Abstract
The interleukin-12 (IL-12) cytokine family plays important roles in the orchestration of innate and adaptive immunity by dendritic cells (DCs). The regulation of IL-12 expression has been thoroughly studied, but little is known about factors governing the expression of IL-23 and IL-27, 2 novel IL-12 family members acting on memory and naive T cells, respectively. We report that the expression of these cytokines by DCs was critically dependent on the mode of activation. DC activation by CD40L predominantly induced IL-12. Ligands of the Toll-like receptor (TLR) 3 and TLR4 induced IL-12 and IL-27, whereas exposure to intact Escherichia coli resulted in high expression of IL-12, IL-27, and IL-23. The nucleotide adenosine triphosphate (ATP) has been shown to inhibit IL-12 production by P2 receptors. We found that ATP also inhibited IL-27 expression but enhanced IL-23 expression. Interestingly, the reciprocal regulation of IL-12/IL-27 and IL-23 by ATP was mediated by 2 distinct P2 receptors and was also induced by prostaglandin E2 by cyclic adenosine monophosphate (cAMP)–elevating EP2/EP4 receptors. As a consequence, DCs were selectively impaired in their ability to induce interferon-γ (IFN-γ) in naive T cells but continued to promote IFN-γ and IL-17 production in memory T cells. These studies identify P2 receptors as promising targets for the design of novel strategies to manipulate specific stages of T-cell responses and to treat IL-12– and IL-23–mediated disorders.
Introduction
The immune system has evolved 2 defense arms to protect the host from microbial attack: a rapidly responding innate immune response to sequester and eliminate pathogens followed by a highly specific adaptive immune response. Dendritic cells (DCs) form a sentinel network that detects pathogen entry through an array of pattern recognition receptors. Signaling through these receptors induces DC maturation, transforming them from cells specialized in antigen uptake to cells that migrate into regional lymph nodes to stimulate T cells.1 Maturation of DCs can be induced by 3 broad classes of stimuli: microbes and their products recognized by Toll-like receptors (TLRs), proinflammatory mediators, and interactions with surface receptors of the tumor necrosis factor (TNF) family, such as CD40/CD40L (for reviews, see Medzhitov,2 Gallucci and Matzinger,3 and van Kooten and Banchereau4 ). Activation of DCs induces the secretion of cytokines. A key cytokine produced by DCs is interleukin-12p70 (IL-12p70), which is a potent inducer of interferon-γ (IFN-γ) production by NK cells, NKT cells, and T cells. IL-12p70 is critical for the differentiation of naive T cells into T-helper 1 (Th1) cells, required for the elimination of intracellular pathogens and tumor cells. In this way, DCs bridge the innate and adaptive immune responses (for reviews, see Brombacher et al5 and Trinchieri et al6 ). IL-12p70 represents the founding member of a family of cytokines that now includes IL-23 and IL-27.
IL-12p70 is a heterodimer composed of a 35-kDa subunit (IL-12p35) and a 40-kDa subunit (IL-12p40) that binds the IL-12 receptor. The p40 subunit is secreted in excess of IL-12p35 and can also associate with p19 to form IL-23.7 IL-23 signals through the IL-23 receptor8 and has functions that overlap with and are distinct from those of IL-12. Unlike IL-12, IL-23 has no effect on naive T cells but selectively activates memory T cells to secrete IFN-γ and IL-17.7,9 IL-23 is more potent than IL-12 in inducing long-lasting T-cell responses to viral antigens10 and can induce tumor-directed cytotoxic T-cell responses.11,12 Furthermore, IL-23 plays a critical role in the pathogenesis of autoimmune disorders, such as autoimmune encephalitis,13 psoriasis vulgaris,14 and arthritis.15 IL-27 is a recently discovered IL-12 family member consisting of a p28 subunit and EBI3 (Epstein-Barr virus–induced gene 3).16 IL-27 signals through the IL-27 receptor17 and appears to play a role in the early stages of Th1 responses by inducing the proliferation and IFN-γ production of naive CD4+ T cells. In addition, IL-27 can attenuate T-cell activation, suggesting an involvement in limiting T cell–mediated immune hyperactivity.18 Expression of the IL-27 receptor on mast cells and monocytes indicates that IL-27 also influences innate immune responses.17
Regulatory mechanisms of IL-12 production have been studied in detail. For instance, IL-12 secretion is enhanced by several cytokines, such as IFN-γ, IL-1β, IL-4, and IL-13.19-22 Furthermore, IL-12 secretion is down-regulated by IL-10,23 transforming growth factor-β,24 and ligand binding to Gαs-linked G protein–coupled receptors (GPCRs), primarily because of their induction of cyclic adenosine monophosphate (cAMP).25 These include EP2/EP4 receptors for prostaglandin E2 (PGE2), the histamine receptor H2, and the adenosine receptor A2a. The efficacy of cAMP signaling as a negative regulatory mechanism of IL-12 production can also be demonstrated with cAMP analogs and cholera toxin, a potent activator of Gαs. Potent inhibition of IL-12 production has also been reported for extracellular nucleotides, such as adenosine triphosphate (ATP), through the activation of membrane-bound P2 receptors.26 P2 receptors are subdivided into P2X, a family of ligand-gated ion channels, and G-protein–coupled P2Y receptors. We and others have previously reported that human DC types express several subtypes of P2X and P2Y receptors.27-31 Initial studies reported an induction of IL-12p40 expression by ATP, especially in synergy with TNF-α, with pharmacologic data using the synthetic nucleotides ATPγS and AR-C67085 pointing to the P2Y11 receptor and cAMP signaling.32,33 With the availability of monoclonal antibody (mAb) against the IL-12p70 heterodimer, it became evident that ATP has a potent inhibitory effect on bioactive IL-12 production induced by lipopolysaccharide (LPS) or CD40L.26 This inhibitory effect also appears to be mediated by the P2Y11 receptor and by cAMP signaling.34 However, an interaction with other P2 receptors and signaling pathways cannot be excluded because ATP activates multiple P2 receptor subtypes in DCs.31
To date, little is known about the regulation of IL-23 or IL-27 expression by human DCs. Expression of IL-23 and IL-27 subunits has been reported for monocyte-derived DCs (MoDCs) activated with combinations of cytokines, CD40L and TLR ligands, or intact bacteria,7,16,35-37 but systematic studies comparing the influence of individual stimuli are lacking, whereas mediators of negative regulation are unknown. The present study provides a systematic analysis of the regulation of expression of IL-12, IL-23, and IL-27 in human MoDCs in response to several classes of stimuli and of how these molecules influence the ability of DCs to activate naive and memory CD4+ T-cell subsets.
Materials and methods
DC culture and activation
Peripheral blood mononuclear cells (PBMCs) from buffy coats of healthy donors (Red Cross Blood Bank, Melbourne, Australia) were prepared by Ficoll-Paque density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). To generate MoDCs, CD14+ monocytes were isolated using MACS magnetic beads (Miltenyi Biotech, Auburn, CA) and were cultured in RPMI/10% fetal calf serum (FCS) supplemented with 20 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF) and 500 U/mL IL-4 for 6 days, as reported.31 MoDCs were replated at 5 × 105 cells/mL in their conditioned media and were stimulated with the following conditions: 1 μg/mL CD40L trimer (kind gift from Immunex, Seattle, WA); a combination of 10 ng/mL TNF-α, 10 ng/mL IL-1β, 10 ng/mL IL-6 (all from Peprotech, Rocky Hill, NJ), and 1 μM PGE2 (Sigma, St Louis, MO); live Escherichia coli (5 × 106/mL); 100 ng/mL E coli–derived LPS (Sigma); 1 μg/mL R-848; 1 μg/mL Pam3Cys; 12.5 μg/mL Poly(I:C); 6 μg/mL cytosine-phosphate-guanosine oligodeoxynucleotide (CpG ODN) 2006 (all from InvivoGen, San Diego, CA); PR-8 virus (25 HAU). Nucleotides ATP, uridine triphosphate (UTP), adenosine diphosphate (ADP), and ATPγ were obtained from Sigma. The P2Y11 receptor agonist AR-C67085 was a generous gift from AstraZeneca UK (London, United Kingdom). Forskolin and the P2 receptor antagonists suramin and pyridoxal-phosphat-6-azophenyl-2′,4′-disulfonic acid (PPADS) were ordered from Sigma. The EP2/EP4 agonist 11-deoxy-PGE1 and the EP1/EP3 agonist sulprostone were obtained from Sapphire Biosciences (Crows Nest, Australia).
Cytokine ELISA
Cytokine enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA; BD Biosciences, San Diego, CA) were used to quantify IL-12p70, IL-12p40, and IFN-γ (BD Biosciences). Human IL-17 was detected using the IL-17 Cytoscreen kit (Biosource Europe SA, Nivelles, Belgium) and mouse IL-17 with the Quantikine M kit (R&D Systems, Minneapolis, MN).
Quantitative real-time PCR
RNA was isolated from MoDCs using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA was synthesized as previously described.31 One microliter cDNA was used as template for quantitative real-time polymerase chain reaction (RT-qPCR). Gene expression levels were quantified using ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Predeveloped assay reagents (PDARs) for IL-12p35 and IL-12p40 were obtained from Applied Biosystems and were used in multiplex reactions with 18S rRNA PDAR (Applied Biosystems) for normalization. Primers and probes were as follows: IL-23p19, probe 6FAM-AGCAGCAACCCTGAGTCCCTAAAGGC-TAMRA, forward 5′-CCGGGTCTTTGCCCATG-3′, reverse 5′-GAGTGCCATCCTTGAGCTGC-3′; EBI3, probe 6FAM-TGGCGGCTCAGGACCTCACAGA-TAMRA, forward 5′-CCGAGCCAGGTACTACGTCC-3′, reverse 5′-CCAGTCACTCAGTTCCCCGT-3′; IL-27p28, probe 6FAM-AGTGAACCTGTACCTCCTGCCCCTGG-TAMRA, forward 5′-GCGGAATCTCACCTGCCA-3′, reverse 5′-GGAAACATCAGGGAGCTGCTC-3′. PCR reactions were set up in 96-well plates and were analyzed using SDS program version 1.9 (Applied Biosystems, Foster City, CA). Relative expression was calculated using the ΔCt method and was expressed relative to a calibrator, in this case MoDCs cultured in the presence of GM-CSF and IL-4: ΔCt = Ctgene – Ct18S; ΔΔCtsample =ΔCtsample –ΔCtGM/IL-4; relative expression = 2–ΔΔCtsample.
Isolation and activation of human T cells
Human CD4+ T cells were positively selected from PBMCs using MACS magnetic beads (Miltenyi Biotech). CD4+ T cells were stained with fluorochrome-labeled anti-CD45RA and anti-CD45RO mAb (BD PharMingen) and were sorted using a MoFlo cell sorter (Cytomation, Fort Collins, CO). T cells (5-10 × 105/mL) were cultured in RPMI 1640/10% FCS with 1% to 20% (vol/vol) supernatant harvested from MoDC cultures 48 hours after activation and were stimulated with CD3/CD28 T-cell Expander Dynabeads (Dynal Biotech, Oslo, Norway) according to the manufacturer's instructions. Supernatant was collected from the T-cell cultures after 4 days for IFN-γ and IL-17 ELISA.
Isolation and activation of mouse splenocytes
Splenocytes were isolated from C57/BL6 mice using Thy 1.2+ (CD90) MACS magnetic beads (Miltenyi Biotech). T cells (106/mL) were cultured for 6 days in 96-well flat-bottom plates with RPMI 1640/10% FCS supplemented with 100 U/mL IL-2 and 1% to 20% (vol/vol) MoDC-derived supernatant. Blocking mAbs against IL-12p70 (clone 20C2; BD PharMingen), IL-12p40/p70 (clone C8.6; BD PharMingen), and an IL-23 receptor/Fc chimeric protein (R&D Systems) were all used at 10 μg/mL. Reagents containing azide were dialyzed with saline before use.
Results
IL-12, IL-23, and IL-27 production by MoDCs in response to specific stimuli
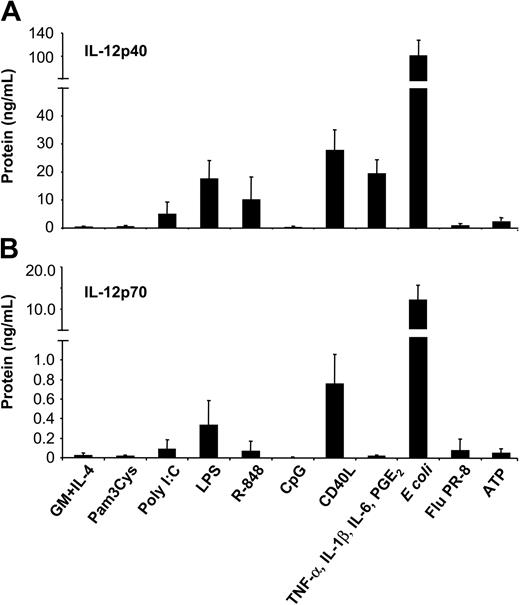
To assess the potency of different classes of stimuli to induce IL-12 production, MoDCs were cultured with intact bacteria (E coli), influenza virus (strain PR-8), Pam3Cys (TLR2 ligand), poly(I:C) (TLR3 ligand), LPS (TLR4 ligand), R-848 (TLR7/8 ligand), CpG ODN2006 (TLR9 ligand), proinflammatory mediators (combination of TNF-α, IL-1β, IL-6, and PGE2), ATP, or CD40L. Secretion of IL-12p40 (Figure 1A) and bioactive IL-12p70 (Figure 1B) was analyzed by ELISA. Striking differences between these stimuli were observed. Stimulation of MoDCs with intact E coli induced the highest levels of IL-12p70 (10.89 ± 4.34 ng/mL). Moderate levels of IL-12p70 were induced by CD40L (0.75 ± 0.30 ng/mL) and LPS (0.33 ± 0.25 ng/mL), whereas low levels were induced by poly(I:C) (0.09 ± 0.09 ng/mL) and R-848 (0.06 ± 0.11 ng/mL). Consistently, the IL-12p40 subunit was produced in excess of IL-12p70. Activation of MoDCs with proinflammatory cytokines induced high levels of IL-12p40 (19.41 ± 4.95 ng/mL) but no detectable IL-12p70. Similarly, ATP induced only IL-12p40 (1.70 ± 0.80 ng/mL). Finally, Pam3Cys (and other TLR2 ligands, such as peptidoglycan; data not shown) and CpG ODN failed to induce significant levels of IL-12p40.
Induction of IL-12p40 and IL-12p70 production by MoDCs in response to various classes of maturation-inducing stimuli. MoDCs (0.5 × 106/mL) were incubated with the indicated stimuli, and supernatants were harvested after 2 days. (A) IL-12p40 and (B) IL-12p70 levels were measured by ELISA from culture supernatants. Data represent mean ± SEM of 5 separate experiments.
Induction of IL-12p40 and IL-12p70 production by MoDCs in response to various classes of maturation-inducing stimuli. MoDCs (0.5 × 106/mL) were incubated with the indicated stimuli, and supernatants were harvested after 2 days. (A) IL-12p40 and (B) IL-12p70 levels were measured by ELISA from culture supernatants. Data represent mean ± SEM of 5 separate experiments.
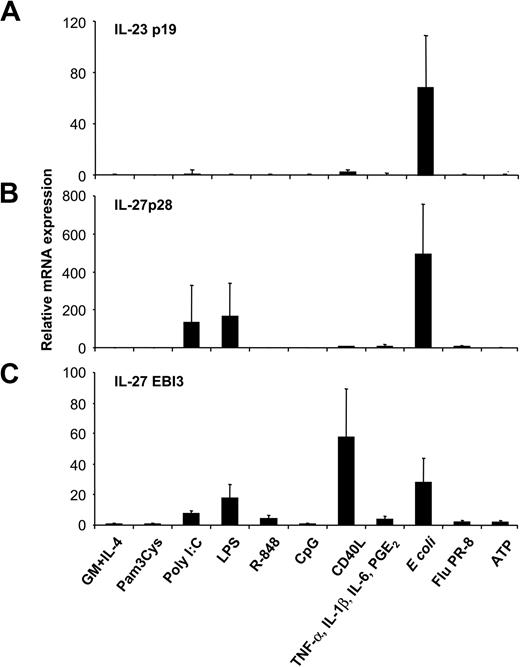
Excessive IL-12p40 levels could reflect the presence of inactive monomers, homodimers, or IL-23p40/p19 heterodimers.7 To assess whether high levels of p40 induced by some stimuli, such as proinflammatory cytokines, reflected IL-23 production, we analyzed IL-23p19 expression of MoDCs activated with the various stimuli by RT-qPCR. Unstimulated, immature MoDCs expressed negligible levels of IL-23p19, equivalent to those of the genomic controls. Surprisingly, neither proinflammatory cytokines, nor ATP, nor CD40L, nor TLR ligands induced IL-23p19 expression in MoDCs (Figure 2A), indicating that the excess IL-12p40 detected in these MoDC cultures was not IL-23. In contrast, activation of MoDCs with intact E coli induced a marked up-regulation of IL-23p19 expression. This effect was strongly down-regulated when E coli were heat inactivated before MoDC stimulation (data not shown). Expression levels of the 2 IL-27 subunits p28 and EBI3 were analyzed next (Figure 2B-C). Intact E coli and the 2 TLR ligands, LPS and poly(I:C), were inducers of both IL-27 subunits, whereas CD40L and, to a lesser extent, R-848 induced only EBI3, not IL-27p28, expression (Figure 2B-C). Pam3Cys, CpG, inflammatory cytokines, ATP, and influenza virus did not induce either IL-27 subunit. Thus, not maturation per se, but the class of stimulus a DC encounters influences the expression pattern of IL-12–related cytokines, with IL-23 and IL-27 expression strongly induced by intact microbes.
Expression of IL-23 and IL-27 subunits by MoDCs activated with several classes of maturation-inducing stimuli. MoDCs were incubated with the indicated stimuli, and (A) IL-23p19, (B) IL-27p28, and (C) EBI3 mRNA expression was quantified by RT-qPCR 14 to 18 hours after stimulation. Data are shown as n-fold increases of mRNA expression compared with unstimulated MoDCs (GM-CSF and IL-4). Data represent mean ± SEM of 4 to 6 separate experiments.
Expression of IL-23 and IL-27 subunits by MoDCs activated with several classes of maturation-inducing stimuli. MoDCs were incubated with the indicated stimuli, and (A) IL-23p19, (B) IL-27p28, and (C) EBI3 mRNA expression was quantified by RT-qPCR 14 to 18 hours after stimulation. Data are shown as n-fold increases of mRNA expression compared with unstimulated MoDCs (GM-CSF and IL-4). Data represent mean ± SEM of 4 to 6 separate experiments.
Regulation of IL-12, IL-23, and IL-27 expression by ATP
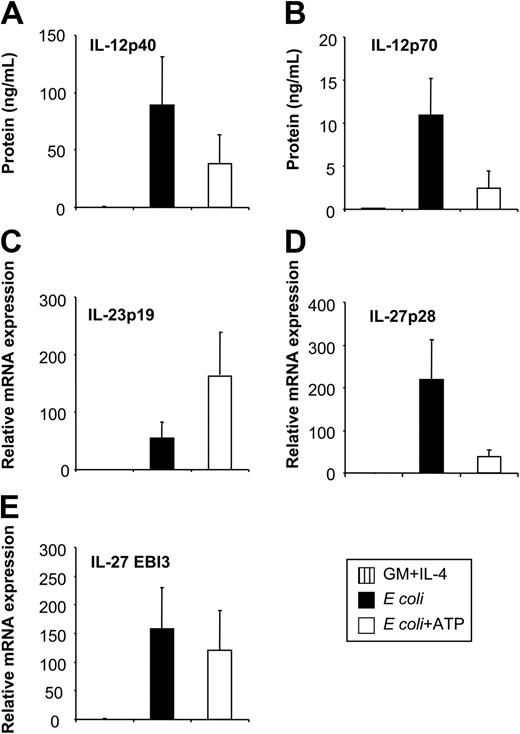
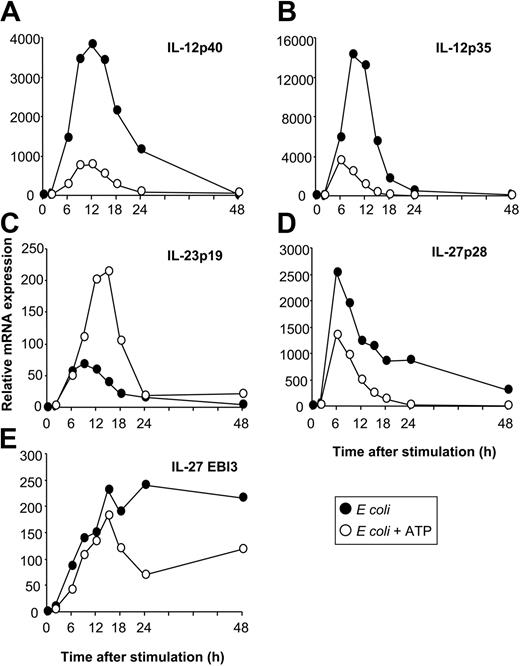
Having identified E coli as a potent inducer of IL-12, IL-23, and IL-27 expression enabled us to investigate the regulation of these 3 cytokines by physiologic modulators of cytokine production. The nucleotide ATP has inhibitory effects on IL-12p70 expression through P2 receptors.26 To investigate whether ATP also affects other IL-12–related cytokines, we stimulated MoDCs with E coli in the absence or presence of ATP. As assessed by RT-qPCR and ELISA, ATP inhibited the expression of IL-12p35 and IL-12p40 (Figures 3A, 4A-B), resulting in reduced levels of IL-12p70 (Figure 3B). In contrast, IL-23p19 expression was enhanced (Figure 3C). Additionally, ATP suppressed IL-27p28 expression (Figure 3D) but had only a minor effect on EBI3 expression (Figure 3E). Thus, the expression of IL-12 and IL-27 is regulated in a manner reciprocal to that of IL-23 by ATP. This regulation pattern of IL-12–related cytokines by ATP was also observed for MoDCs stimulated with CD40L or LPS (data not shown). To exclude that these observations were attributed to disparities in the kinetics of cytokine synthesis, we analyzed cytokine expression over a 48-hour time period (Figure 4). The expression of IL-12p40, IL-12p35, and IL-23p19 was up-regulated within the first 6 hours after activation with E coli, peaking at approximately 9 to12 hours and decreasing to background levels by 24 to 48 hours (Figure 4A-C). In contrast, the up-regulation of IL-27p28 expression was more rapid, peaking at 6 hours and rapidly decreasing thereafter, whereas EBI3 expression increased gradually and was sustained during the 48 hours (Figure 4D-E). Inhibitory and stimulatory effects of ATP on IL-12, IL-27, and IL-23 expression mirrored the kinetic profile observed in the absence of ATP, arguing against disparities in the kinetics of cytokine synthesis.
Influence of extracellular ATP on IL-12, IL-23, and IL-27 expression of E coli–activated MoDCs. MoDCs were left untreated or were incubated with E coli in the absence or presence of ATP (250 μM). (A) Secreted IL-12p40 and (B) IL-12p70 levels were analyzed by ELISA 2 days after stimulation. (C) Expression of IL-23p19, (D) IL-27p28, and (E) EBI3 was analyzed by RT-qPCR 14 to 18 hours after stimulation. Data represent mean ± SEM of 5 to 6 separate experiments.
Influence of extracellular ATP on IL-12, IL-23, and IL-27 expression of E coli–activated MoDCs. MoDCs were left untreated or were incubated with E coli in the absence or presence of ATP (250 μM). (A) Secreted IL-12p40 and (B) IL-12p70 levels were analyzed by ELISA 2 days after stimulation. (C) Expression of IL-23p19, (D) IL-27p28, and (E) EBI3 was analyzed by RT-qPCR 14 to 18 hours after stimulation. Data represent mean ± SEM of 5 to 6 separate experiments.
Kinetics of IL-12, IL-23, and IL-27 expression. MoDCs were activated with E coli in the absence or presence of ATP (250 μM), and cytokine expression was analyzed by RT-qPCR at the indicated time points after stimulation. (A) IL-12p40. (B) IL-12p35. (C) IL-23p19. (D) IL-27p28. (E) EBI3. Data are representative of 3 separate experiments.
Kinetics of IL-12, IL-23, and IL-27 expression. MoDCs were activated with E coli in the absence or presence of ATP (250 μM), and cytokine expression was analyzed by RT-qPCR at the indicated time points after stimulation. (A) IL-12p40. (B) IL-12p35. (C) IL-23p19. (D) IL-27p28. (E) EBI3. Data are representative of 3 separate experiments.
Regulation of IL-12 and IL-23 expression by ATP is mediated by different P2 receptors
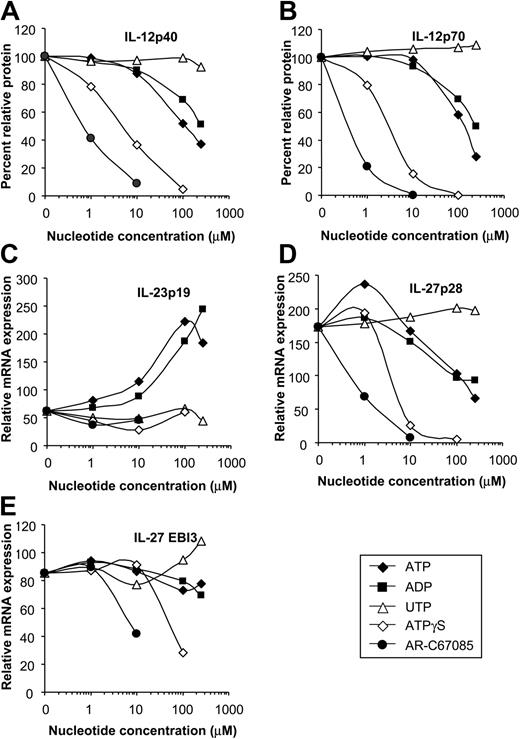
DCs express different subtypes of P2 receptors with distinct pharmacologic properties. To identify the P2 receptors involved in the regulation of IL-12, IL-23, and IL-27 expression, we assessed the rank order of potency of various nucleotides on cytokine expression. ATP inhibited IL-12p40 and IL-12p70 production of E coli–stimulated MoDCs in a dose-dependent fashion (concentration producing 50% of the maximum rate of inhibition [EC50], approximately 100 μM) (Figure 5A-B). Less efficient IL-12 inhibition was seen for ADP (EC50, approximately 250 μM), whereas UTP was ineffective. Nucleotides with a high affinity for the P2Y11 receptor, such as AR-C67085 (EC50, approximately 0.4 μM) and ATPγS (EC50, approximately 3 μM), had the highest inhibitory potency (Figure 5A-B). Next, we assessed the influence of the P2 receptor antagonists suramin and PPADS on cytokine expression. No cytokine induction was seen for these inhibitors (data not shown). The ATP effect on IL-12 expression was sensitive to the P2 receptor antagonist suramin but not to PPADS (Figure 6A-B). This pharmacologic profile is characteristic for the P2Y11 receptor.38 As with the IL-12 subunits, expression of the IL-27 subunit p28 was inhibited most effectively by nucleotides with high affinity for the P2Y11 receptor (Figure 5D-E). In contrast, IL-23p19 expression was up-regulated by ATP and ADP in dose-dependent fashion, whereas specific P2Y11 receptor agonists and UTP were ineffective (Figure 5C). Furthermore, the ATP effect on IL-23p19 expression was not antagonized by suramin but was antagonized by PPADS (P = .01) (Figure 6C). Because PPADS can also interfere with ecto-nucleotidases, which hydrolyze ATP and ADP to adenosine, we investigated whether adenosine affects IL-23p19 expression. However, adenosine had no significant effect on IL-23p19 expression at concentrations up to 100 μM (data not shown). These findings indicate that the reciprocal regulation of IL-12 and IL-23 is mediated by distinct P2 receptors.
Influence of various P2 receptor agonists on IL-12, IL-23, and IL-27 expression. MoDCs were activated with E coli in the absence or presence of ATP, ADP, UDP, ATPγS, or AR-C67085 (P2Y11 receptor agonist) at the indicated concentrations. (A) IL-12p40 and (B) IL-12p70 levels were determined by ELISA. Cytokine production in the absence of nucleotides was normalized to 100%. Data are mean values of 7 experiments. (C) IL-23p19, (D) IL-27p28, and (E) EBI3 expression was quantified by RT-qPCR and is expressed as n-fold increase compared with MoDCs left untreated. Data are mean values of 4 experiments.
Influence of various P2 receptor agonists on IL-12, IL-23, and IL-27 expression. MoDCs were activated with E coli in the absence or presence of ATP, ADP, UDP, ATPγS, or AR-C67085 (P2Y11 receptor agonist) at the indicated concentrations. (A) IL-12p40 and (B) IL-12p70 levels were determined by ELISA. Cytokine production in the absence of nucleotides was normalized to 100%. Data are mean values of 7 experiments. (C) IL-23p19, (D) IL-27p28, and (E) EBI3 expression was quantified by RT-qPCR and is expressed as n-fold increase compared with MoDCs left untreated. Data are mean values of 4 experiments.
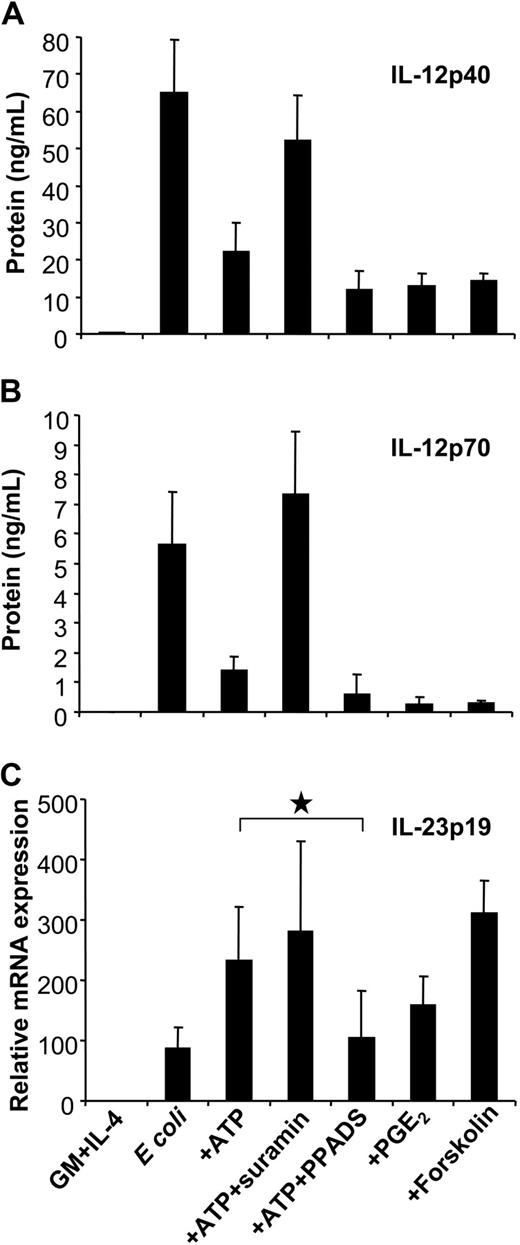
Influence of P2 receptor antagonists and the cAMP pathway on IL-12 and IL-23 expression. MoDCs were left untreated or were stimulated with E coli in the absence or presence of ATP (250 μM). Where indicated, MoDCs were pretreated with suramin or PPADS. To assess the influence of the cAMP pathway on cytokine expression, MoDCs were stimulated in the presence of PGE2 or forskolin. (A) IL-12p40 and (B) IL-12p70 levels were measured by ELISA. (C) IL-23p19 expression was analyzed by RT-qPCR Mean ± SEM of 5 to 7 different experiments are shown. *P = .01.
Influence of P2 receptor antagonists and the cAMP pathway on IL-12 and IL-23 expression. MoDCs were left untreated or were stimulated with E coli in the absence or presence of ATP (250 μM). Where indicated, MoDCs were pretreated with suramin or PPADS. To assess the influence of the cAMP pathway on cytokine expression, MoDCs were stimulated in the presence of PGE2 or forskolin. (A) IL-12p40 and (B) IL-12p70 levels were measured by ELISA. (C) IL-23p19 expression was analyzed by RT-qPCR Mean ± SEM of 5 to 7 different experiments are shown. *P = .01.
Reciprocal regulation of IL-12 and IL-23 expression is mediated by the cAMP pathway
GPCRs linked to Gαs inhibited IL-12 production by enhancing levels of intracellular cAMP. To assess whether cAMP mediated the up-regulation of IL-23p19, we examined p35, p40, and p19 expression of E coli–activated MoDCs in response to PGE2, which enhances cAMP levels through EP2/EP4 receptors. As previously reported, PGE2 effectively inhibited IL-12p40 and p70 production induced by E coli (Figure 6A-B).39 Interestingly, as observed for ATP, PGE2 enhanced IL-23p19 expression (Figure 6C). The same effect was induced by the EP2/EP4 agonist 11-deoxy-PGE1 but not by the EP1/EP3 agonist sulprostone (data not shown). The role of cAMP signaling in the reciprocal regulation of IL-12 and IL-23 expression was further supported using forskolin (Figure 6A-C).
MoDCs activated with E coli and ATP secrete high levels of bioactive IL-23
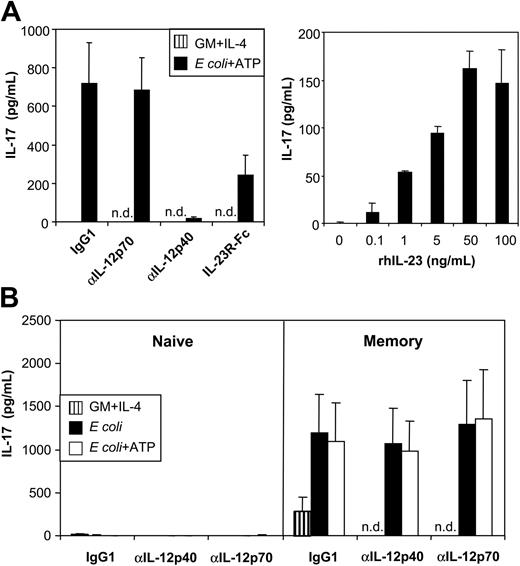
Next, we attempted to confirm whether ATP-mediated up-regulation of IL-23p19 expression in MoDCs, as detected by RT-qPCR, correlated with the secretion of bioactive IL-23. Because an IL-23–specific ELISA was unavailable, we assessed IL-23 bioactivity by the induction of IL-17 by mouse splenocytes.9 Mouse T cells isolated from spleens were incubated with human MoDC supernatants, and murine IL-17 was measured by ELISA. Supernatants of unstimulated MoDCs (cultured with GM-CSF and IL-4) did not induce detectable levels of IL-17, correlating with the lack of IL-23p19 expression found by RT-qPCR (Figure 7A, left graph). In contrast, MoDCs stimulated with E coli and ATP induced high levels of IL-17, which were in the range of those maximally obtained with recombinant human IL-23 (rhIL-23) added to T-cell cultures (Figure 7A, right graph). To examine whether IL-17 production was IL-23 specific, we performed experiments in the presence of neutralizing mAbs against IL-12p70 or IL-12p40, the latter blocking IL-12 and IL-23. Blocking antibodies against p40, but not p70, abrogated IL-17 production. Furthermore, adding an IL-23R/Fc chimeric protein (with IL-23–neutralizing activity) also inhibited IL-17 production (Figure 7A, left graph). Thus, MoDCs activated with E coli and ATP produced high levels of bioactive IL-23.
MoDCs activated with E coli and ATP secrete high levels of bioactive IL-23. (A) (left graph) Murine splenocytes were incubated with supernatant of unactivated or E coli plus ATP–activated MoDCs in the absence or presence of anti–IL-12p70 or anti–IL-12/23p40 mAb or an IL-23R/Fc fusion protein. (right graph) Murine splenocytes were incubated with different concentrations of recombinant human IL-23. Murine IL-17 production was measured by ELISA. (B) Human CD4+ T cells were sorted into naive and memory T-cell populations based on their expression of CD45RA and CD45RO, respectively, and were cultured in the presence of MoDC supernatants and CD3/CD28 T-cell expander beads. Isotype (IgG1), anti–IL-12p70, or anti–IL-12/23p40 mAbs were added as indicated. IL-17 was measured by ELISA. Data represent mean ± SEM of 5 separate experiments, n.d. indicates not done.
MoDCs activated with E coli and ATP secrete high levels of bioactive IL-23. (A) (left graph) Murine splenocytes were incubated with supernatant of unactivated or E coli plus ATP–activated MoDCs in the absence or presence of anti–IL-12p70 or anti–IL-12/23p40 mAb or an IL-23R/Fc fusion protein. (right graph) Murine splenocytes were incubated with different concentrations of recombinant human IL-23. Murine IL-17 production was measured by ELISA. (B) Human CD4+ T cells were sorted into naive and memory T-cell populations based on their expression of CD45RA and CD45RO, respectively, and were cultured in the presence of MoDC supernatants and CD3/CD28 T-cell expander beads. Isotype (IgG1), anti–IL-12p70, or anti–IL-12/23p40 mAbs were added as indicated. IL-17 was measured by ELISA. Data represent mean ± SEM of 5 separate experiments, n.d. indicates not done.
To examine whether MoDC-derived IL-23 also induces IL-17 production by human T cells, we sorted human CD4+ T cells into naive (CD45RA+) and memory (CD45RO+) T-cell populations and stimulated these with beads coated with anti-CD3/CD28 mAbs in the absence or presence of MoDC supernatants. No IL-17 was detected in cultures of naive CD4+ T cells. In contrast, bead-stimulated memory T cells produced significant levels of IL-17 even in the absence of MoDC supernatant (Figure 7B), indicating that, in contrast to production by mouse T cells, IL-17 production by human T cells was not strictly dependent on IL-23. Supernatant of MoDCs activated with E coli in the absence or presence of ATP strongly enhanced IL-17 production (Figure 7B). In contrast again to mouse cells, IL-17 production by human memory T cells was not neutralized by p40- or p70-blocking mAbs (Figure 7B). Thus, once more in contrast to mouse T cells, IL-23 does not appear to be required for IL-17 production by human memory T cells.
ATP reduces the ability of E coli–activated MoDCs to induce IFN-γ by naive T cells but not by memory T cells
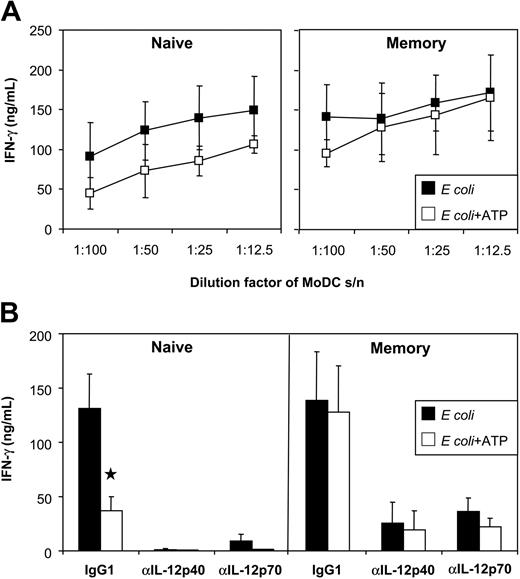
In humans, naive T cells produce IFN-γ in response to IL-12 and IL-27, whereas memory T cells produce IFN-γ in response to IL-12 and, to a lesser extent, in response to IL-23 (for a review, see Trinchieri et al6 ). Because ATP reduced the expression of IL-12 and IL-27 in E coli–activated MoDCs, we speculated that the ability of these DC to activate naive T cells would be impaired. The impact of ATP on memory T-cell activation was difficult to predict because both IL-12 and IL-23 target this T-cell subset in humans. To examine the impact, naive and memory CD4+ T cells were stimulated with anti-CD3/CD28 mAb-coated beads in the absence or presence of MoDC supernatant, and IFN-γ induction was analyzed. Supernatants from E coli–activated MoDCs (but not unstimulated MoDCs) induced high levels of IFN-γ by naive and memory T cells. MoDCs exposed to ATP showed a reduced capacity for inducing IFN-γ production by naive T cells (Figure 8A-B). The importance of DC-derived IL-12 on naive T-cell IFN-γ was confirmed by blocking IFN-γ induction with anti–IL-12p70 mAb (Figure 8B). In contrast, the ability of MoDCs to induce memory T-cell IFN-γ was not significantly inhibited by ATP. Even when the DC supernatant was diluted to 1%, memory T-cell activation was still highly efficient (Figure 8A). The influence of IL-12 and IL-23 on memory T-cell IFN-γ induction was next assessed. Both p40- and p70-blocking mAbs effectively blocked memory T-cell IFN-γ. Thus, as observed for naive human T cells, memory human T-cell IFN-γ production depended primarily on IL-12.
Influence of ATP on the ability of MoDCs to induce IFN-γ by naive and memory T cells. (A) Human naive and memory CD4+ T cells were cultured with CD3/CD28 T-cell expander beads in the presence of MoDC supernatants (stimulated with E coli in the presence or absence of ATP). IFN-γ was measured by ELISA. (B) As indicated, isotype (IgG1), anti–IL-12p70, or anti–IL-12/23p40 mAbs were added to the T-cell cultures. Data represent mean ± SEM of 6 experiments (using supernatants of 3 DC donors).
Influence of ATP on the ability of MoDCs to induce IFN-γ by naive and memory T cells. (A) Human naive and memory CD4+ T cells were cultured with CD3/CD28 T-cell expander beads in the presence of MoDC supernatants (stimulated with E coli in the presence or absence of ATP). IFN-γ was measured by ELISA. (B) As indicated, isotype (IgG1), anti–IL-12p70, or anti–IL-12/23p40 mAbs were added to the T-cell cultures. Data represent mean ± SEM of 6 experiments (using supernatants of 3 DC donors).
Discussion
It has recently become evident from mouse studies that 2 novel members of the IL-12–related cytokine family, IL-23 and IL-27, play crucial roles in the regulation of innate and adaptive immune responses. We investigated how the expression of these cytokines is regulated in human DCs and found that the expression of IL-12, IL-23, and IL-27 was critically dependent on the mode of DC activation. A mixture of proinflammatory mediators (IL-1β, IL-6, TNF-α, and PGE2), commonly used to mature MoDCs, induced the expression of the shared IL-12/IL-23 p40 subunit but not IL-12p70 or IL-23p19. CD40L and specific TLR ligands induced either predominantly IL-12 or IL-12 and IL-27, whereas intact E coli induced IL-12, IL-27, and IL-23. Expression of these 3 IL-12–related cytokines was recently reported for MoDCs stimulated with intact Streptococcus pyogenes.35 Thus, IL-23 production by MoDCs can be induced by Gram-negative and Gram-positive bacteria. In line with a previous report,35 to exhibit this activity the bacteria had to be intact, indicating that DC activation by bacteria differs from DC activation by TLR ligands, possibly because of a combined effect of engaging multiple TLR or of as yet unidentified mechanisms requiring active bacterial metabolism.
MoDCs activated with E coli were used to study the regulation of IL-12–related cytokine expression by physiologic modulators of cytokine production. ATP has previously been shown to influence IL-12 production of MoDCs in 2 ways: in synergy with TNF-α, ATP up-regulates IL-12p40 expression32,33 but inhibits IL-12p70 production in response to LPS or CD40L.26,34 In this respect, ATP has similarities with PGE2.39-41 Our study confirmed that ATP inhibits IL-12p70 production in response to potent IL-12–inducing stimuli, such as E coli, LPS, or CD40L (data for CD40L and LPS not shown). In addition, we found that ATP suppressed IL-27, but enhanced IL-23, expression. The production of high levels of bioactive IL-23 heterodimers was confirmed by the ability of DC supernatants to induce IL-17 in mouse splenocytes in an IL-23–specific manner. The lack of IL-23–specific mAbs for use in ELISA did not allow exact IL-23 quantification; however, IL-23 levels can be estimated by the bioactivity of DC supernatants, which induced IL-17 levels similar to those maximally obtained with rhIL-23 (greater than 50 ng/mL). Thus, ATP had a selective effect on the expression profile of IL-12–related cytokines, shifting the ratios of IL-12/IL-23 and IL-27/IL-23 toward IL-23.
What is the physiologic relevance of our findings in vivo? ATP is stored in the cytosol at millimolar concentrations and is released into the extracellular space on cell activation or by leakage from damaged cells.42-44 ATP accumulates in inflamed tissues as a result of enhanced release and reduced degradation through the down-regulation of ecto-ATP/ADPase (CD39), which dephosphorylates ATP to AMP.45 Extracellular ATP binds to P2 receptors expressed by various cell types and induces numerous effects on immune and inflammatory responses (for a review, see Di Virgilio et al46 ). The in vivo relevance of extracellular nucleotides on immune function has been demonstrated with CD39-deficient mice, which exhibit impaired DC function and altered cellular immune responses.47 Human DCs express several P2 receptor subtypes, and signaling through these receptors modulates DC functions. ATP enhances antigen uptake, the expression of activation markers, and the T-cell stimulatory capacity of DCs.27,32 ATP regulates DC migration to lymph node–directing chemokines31,48,49 and inhibits TNF-α and IL-12p70 production and, thus, Th1 polarization of naive T cells.26,49 At high concentrations (millimolar range), ATP induces apoptosis.28,29 Thus, nucleotides can influence DC-mediated immune responses at multiple levels, and the present study has identified that this can be at the level of regulation of DC cytokine production and interaction with specific stages of T cells.
Expression of multiple P2 receptor subtypes by MoDCs complicates the identification of the precise mechanism mediating the ATP effect on cytokine production. A recent study indicated that the inhibitory effect of ATP on IL-12p70 production is mediated by the P2Y11 receptor and involves cAMP signaling.34 Our study confirmed a role for the P2Y11 receptor in IL-12 regulation. First, 2 nucleotides with high affinity for the P2Y11 receptor, AR-C67085 and ATPγ-S, were the most potent inhibitors of IL-12p70 production. Second, the P2 receptor antagonist suramin, but not PPADS, inhibited the ATP effect. These findings are consistent with the pharmacologic profile of the cloned P2Y11 receptor.50 Inhibition of IL-27p28 expression was also most pronounced for P2Y11 receptor agonists, indicating that IL-12 and IL-27 expression are regulated by the P2Y11 receptor. Interestingly, IL-23p19 expression was not influenced by P2Y11 receptor agonists but was strongly up-regulated by ATP and ADP. Furthermore, the ATP effect on IL-23p19 expression was not inhibited by suramin but was inhibited by the P2 receptor antagonist PPADS. Given that we could exclude adenosine, which accumulates during ATP/ADP degradation and binds to P1 receptors, as the mechanism for IL-23p19 up-regulation, our findings indicated that the reciprocal regulation of IL-12/IL-27 and IL-23 was mediated by distinct P2 receptors. The effectiveness of ADP pointed to a P2 receptor with affinity to ADP, such as P2Y1, P2Y2, P2Y12, or P2Y13, which are all expressed by MoDC.31 Interestingly, forskolin and PGE2, which enhanced cAMP levels through EP2/EP4 receptors, also shifted the IL-12/IL-23 ratio toward IL-23. Thus, cAMP signaling appeared to mediate the reciprocal regulation of IL-12 and IL-23 expression. It is thus surprising that the P2Y11 receptor, which is positively coupled to adenylate cyclase (and to phospholipase C),38,50 had no influence on IL-23 expression. We conclude that PGE2 and ATP regulated IL-23p19 expression through distinct pathways, which, for ATP, might have involved cross-talk between different P2 receptors and their downstream signaling cascades.
What are the consequences of exposure to ATP or PGE2 on the capacity of MoDCs to regulate T-cell responses? IL-12 is a key cytokine for the activation of innate effector cells, such as NK cells, and for the induction of Th1 responses. IL-27 appears to be particularly important in the early stages of Th1 responses.5 Thus, inhibition of IL-12 and IL-27 by ATP or PGE2 may provide a negative regulatory mechanism for the limitation of excessive T-cell activation. Consistent with this hypothesis is our finding that MoDCs exposed to ATP- or PGE2-containing stimuli had a reduced capacity to stimulate IFN-γ in naive T cells. Of note, a deviation toward Th2 cytokines, such as IL-4 or IL-5, was not observed (data not shown; also Luft et al39 and Scandella et al40 ). The up-regulation of IL-23 expression by nucleotides may serve a different purpose. Several studies provide evidence that IL-23 plays an important role in the induction of T-cell memory in mice.10-12,51 IL-23 has also been shown to induce IFN-γ production by human memory T cells, though far less efficiently than IL-12 (an important difference to mouse memory T cells, which do not express the IL-12 receptor complex).7 Interestingly, despite the reduction of IL-12 levels, ATP-activated MoDCs were still highly efficient in stimulating IFN-γ and IL-17 in memory T cells in our study, which for IFN-γ occurred in an IL-12–dependent fashion. This could be explained by a high sensitivity of memory T cells to IL-12 or by a synergistic effect between IL-23 and IL-12, which may reduce the IL-12 requirement. The implication is that, in contrast to reports suggesting that IL-12–deficient DCs skew Th2,52 these DCs, in fact, continue to promote Th1-type T-cell responses on T cells that are at a later stage of the life cycle. Another important finding of the study was that human and mouse memory T cells differed in their requirements for IL-23 to secrete IL-17. Unlike their murine counterpart, human memory T cells produced IL-17 in response to MoDC supernatants in an IL-23–independent fashion. Thus, these species-specific differences have to be taken into account when extrapolating from murine studies to humans.
In summary, we describe a novel role for P2 receptors and the cAMP signaling pathway in the regulation of DC function. P2 receptors were found to be potent regulators of IL-12–related cytokines in human DCs, limiting IL-12 and IL-27 expression, and enhancing IL-23 expression. Because IL-23 appears to be critical for the generation of T-cell memory and autoimmunity, P2 receptors may serve as promising targets for the development of new treatment modalities for infections, autoimmune disorders, and cancer.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-05-1718.
Supported by a program grant from the Australian National Health and Medical Research Council (NH&MRC) and the Ludwig Institute for Cancer Research and by the Mildred Scheel Stiftung (M.S.). E.M. is an employee of CSL Limited and an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research.
J.C. and E.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Immunex, an Amgen company, for supplying the soluble CD40L trimer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal