. Apoptotic cells containing condensed chromatin fragments were scored and expressed as a percentage of the total cell numbers measured. Results from 3 independent experiments are shown as means ± SEM. IR indicates ionizing radiation. (C) Cross-resistance to the phytosphingosine in radiation-resistant clones. Radiation-resistant Jurkat clones were treated with 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. ▪ indicates control; □, phytosphingosine. (D) Phytosphingosine sensitizes radiation-resistant Jurkat clones to radiation-induced apoptotic cell death. Radiation-resistant Jurkat clones were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL). After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (E) Mitochondrial transmembrane potential was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amounts of retained DioC3(6) were measured by flow cytometry. (F) Cell lysates of clone no. 6 treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine were subjected to Western blot analysis with anti-caspase-8, -Bid, -caspase-9, -caspase-3, and -PARP antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (G) Cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM z-VAD-fmk. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells. (A) Selection of radiation-resistant Jurkat clones. Single cells in limiting dilution condition (0.2 cells/well) were incubated in 96-well plates for 2 months and then individual clones were irradiated with increasing doses of γ-radiation. Cells were allowed to grow on soft agar for 10 to 14 days and were stained with 0.5% crystal violet and scored for colony formation. Results are given as means ± SEM of 3 independent experiments. (B) Individual clones were treated with 10 Gy of γ-radiation and cultured for 24 or 48 hours. Cells were stained with Hoechst 33258, and apoptotic cells were analyzed by fluorescence microscopy. Apoptotic cells containing condensed chromatin fragments were scored and expressed as a percentage of the total cell numbers measured. Results from 3 independent experiments are shown as means ± SEM. IR indicates ionizing radiation. (C) Cross-resistance to the phytosphingosine in radiation-resistant clones. Radiation-resistant Jurkat clones were treated with 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. ▪ indicates control; □, phytosphingosine. (D) Phytosphingosine sensitizes radiation-resistant Jurkat clones to radiation-induced apoptotic cell death. Radiation-resistant Jurkat clones were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL). After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (E) Mitochondrial transmembrane potential was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amounts of retained DioC3(6) were measured by flow cytometry. (F) Cell lysates of clone no. 6 treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine were subjected to Western blot analysis with anti-caspase-8, -Bid, -caspase-9, -caspase-3, and -PARP antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (G) Cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM z-VAD-fmk. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
We next examined whether phytosphingosine treatment in combination with radiation had a sensitizing effect on cell death in radiation-resistant Jurkat clones. As shown in Figure 1D, the combination treatment with phytosphingosine and γ-radiation indeed synergistically enhanced the apoptotic cell death of radiation-resistant Jurkat clones. However, in parental Jurkat T cells, phytosphingosine did not show synergistic effect on cell death when simultaneously treated with γ-radiation. Instead, phytosphingosine treatment alone showed a significant cytotoxic effect. To determine the contribution of the mitochondrial pathway to the induction of apoptotic cell death seen after the combination treatment of phytosphingosine with γ-radiation, we examined changes in mitochondrial membrane potential (Δψm). Figure 1E shows a marked loss of Δψm in radiation-resistant cells treated with γ-radiation and phytosphingosine. We next investigated whether caspase activities are required for enhancement of apoptotic cell death of radiation-resistant cells treated with γ-radiation and phytosphingosine. As shown in Figure 1F, the combination treatment with γ-radiation and phytosphingosine did not affect the caspase activities. Moreover, a broad-spectrum caspase inhibitor, z-VAD-fmk, did not attenuate the cell death induced by combination treatment (Figure 1G). These findings suggest that phytosphingosine in combination with ionizing radiation synergistically enhances apoptotic cell death in ionizing radiation-resistant cells in a caspase-independent manner.
Combination treatment of γ-radiation with phytosphingosine enhances apoptotic cell death through AIF translocation to nucleus
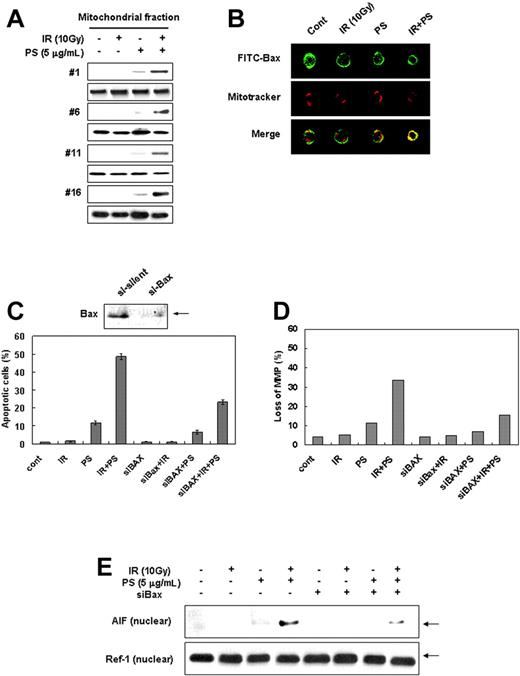
Since apoptosis-inducing factor (AIF) is known to be involved in induction of apoptotic cell death through a caspase-independent pathway,8,44 we next examined whether AIF plays a role in apoptotic cell death induced by the combination treatment. AIF is a mitochondrialocalized flavoprotein that is released and translocated to the nucleus and causes nuclear condensation in response to death stimuli.8,44 Subcellular fractionation showed that combination treatment of radiation-resistant cells with γ-radiation and phytosphingosine dramatically redistributed AIF from mitochondria to the nucleus (Figure 2A). Confocal microscopy also clearly revealed that AIF was translocated to the nucleus and caused nuclear condensation after the combination treatment (Figure 2B). To test whether redistribution of AIF occurs in a caspase-independent manner, we examined the effect of z-VAD-fmk, a broad-spectrum caspase inhibitor, on AIF translocation to nucleus. The z-VAD-fmk treatment did not affect intracellular redistribution of AIF and nucleus condensation seen after the combination treatment (Figure 2B). Furthermore, experiments with siRNA showed that siRNA targeting of AIF effectively attenuated the combination treatment-induced cell death (Figure 2C). These results suggest that translocation of AIF from mitochondria to nucleus is required for caspase-independent cell death in radiation-resistant cells induced by combination treatment of γ-radiation with phytosphingosine.
Combination treatment of phytosphingosine with ionizing radiation induces AIF translocation to nucleus. (A) Analysis of AIF translocation by subcellular fractionation. Nuclear fraction was obtained from the Jurkat clones treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) and was subjected to Western blot analysis with anti-AIF (top blot of each pair) and -Ref-1 (bottom blot of each pair) antibodies. Ref-1 was used as a nuclear marker protein. (B) Representative confocal images for translocation of AIF to the nucleus and nuclear condensation after combination treatment in clone no. 6 in the absence or presence of 30 μM z-VAD-fmk. The nuclear translocation of AIF is demonstrated by the overlap of AIF (green) and nuclear staining (red), as noted by yellow color. (C) siRNA targeting of AIF attenuates combination treatment-induced cell death. The clone no. 6 transfected with AIF siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
Combination treatment of phytosphingosine with ionizing radiation induces AIF translocation to nucleus. (A) Analysis of AIF translocation by subcellular fractionation. Nuclear fraction was obtained from the Jurkat clones treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) and was subjected to Western blot analysis with anti-AIF (top blot of each pair) and -Ref-1 (bottom blot of each pair) antibodies. Ref-1 was used as a nuclear marker protein. (B) Representative confocal images for translocation of AIF to the nucleus and nuclear condensation after combination treatment in clone no. 6 in the absence or presence of 30 μM z-VAD-fmk. The nuclear translocation of AIF is demonstrated by the overlap of AIF (green) and nuclear staining (red), as noted by yellow color. (C) siRNA targeting of AIF attenuates combination treatment-induced cell death. The clone no. 6 transfected with AIF siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
Combination treatment of γ-radiation with phytosphingosine induces Bax translocation to mitochondria, dissipation of mitochondrial membrane potential (Δψm), and AIF translocation to nucleus
Since it has been shown that translocation of Bax from the cytosol to the mitochondria causes a decline of Δψm,45-47 we investigated whether the combination treatment with γ-radiation and phytosphingosine induces mitochondrial translocation of Bax. As shown in Figure 3A, the combination treatment redistributed Bax from cytosol to the mitochondria without changing the protein expression levels of Bcl-2 and Bax (data not shown). Confocal microscopy also clearly revealed that Bax was translocated to the mitochondria (Figure 3B). Furthermore, siRNA targeting of Bax effectively attenuated combination treatment-induced depolarization of Δψm and cell death (Figure 3C-D). We also showed that siRNA targeting of Bax effectively inhibited nuclear translocation of AIF induced by the combination treatment (Figure 3E). These results suggest that combination treatment with radiation and phytosphingosine induces Bax redistribution to mitochondria and subsequently promotes the loss of Δψm and translocation of AIF to nucleus.
Combination treatment of ionizing radiation with phytosphingosine enhances Bax translocation to mitochondria. (A) Analysis of Bax translocation by subcellular fractionation. Mitochondrial fractionation was performed with Jurkat clones treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL). After 3 hours, proteins were subjected to Western blot analysis with anti-Bax (top blot of each pair) and HSP 60 (bottom blot of each pair) antibodies. HSP 60 was used as a mitochondrial marker protein. (B) Representative confocal images for translocation of Bax to the mitochondria. Clone no. 6 was incubated with Mitotracker and stained with anti-Bax antibody and analyzed by confocal laser scanning microscopy. Mitochondrial localization of Bax was defined by yellow spots, indicating overlap of fluorescein isothiocyanate (Bax) and Mitotracker-Red. (C) siRNA targeting of the Bax attenuates combination treatment-induced cell death. The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation alone and/or 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (D) The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, mitochondrial transmembrane potential of these cells was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amount of retained DioC3(6) were measured by flow cytometry. (E) The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, nuclear fraction was prepared and was subjected to Western blot analysis with anti-AIF and -Ref-1 antibodies. Ref-1 was used as a nuclear marker protein.
Combination treatment of ionizing radiation with phytosphingosine enhances Bax translocation to mitochondria. (A) Analysis of Bax translocation by subcellular fractionation. Mitochondrial fractionation was performed with Jurkat clones treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL). After 3 hours, proteins were subjected to Western blot analysis with anti-Bax (top blot of each pair) and HSP 60 (bottom blot of each pair) antibodies. HSP 60 was used as a mitochondrial marker protein. (B) Representative confocal images for translocation of Bax to the mitochondria. Clone no. 6 was incubated with Mitotracker and stained with anti-Bax antibody and analyzed by confocal laser scanning microscopy. Mitochondrial localization of Bax was defined by yellow spots, indicating overlap of fluorescein isothiocyanate (Bax) and Mitotracker-Red. (C) siRNA targeting of the Bax attenuates combination treatment-induced cell death. The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation alone and/or 5 μg/mL of phytosphingosine. After 3 hours, cells were stained with Hoechst 33258 and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (D) The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, mitochondrial transmembrane potential of these cells was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amount of retained DioC3(6) were measured by flow cytometry. (E) The clone no. 6 transfected with Bax siRNA was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine. After 3 hours, nuclear fraction was prepared and was subjected to Western blot analysis with anti-AIF and -Ref-1 antibodies. Ref-1 was used as a nuclear marker protein.
Role of ROSs in apoptotic cell death of radiation-resistant cancer cells induced by combination treatment with γ-radiation and phytosphingosine
Since sphingolipid metabolites have been implicated in intracellular ROS accumulation, which in turn can activate several pathways important for the induction of apoptosis,48-50 we examined involvement of ROSs in combination treatment-induced cell death of radiation-resistant cells. As shown in Figure 4A, ROS levels were dramatically increased after the combination treatment with γ-radiation and phytosphingosine in radiation-resistant clones and the increase in ROS level was effectively blocked by N-acetyl-L-cysteine (NAC). To determine the direct relationship between the increased intracellular ROS level and mitochondrial activation-mediated cell death pathway, cells were pretreated with antioxidant N-acetyl-L-cysteine (NAC) before combination treatment. As shown in Figure 4B-C, NAC significantly attenuated the combination treatment-induced loss of Δψm and cell death, suggesting that ROS plays an important role in enhancement of γ-radiation-induced cell death by phytosphingosine. Furthermore, NAC effectively blocked nuclear translocation of AIF induced by combination treatment with γ-radiation and phytosphingosine in radiation-resistant cells (Figure 4D). Moreover, NAC also effectively blocked the combination treatment-induced Bax translocation to mitochondria (Figure 4E). These observations suggest that ROS contributes to Bax-mediated AIF release and cell death induced by the combination treatment.
Enhancement of ROS production by combination treatment of ionizing radiation with phytosphingosine. Radiation-resistant Jurkat clones were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. (A) After 3 hours, cells were incubated with 10 μM of H2DCF-DA for 30 minutes and analyzed by flow cytometry as described in “Materials and methods.” (B) Mitochondrial transmembrane potential was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amount of retained DioC3(6) were measured by flow cytometry. (C) Cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (D) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mLof phytosphingosine in the presence or absence of 10 mM NAC. After 3 hours, nuclear fraction was prepared and subjected to Western blot analysis using anti-AIF or -Ref-1 antibody. Ref-1 was used as a nuclear marker protein. (E) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in presence or absence of 10 mM NAC. After 3 hours, mitochondrial fraction was prepared and was subjected to Western blot analysis with anti-Bax and -HSP 60 antibodies. HSP 60 was used as a mitochondrial marker protein.
Enhancement of ROS production by combination treatment of ionizing radiation with phytosphingosine. Radiation-resistant Jurkat clones were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. (A) After 3 hours, cells were incubated with 10 μM of H2DCF-DA for 30 minutes and analyzed by flow cytometry as described in “Materials and methods.” (B) Mitochondrial transmembrane potential was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amount of retained DioC3(6) were measured by flow cytometry. (C) Cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (D) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mLof phytosphingosine in the presence or absence of 10 mM NAC. After 3 hours, nuclear fraction was prepared and subjected to Western blot analysis using anti-AIF or -Ref-1 antibody. Ref-1 was used as a nuclear marker protein. (E) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in presence or absence of 10 mM NAC. After 3 hours, mitochondrial fraction was prepared and was subjected to Western blot analysis with anti-Bax and -HSP 60 antibodies. HSP 60 was used as a mitochondrial marker protein.
Phytosphingosine treatment in combination with radiation induces PARP-1 activation leading to AIF-mediated cell death
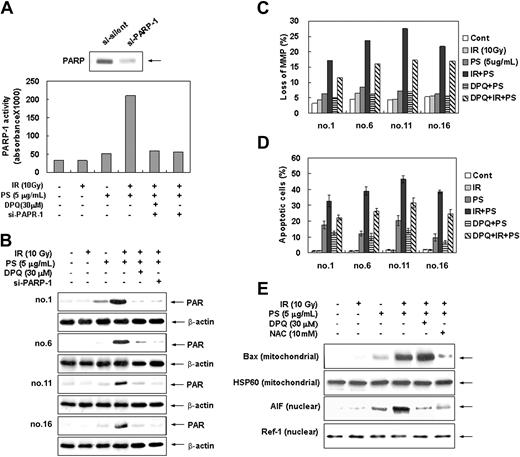
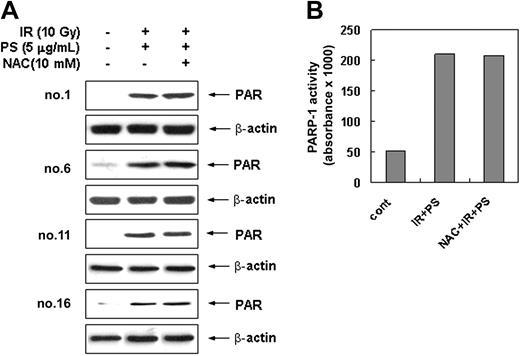
Since it has been shown that ROS-mediated DNA damage triggers activation of the PARP-1 and subsequent cell death,30,51 we investigated whether PARP-1 is involved in combination treatment-induced apoptotic cell death. Intracellular PARP-1 enzyme assay and immunoblot analysis with the antibody against PARP-1 shows that combination treatment with radiation and phytosphingosine dramatically enhanced PARP-1 activity and PAR polymer formation in radiation-resistant cells (Figure 5A-B). Pretreatment of DPQ, a specific PARP-1 inhibitor, or siRNA targeting of the PARP-1 effectively blocked PAPR-1 activation and PAR polymer formation induced by the combination treatment. Furthermore, pretreatment of DPQ or PARP-1 siRNA attenuated the combination treatment-induced loss of Δψm and subsequent cell death (Figure 5C-D) as well as PARP-1 activation and PAR formation (Figure 5A-B). In addition, translocation of AIF to nucleus was effectively blocked by DPQ pretreatment (Figure 5E). Nevertheless, DPQ pretreatment did not affect the Bax translocation to mitochondria (Figure 5E). Moreover, the pretreatment of antioxidant NAC has no effect on combination treatment-induced PARP-1 activation (Figure 6A-B). These observations indicate that the PARP-1 activation, similarly to intracellular ROSs, induces mitochondrial membrane potential loss, AIF translocation, and cell death in a ROS-independent manner upon combination treatment. Taken together, these results suggest that apoptotic cell death induced by the combination treatment of phytosphingosine with γ-radiation is regulated by 2 pathways: one is ROS-dependent Bax redistribution that mediates AIF release and translocation to nucleus, and the other is ROS-independent PARP-1 activation that mediates nuclear translocation of AIF.
Enhancement of PARP-1 activation by combination treatment of ionizing radiation with phytosphingosine. (A) Clone no. 6 was treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 30 μM DPQ or PARP-1 siRNA. After 3 hours, PARP enzyme activity was measured using a commercial kit under guidance of manufacturer (see “Materials and methods”). (B) Radiation-resistant cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ or PARP-1 siRNA. After 3 hours, cell lysates were subjected to Western blot analysis with anti-PARP-1 and β-actin antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (C) Radiation-resistant cells were treated with 10 Gy of γ-radiation and 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ. After 3 hours, mitochondrial transmembrane potential of these cells was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amounts of retained DioC3(6) were measured by flow cytometry. (D) Radiation-resistant cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (E) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ and 10 mM NAC. After 3 hours, mitochondrial or nuclear fractions were prepared. Mitochondrial protein fraction was subjected to Western blot analysis with anti-Bax and -HSP 60 antibodies, and nuclear protein fraction was subjected to Western blot analysis with anti-AIF and -Ref-1 antibodies. HSP 60 and Ref-1 were used as mitochondria and nuclear marker proteins, respectively.
Enhancement of PARP-1 activation by combination treatment of ionizing radiation with phytosphingosine. (A) Clone no. 6 was treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 30 μM DPQ or PARP-1 siRNA. After 3 hours, PARP enzyme activity was measured using a commercial kit under guidance of manufacturer (see “Materials and methods”). (B) Radiation-resistant cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ or PARP-1 siRNA. After 3 hours, cell lysates were subjected to Western blot analysis with anti-PARP-1 and β-actin antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (C) Radiation-resistant cells were treated with 10 Gy of γ-radiation and 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ. After 3 hours, mitochondrial transmembrane potential of these cells was determined by retention of DioC3(6) added during the last 30 minutes of treatment. After removal of the medium, the amounts of retained DioC3(6) were measured by flow cytometry. (D) Radiation-resistant cells were treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM. (E) Clone no. 6 was treated with 10 Gy of γ-radiation and/or 5 μg/mL of phytosphingosine in the presence or absence of 30 μM DPQ and 10 mM NAC. After 3 hours, mitochondrial or nuclear fractions were prepared. Mitochondrial protein fraction was subjected to Western blot analysis with anti-Bax and -HSP 60 antibodies, and nuclear protein fraction was subjected to Western blot analysis with anti-AIF and -Ref-1 antibodies. HSP 60 and Ref-1 were used as mitochondria and nuclear marker proteins, respectively.
PARP-1 activation induced by combination treatment of ionizing radiation with phytosphingosine is independent from ROS generation. (A) Radiation-resistant cells were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. After 3 hours, cell lysates were subjected to Western blot analysis with anti-PAR and β-actin antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (B) Clone no. 6 was treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. After 3 hours, PARP enzyme activity was measured using a commercial kit under guidance of manufacturer (see “Materials and methods”).
PARP-1 activation induced by combination treatment of ionizing radiation with phytosphingosine is independent from ROS generation. (A) Radiation-resistant cells were treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. After 3 hours, cell lysates were subjected to Western blot analysis with anti-PAR and β-actin antibodies. The data represent a typical experiment conducted at least 3 times with similar results. (B) Clone no. 6 was treated with 10 Gy of γ-radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC. After 3 hours, PARP enzyme activity was measured using a commercial kit under guidance of manufacturer (see “Materials and methods”).
To confirm the hypothesis that combination treatment with γ-radiation and phytosphingosine use 2 separate pathways to induce cell death in the γ-radiation-resistant cells, we treated NAC to the cells transfected with PARP-1 siRNA before combination treatment with γ-radiation and phytosphingosine. As shown in Figure 7, the cells simultaneously treated with PARP-1 siRNA and NAC show more dramatic attenuation of the apoptotic cell death than the cells treated with either reagent alone.
Enhancement of cell death after combination treatment of ionizing radiation with phytosphingosine is mediated both by ROS generation and PARP-1 activation pathways. Clone no. 6 was treated with 10 Gy of radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC or siRNA for PARP-1. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
Enhancement of cell death after combination treatment of ionizing radiation with phytosphingosine is mediated both by ROS generation and PARP-1 activation pathways. Clone no. 6 was treated with 10 Gy of radiation alone, 5 μg/mL of phytosphingosine alone, or combination of γ-radiation (10 Gy) and phytosphingosine (5 μg/mL) in the presence or absence of 10 mM NAC or siRNA for PARP-1. After 3 hours, cells were stained with Hoechst 33258, and apoptotic cells were quantitated by fluorescence microscopy. Results from 3 independent experiments are shown as means ± SEM.
Discussion
Previously, we have shown that phytosphingosine, a member of sphingolipid metabolites, had an anticancer effect on Jurkat T-cell lymphoma and NCI-H460, a human non-small cell lung cancer cell line.42,43 One of the important points in the development of a new anticancer drug is the understanding of its potential for inclusion in combination treatment regimens. In the present study, we examined whether the use of phytosphingosine in combination with γ-radiation may increase the therapeutic effect on cancer by overcoming a high apoptotic threshold. We demonstrated that combination treatment of phytosphingosine with γ-radiation synergistically enhanced caspase-independent apoptotic cell death of radiation-resistant Jurkat T-cell clones. We also showed that nuclear translocation of AIF from mitochondria was necessary for enhancement of cell death by combination treatment and that ROS-mediated Bax translocation and ROS-independent PARP-1 activation were associated with AIF release from mitochondria.
We first established radiation-resistant variant clones from Jurkat cells, which showed resistance to radiation compared with the parental Jurkat T cells in both clonogenic survival and apoptotic cell death. The greatest difference in radiation-induced cell death between parental Jurkat and resistant cells was observed at 48 hours. Previously, we have reported that phytosphingosine can potently induce apoptotic cell death in Jurkat T cells.42,43 Consistently, phytosphingosine treatment alone showed a strong induction of cell death (close to 30%) in parental Jurkat cells at 3 hours. However, in γ-radiation-resistant Jurkat T-cell clones, phytosphingosine did not effectively induce cell death, which means that radiation-resistant clones show cross-resistance to phytosphingosine. Nevertheless, interestingly, combination treatment with γ-radiation and phytosphingosine strongly enhanced apoptotic cell death and disruption of mitochondrial membrane potential measured at 3 hours after the treatment in radiation-resistant Jurkat clones. However, phytosphingosine did not show synergistic effect on cell death in parental Jurkat T cells upon combination treatment with γ-radiation at the same experimental condition as described in “Discussion.” Moreover, in the previous study, we have shown that down-regulation of the extracellular signal-related kinase (ERK) pathway is critical in the death receptor-independent activation of caspase-8 and activation of p38 mitogenactivated protein kinase (MAPK) is essential for mitochondria-mediated caspase-9 activation-dependent cell death in phytosphingosine-treated Jurkat cells43 ; in marked contrast, we failed to detect any changes in ERK and p38 MAPK activity in radiation-resistant Jurkat clones treated with phytosphingosine alone or in combination with γ-radiation (data not shown). These results suggest that differential regulatory mechanisms of cell death are operating in parental Jurkat cells compared with radiation-resistant Jurkat clones. Moreover, the combination treatment with radiation and phytosphingosine did not affect the activity of caspases in radiation-resistant clones and the caspase inhibitors failed to attenuate cell death, suggesting that the apoptotic cell death induced by the combination treatment is caspase independent in these variant clones, thus explaining the different outcomes of combination treatment between the parental cells and variant resistant clones.
It has been reported that AIF mediates cell death through a caspase-independent pathway. Mitochondrial AIF translocates to the nucleus upon death stimuli and initiates nuclear condensation.8,11 Once nucleus condenses, this leads to large-scale chromatin fragmentation followed by the cell death.8,11 Consistent with these findings, we found translocation of AIF from mitochondria to the nucleus and nuclear shrinkage after the combination treatment of phytosphingosine with radiation in radiation-resistant clones. Furthermore, siRNA knockdown of AIF in radiation-resistant clones effectively attenuated cell death induced by the combination treatment. Recently, several reports proposed that AIF is indeed a caspase-dependent death effector,52 implicating that the translocation of AIF to the nucleus is caspase dependent. However, we found that caspase inhibitors failed to attenuate AIF translocation and nuclear shrinkage, suggesting that the combination treatment of phytosphingosine with γ-radiation induces nuclear redistribution of AIF in a caspase-independent manner during apoptotic process.
In response to stimuli such as transforming growth factor β (TGF-β), etoposide, nitric oxide (NO), staurosporine, and UV, which require mitochondria-dependent pathway for apoptosis, Bax become activated, translocated to the outer membrane of mitochondria, and oligomerized therein.53-57 The mitochondrial membrane permeabilization and the release of mitochondrial apoptogenic molecules into the cytosol ensue. We also found the combination treatment with γ-radiation and phytosphingosine redistributed Bax from cytosol to the mitochondria. Moreover, siRNA targeting of Bax effectively attenuated mitochondrial membrane potential (Δψm) loss and AIF translocation to the nucleus seen after the combination treatment. These results suggest that mitochondrial redistribution of Bax may trigger Δψm loss and causes subsequent AIF translocation to the nucleus following the combined treatment with γ-radiation and phytosphingosine in radiation-resistant cancer cells.
ROS production frequently occurs in cells exposed to UV light, ionizing radiation, H2O2, or cytokines,58-60 and accumulation of intracellular ROSs leads to disruption of the Δψm and subsequent activation of cell death machinery.61,62 Moreover, experiments using antioxidants indicated that ROSs act upstream of Bax relocalization and mitochondrial membrane depolarization.58 Similarly, an early increase in ROS levels has been found to precede mitochondrial membrane permeabilization and in some cases to be independent of caspases in various models including apoptosis induced by Fas,63 p53,64 ischemia,65 DNA alkylation,66 or etoposide.54 In this study, we provided further evidence that increased level of ROSs is involved in the Bax translocation to the mitochondria, Δψm loss, AIF release, and cell death triggered by the combination treatment of phytosphingosine with γ-radiation in radiation-resistant Jurkat clones. Complete inhibition of Bax translocation to the mitochondria by thiol-containing antioxidant, NAC, suggests that increased intracellular ROS level is critical for the Bax relocalization after the combination treatment. However, as the AIF translocation to the nucleus and cell death induced by the combination treatment is not fully inhibited by NAC, it is possible that undefined signals other than ROSs also play an important role in combination treatment-induced AIF translocation and subsequent cell death.
PARP-1 is a nuclear enzyme that facilitates DNA repair in response to DNA damage.26,27 Recently it has been shown that excessive activation of PARP-1 results in depletion of cellular NAD and ATP, eventually leading to cell death.30,31 Although PARP-1-mediated cell death is thought to be necrotic,67,68 recent reports have demonstrated that PARP-1-mediated cell death also has many features in common with apoptotic forms of the cell death.27,30 These studies suggested that translocation of AIF from mitochondria to the nucleus is required for PARP-1-mediated cell death. We also found that PARP-1 activation is involved in the nuclear translocation of AIF and subsequent cell death induced by the combination treatment with γ-radiation and phytosphingosine in radiation-resistant Jurkat clones. Pretreatment of cells with PARP-1 inhibitor or transfection of PARP-1 siRNA efficiently attenuated the disruption of mitochondrial membrane potential, AIF translocation, nuclear condensation, and subsequent cell death induced by the combination treatment. However, Bax translocation to mitochondria was not blocked by inhibition of PARP-1, indicating that the Bax translocation is independent of PARP-1 activation during the apoptotic process induced by the combination treatment. It is possible that PARP-1 activation by combination treatment in our experimental system is caused by ROS-dependent DNA damage. Recently it has been shown that ROS-mediated DNA damage triggers activation of PARP-1 and subsequent cell death.69 Consistent with these findings, we found that combination treatment induces a significant degree of DNA damage as assessed by the phosphorylation level of histone H2AX (γ-H2AX) and its foci formation (data not shown), an established marker for doublestrand breakages (DSBs) in chromosomal DNA.70,71 However, a thiol-containing antioxidant, NAC, failed to block PARP-1 activation and DNA damage (data not shown) induced by the combination treatment, indicating that PARP-1 activation and DNA damage by combination treatment in our experimental system is independent on ROS production. In this regard, it has been reported that ROS generation by UVB is not involved in UVB-induced DNA damage.72 However, the mechanism by which ROS-independent DNA damage and activation of PARP-1 occur remains unclear.
Taken together, we have shown that combination treatment with γ-radiation and phytosphingosine can overcome the radiation resistance through the caspase-independent cell death pathway by enhancing ROS generation and PARP-1 activation. The enhancement of intracellular ROS level by combination treatment promotes mitochondrial translocation of Bax leading to the collapse of mitochondria membrane potential and subsequent AIF release. PARP-1 activation also affects mitochondria membrane potential loss and subsequent AIF release. Interestingly, phytosphingosine appears to trigger 2 independent cell death pathways to overcome radiation resistance in Jurkat variant clones. Our results suggest that the combination treatment of phytosphingosine and γ-radiation is potentially an effective way of treating cancers refractive to conventional radiation therapy.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-07-2938.
Supported by Nuclear Research and Development Program from the Ministry of Science and Technology in Korea.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal