Abstract
Posttransplantation lymphoproliferative disorder (PTLD) is a devastating post-transplantation complication often associated with Epstein-Barr virus (EBV). Although the type and length of immunosuppression are risk factors, a patient's inherent immune capacity also likely contributes to this disorder. This report uses severe-combined immunodeficient mice given injections of human peripheral blood leukocytes (hu PBL-SCID [Severe Combined Immunodeficient] mice) to test the hypothesis that cytokine genotype associates with the development of EBV-associated lymphoproliferative disease (LPD). We observed that the A/A (adenosine/adenosine) genotype for base + 874 of the interferon γ (IFN-γ) gene was significantly more prevalent in PBLs producing rapid, high-penetrance LPD in hu PBL-SCID mice, compared to PBLs producing late, lowpenetrance LPD or no LPD. In examining the relationship between genotype and cytolytic T-lymphocyte (CTL) function, transforming growth factor β (TGF-β) inhibited restimulation of CTLs in PBLs with adenosine at IFNG base + 874, but not in PBLs homozygous for thymidine. Importantly, neutralization of TGF-β in hu PBL-SCID mice injected with A/A genotype PBLs resulted in reduced LPD development and expanded human CD8+ cells. Thus, our data show that TGF-β may promote tumor development by inhibiting CTL restimulation and expansion. Further, our data indicate that IFNG genotype may provide valuable information for both identifying transplant recipients at greater risk for PTLD and developing preventive and curative strategies.
Introduction
Posttransplantation lymphoproliferative disorder (PTLD) is a devastating complication of solid organ and stem cell transplantation that can have a 70% to 80% mortality rate.1 PTLD is often associated with Epstein-Barr virus (EBV), a ubiquitous herpes virus that establishes latent infection in the majority of healthy adults. Primary infection with EBV and elevated viral load in the posttransplantation period are significant risk factors for developing PTLD, particularly in pediatric patients.2-4 However, the majority of adults are EBV seropositive, so additional risk factors for PTLD have been difficult to determine. The incidence of PTLD varies according to the organ transplanted, as well as the intensity and duration of immunosuppression. In recipients of renal transplants, PTLD occurs in 1% to 2% of patients, but the incidence is as high as 20% in recipients of bone marrow and lung transplants.1 There is no accepted standard of therapy for PTLD.
Cytolytic T-lymphocyte (CTL) activity is crucial for prevention or sustained recovery from PTLD.5-8 It is thought that immunosuppression inhibits the EBV-specific cellular immunity that normally prevents the progression of EBV-driven transformation of latently infected cells. Reduction of immunosuppression is effective in treating some, but not all, patients with PTLD,1,8 and increases the likelihood of developing acute rejection episodes that can result in graft loss. Current clinical trials for treatment of PTLD include cellular and monoclonal antibody therapies; however, recurrences may occur after these treatments.9,10
Interferon γ (IFN-γ) is an important cytokine in cellular immunity and CTL function. One polymorphism in the IFNG gene is a single nucleotide polymorphism at position + 874 containing either a thymidine (T) or an adenosine (A). The presence of the thymidine at + 874 correlates with microsatellite repeats associated with high cytokine production and creates a nuclear factor κB (NF-κB)–binding site.11-13 The T/T genotype is often referred to as a “high producer” and A/A genotype as “low producer,”13 although in vitro cytokine production can only be reliably inferred from genotype if T cells are maximally stimulated14 or a large sample size is used.15 In general, the relationship between IFNG polymorphisms and disease states has not been extensively studied, although there are reported links to graft-versus-host disease (GVHD), chronic hepatitis infection, and tuberculosis.15-18 We recently observed in a small study that a majority of patients with PTLD exhibit the A/A cytokine genotype for IFNG and that this is significantly increased compared to the frequency seen in non-PTLD renal transplant patients.19
Transforming growth factor β (TGF-β) is a ubiquitous, pluripotent cytokine that can suppress multiple T-cell and antigenpresenting cell (APC) functions (for reviews, see Letterio and Roberts20 and Gold21 ), including EBV-specific CTL effector function.22 TGF-β has been linked to EBV activation and replication,23-26 and increased transformation.27,28 These data suggest that TGF-β may be important in EBV-driven cancers. By activating viral replication and suppressing viral-specific CTL function, the increased viremia and B-cell transformation combined with simultaneously suppressed T-cell responses could promote PTLD. TGF-β and IFN-γ are antagonistic and counter-regulatory (for reviews, see Strober et al,29 Cavaillon,30 and Leash and Abraham31 ), in that both in vitro32-35 and in vivo36-38 studies have shown that IFN-γ can inhibit TGF-β activity, and vice versa. Thus, high levels of IFN-γ may protect against the immunosuppressive effects of TGF-β, or reducing TGF-β may allow increased IFN-γ activity.
The hu PBL-SCID mouse, in which human (hu) peripheral blood leukocytes (PBLs) from healthy EBV-seropositive donors are injected into severe combined immunodeficiency (SCID) mice, is a reproducible model of spontaneous EBV-driven lymphoproliferative disease (LPD). EBV+ B-cell tumors spontaneously arising in hu PBL-SCID mice are phenotypically and genotypically very similar to PTLD.39,40 Previous reports indicated donor-derived variability in LPD development in the hu PBL-SCID mouse39 ; however, factors contributing to this variability are largely undefined. We hypothesized that the low-producer A/A IFN-γ genotype would associate with rapid LPD in the hu-PBL-SCID mouse model. Here, we demonstrate that PBLs from individuals with specific IFNG genotypes differ in their ability to rapidly develop high-penetrance LPD in the hu PBL-SCID mouse. In examining the relationship between IFN-γ genotype and CTL function, we demonstrate that restimulation of CTLs from donors of the A/A or A/T IFNG genotypes is inhibited following exposure to TGF-β in vitro. To further assess the contribution of TGF-β to the LPD process, we neutralized TGF-β in hu PBL-SCID mice. Effective TGF-β neutralization resulted in protection from LPD and an expansion of human CD8+ cells. Given that CTL activity is crucial for prevention or sustained recovery from PTLD,5-8 our data suggest that increased susceptibility to TGF-β may provide an explanation for the relationship between IFNG genotype and development of PTLD.
Materials and methods
PBLs
PBLs were obtained from American Red Cross leukopacks or from volunteers using protocols approved by the Institutional Review Board. PBLs were isolated by Ficoll-Hypaque according to standard methods. Donors were tested for EBV reactivity by enzyme-linked immunosorbent assay (ELISA; Meridian, Cincinnati, OH) and EBV-reactive trans vivo delayed-type hypersensitivity assays41,42 prior to injection into SCID mice or use in CTL restimulation cultures. Approval was obtained from the Ohio State University Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Genotyping assays
DNA was isolated from PBLs using Qiagen (Valencia, CA) DNA extraction kits. HLA analysis was done using Pel-Freez Clinical Systems AB/DR PCR-SSP unitrays (Brown Deer, WI). Cytokine genotyping for TGF-β, tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), IL-10, and IFN-γ was accomplished using Cytgen cytokine genotyping trays from One Lambda (Canoga Park, CA). Polymerase chain reaction (PCR) products were run on 2% agarose gels and visualized with ethidium bromide. Banding patterns were interpreted using manufacturer's templates and compared to internal controls in each lane.
hu PBL-SCID model
Female Balb/c or CB.17 scid/scid (SCID) mice were purchased from Charles River (Wilmington, MA) or Taconic Farms (Germantown, PA). Mice were housed and treated in accordance with National Institutes of Health (NIH) and institutionally approved guidelines. Mice received 50 × 106 human PBLs intraperitoneally in saline. PBLs from each donor were injected into 3 to 5 separate mice. Human PBL engraftment was monitored with biweekly ELISAs for the presence of human IgG in mouse serum, as previously described.40 Mice included in this study had more than 750 μg/mL human IgG, which increased to more than 1 mg/mL when tumors were detected. Latency was defined as the time after injection until mice became moribund or died.39 All animals were inspected at death for the presence of tumors, and these tumors were confirmed to be of human B-cell origin using flow cytometry. Only mice with confirmed human tumors were considered to have LPD.
Flow cytometry
Splenocytes and tumor cells from hu PBL-SCID mice were analyzed via flow cytometry to assess CD8+ T-cell levels and T-cell activation. All antibodies and isotype control antibodies were directly conjugated and obtained from BD PharMingen (San Diego, CA). Samples were read on a FACScan (BD PharMingen) and analyzed using Cell Quest software.
In vivo TGF-β neutralization
The hu PBL-SCID mice received 125 μg anti–TGF-β monoclonal antibody (A411; obtained from Dr P. Heeger, Cleveland Clinic Foundation, Cleveland, OH) intraperitoneally 3 times per week or 100 μg every other day for 8 weeks. Control animals received phosphate-buffered saline (PBS) or 100 μg mouse IgG. Neutralization of endogenous TGF-β was confirmed by TGF-β ELISA of serum from hu PBL-SCID mice tested at 4, 6, and 8 weeks after cell injection. In the survival trial, animals received 100 μg anti–TGF-β (1D11; Genzyme, Cambridge, MA), isotype control antibody, or PBS intraperitoneally every other day for the duration of the study.
LCLs and culture
EBV-transformed lymphoblastoid cell lines (LCLs) were derived from hu PBL-SCID tumors or by in vitro infection with EBV-containing supernatant of the B89.5 cell line using standard protocols. The LCL with defined HLA molecules was the kind gift of Dr W. Hildebrand (B62 → .221, Oklahoma Medical Research Foundation, Oklahoma City, OK).
CTL restimulation
PBLs were depleted of natural killer (NK) cells using Miltenyi Biotech (Auburn, CA) anti-CD56 magnetic beads and LD columns. PBLs were plated at 2 × 106 cells/mL. LCL tumor lines used as stimulator cells were γ irradiated at a dose of 20 000 rads or treated with mitomycin C (5 μg per million cells). There were no observed differences in CTL function using either irradiated or mitomycin-treated LCLs as stimulator cells. LCLs were plated at 1 × 105 cells/mL. PBLs and LCLs were cocultured with cytokine for 5 days and viable PBLs were recovered and used in cytolysis assays. After 5 days, restimulation cultures contain a mix of CD3+CD4+ and CD3+CD8+ cells, with no CD3+CD56+ lymphokine-activated killer cells detectable. The HLA typing of the donors and LCL stimulators are shown in Table 1. PBLs were stimulated with autologous LCLs, except for donor 6J and donors 1P, 2A, 3M, and 5C. Donor 6J was stimulated with LCLs expressing only B62 and DR1, whereas donors 1P, 2A, 3M, and 5C were stimulated with LCLs expressing only A1, B8, and DR3. Thus, LCL stimulators did not express HLA-A/-B molecules different from the PBL donor.
HLA typing of PBL donors and LCL stimulators
Donor . | HLA typing of donor . | HLA typing of LCL stimulator . |
|---|---|---|
| 1P | A1, 24 B8,51 DR2,5 | A1, B8, DR3 |
| 2A | A1,2, B8, DR3, 13 | A1, B8, DR3 |
| 3M | A1,24 B7,8DR15,17* | A1, B8, DR3 |
| 4D | A30,32 B8,18 DR4,7 | A30,32 B8,18 DR4,7 |
| 5C | A1,24 B8,27 DR3,4 | A1, B8, DR3 |
| 6J | A1,2 B17,62 DR1,7 | A(null), B62, DR1 |
| 7AO | A23,30 B27,44 DR1,7 | A23,30 B27,44 DR1,7 |
| 8G | A2,31 B50,60 DR4 | A2,31 B50,60 DR4 |
| 9J | A23,32 B37,49 DR1,15 | A23,32 B37,49 DR1,15 |
| 10E | A1,31 B18,27 DR11 | A1,31 B18,27 DR11 |
| 11T | A2,11 B7,71 DR13,15 | A2,11 B7,71 DR13,15 |
| 12G | A2, B7,38 | A2, B7,38 |
Donor . | HLA typing of donor . | HLA typing of LCL stimulator . |
|---|---|---|
| 1P | A1, 24 B8,51 DR2,5 | A1, B8, DR3 |
| 2A | A1,2, B8, DR3, 13 | A1, B8, DR3 |
| 3M | A1,24 B7,8DR15,17* | A1, B8, DR3 |
| 4D | A30,32 B8,18 DR4,7 | A30,32 B8,18 DR4,7 |
| 5C | A1,24 B8,27 DR3,4 | A1, B8, DR3 |
| 6J | A1,2 B17,62 DR1,7 | A(null), B62, DR1 |
| 7AO | A23,30 B27,44 DR1,7 | A23,30 B27,44 DR1,7 |
| 8G | A2,31 B50,60 DR4 | A2,31 B50,60 DR4 |
| 9J | A23,32 B37,49 DR1,15 | A23,32 B37,49 DR1,15 |
| 10E | A1,31 B18,27 DR11 | A1,31 B18,27 DR11 |
| 11T | A2,11 B7,71 DR13,15 | A2,11 B7,71 DR13,15 |
| 12G | A2, B7,38 | A2, B7,38 |
Matching HLAs are shown underlined.
DR17 is a split of DR3.
Cytokines
Recombinant human TGF-β was purchased from R&D Systems (Minneapolis, MN) or Leinco (St Louis, MO) and used at 10 ng/mL.
Cytolysis assays
Standard nonradioactive cytotoxicity assays43 were set up using PBLs from 5- to 7-day restimulation cultures and either HLA-matched or HLA-mismatched LCLs at various effector-to-target (E/T) ratios, with target cells plated at 5 × 104 to 1 × 105 cells/mL. All samples were plated in triplicate. Alamar blue (Biosource, Camarillo, CA) was used at a dilution of 1:10. Cells were cultured for 24 hours and read on a Cytofluor II fluorescent multiwell plate reader (Perseptive Biosystems, Foster City, CA) at an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Percent lysis was determined as follows: (targets alone – [(E+T) – (E alone)]/targets alone]. Lytic units (LUs) are arbitrarily defined as the number of lymphocytes required to yield the selected lysis value (in this case, 30%). To define LUs, all curves must pass through this lysis value, and it must be in the linear portion of the curve. The number of LUs per million cells is calculated using the following formula: LU30 per million cells = 106/[(no. effectors/percent lysis) × (30)].
Growth inhibition assays
Long-term tumor growth inhibition assays were performed similarly to Wilson et al.44 Briefly, LCL targets were plated at 1 × 105 cells/mL into 96-well plates and serially diluted 1: 2. PBLs were harvested from 5-day restimulation cultures and plated at 1 × 104 cells/well. On day 14, Alamar blue was added, and plates were read as described (see “Cytolysis assays”). Percent inhibition of proliferation was calculated for each effector cell population and compared to the proliferation of LCL targets alone as follows: [(proliferation of effectors + targets)/(proliferation of targets)] × 100. As an additional control, viable cells were subjected to flow cytometry and shown to be CD19/CD20+, demonstrating outgrowth of LCLs rather than CTLs.
Cytokine ELISAs for IFN-γ and TGF-β
Levels of IFN-γ in culture supernatants (SNs) were measured by ELISA using antibody pairs from Endogen (Rockford, IL), and TGF-β in serum was measured using antibody pairs from BD PharMingen or a TGF-β ELISA kit (R&D Systems) according to the manufacturers' instructions. Briefly, microtiter plates were coated with anti–IFN-γ or anti–TGF-β (2 μg/mL) overnight and blocked with borate-buffered saline buffer containing 5% bovine serum albumin and 1% normal goat serum for 2 hours. Cytokine standards or SNs were added at titrated dilutions for 4 hours at room temperature. Detecting antibody (0.5 μg/mL for IFN-γ, 2 μg/mL for TGF-β) was added for 2 hours. Streptavidin-horseradish peroxidase (1:1000) was added for 30 minutes. After washing, 2,2′-azinobis(3-ethylbenz-thiazoline-6-sulfonic acid) substrate was added for 20 minutes. Optical density (OD) was measured at 405 nm using a Bio-Rad (Hercules, CA) ELISA reader. IFN-γ and TGF-β concentrations were determined by comparing the OD of dilutions in the linear phase of the curve to the standard curve run on the same plate.
Statistical methods
The genotypes of rapid tumor producers for specific cytokines were compared to other subjects using the Fisher exact test. For TNFA, the presence of the A allele at base — 308 (high TNFA) was compared to other genotypes. For IL-10, the absence of the G allele at base — 1082 (low IL-10) was compared to the other genotypes. In the IL-6 gene, homozygosity for the C allele at base — 174 (low IL-6) was compared to other genotypes. For TGFB, the genotype-associated low-TGF-β production (changes in the leader sequence resulting in proline residues at codons 10 and 25) was compared to high/intermediate TGFB genotypes. Finally, for IFNG, homozygosity for the A allele at base + 874 as well as the presence of the A allele were compared to other genotypes for the 3 LPD groups (rapid versus late versus no LPD). Median time to LPD onset (latency) and median penetrance (percentage of mice developing human tumors) were compared using the exact Wilcoxon rank sum test. CTL activity was compared using Student t test. Survival times were compared using the log-rank test. For all statistical tests, P < .05 was considered significant.
Results
Heterogeneity in LPD development in hu-PBL-SCID mice
PBLs from each of 49 EBV-reactive donors were injected into 3 to 5 SCID mice per donor. Recipient mice were monitored for up to 6 months for engraftment by human cells (as evidenced by human IgG in the serum) and development of LPD (human CD45+ CD19/CD20+ tumors infiltrated with small numbers of CD45+CD3+ cells). As shown in Table 2, PBLs from 47% (23 of 49) of the donors produced no LPD after 20 weeks, whereas 24% (12 of 49) developed LPD tumor rapidly (median time to LPD, 8 weeks) and with high penetrance (median, 100%; range, 80%-100%). PBLs from the remainder of the donors (29%; 14 of 49) produced LPD later (median 12 weeks), and in fewer mice (median penetrance, 55%; range, 33%-100%). As determined by the exact Wilcoxon rank sum test, the differences in latency and penetrance between the rapid and intermediate/late groups are statistically significant (P < .0001).
LPD development and penetrance
Donor group . | No. . | Median onset, wk . | Range, wk to LPD . | Median LPD Penetrance, % . | Range of LPD penetrance, % . |
|---|---|---|---|---|---|
| Rapid LPD | 12 | 8* | 6-10 | 100† | 80-100 |
| Late LPD | 14 | 12 | 10-18 | 55 | 33-100 |
| No LPD | 23 | > 20 | Not applicable | Not applicable | Not applicable |
Donor group . | No. . | Median onset, wk . | Range, wk to LPD . | Median LPD Penetrance, % . | Range of LPD penetrance, % . |
|---|---|---|---|---|---|
| Rapid LPD | 12 | 8* | 6-10 | 100† | 80-100 |
| Late LPD | 14 | 12 | 10-18 | 55 | 33-100 |
| No LPD | 23 | > 20 | Not applicable | Not applicable | Not applicable |
P < .0001 compared to late LPD group.
P < .0001 compared to late LPD group.
Cytokine genotype and the development of LPD in hu PBL-SCID mice
To test our hypothesis that cytokine genotypes correlate with LPD development, we assessed the distribution of cytokine genotypes for IFNG, TNFA, IL-6, IL-10, and TGFB in the PBLs used to produce EBV-LPD in hu PBL-SCID mice.
We compared rapid, high-penetrance LPD producers with intermediate/late LPD producers and with donors whose PBLs did not produce LPD (as determined in Table 2). Table 3 demonstrates that analysis of the distribution of polymorphisms for IFN-γ demonstrated statistically significant differences between rapid LPD producers and the other 2 groups. Of the 12 rapid LPD producers, none were of the T/T genotype, 5 were T/A genotype (41.7%), and 7 were A/A genotype (58.3%). In contrast, donors whose PBLs produced intermediate/late LPD or not at all exhibited a more heterogeneous distribution of genotypes (14 T/T, 37.8%; 15 T/A, 43.3%; and 8 A/A, 18.9%). Statistical analyses of these data indicate that the A/A genotype was significantly more frequent in the rapid LPD producers compared to the intermediate/late LPD producers and the no LPD producers (P = .0144). The absence of the T/T genotype among the rapid LPD producers was also striking, suggesting that the presence of the T allele correlated with a lack of LPD development in hu PBL-SCID mice. All (12 of 12) of the rapid LPD producers had at least one A allele present, contrasted to the intermediate/late LPD producers (8 of 14) and no LPD producers, where 15 of 23 donors had at least one A allele present. This is a statistically significant difference among the 3 groups (P = .0257). When the cytokine polymorphism distributions for TNFA, IL-6, TGFB, and IL-10 were analyzed, no statistical differences were observed among the groups of donors. Similar to the reported distributions for TGF-β genotypes,45 the majority of our donors exhibited genotypes for high TGF-β production. Indeed, 48 of the 49 PBL donors, and all of those producing rapid LPD, had genotypes linked to high TGF-β production.
IFNG genotypes and LPD development in hu PBL-SCID mice
. | . | Intermediate/late . | . | |
|---|---|---|---|---|
| Genotype . | Rapid LPD, % . | LPD, % . | No LPD, % . | |
| A/A* | 58.3 | 7.1 | 26.1 | |
| T/A | 41.7 | 50.0 | 39.1 | |
| T/T† | 0 | 42.9 | 34.8 | |
. | . | Intermediate/late . | . | |
|---|---|---|---|---|
| Genotype . | Rapid LPD, % . | LPD, % . | No LPD, % . | |
| A/A* | 58.3 | 7.1 | 26.1 | |
| T/A | 41.7 | 50.0 | 39.1 | |
| T/T† | 0 | 42.9 | 34.8 | |
Three to 5 SCID mice were injected per PBL donor, and all mice were engrafted, as evidenced by more than 750 μg/mL human IgG in the sera. For rapid LPD, n = 12; for intermediate/late LPD, n = 14; and for no LPD, n = 23.
A/A genotype is significantly more prevalent in the rapid LPD group, P = .0144.
The presence of the A allele (A/A + T/A) is significantly more prevalent in the rapid LPD group, P = .0257.
TGF-β inhibition of CTL activity is associated with IFN-γ genotype
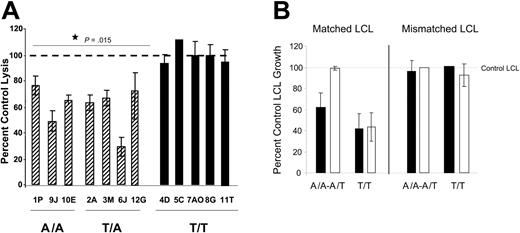
To further examine the relationship between IFN-γ genotype and CTL function, we next tested whether TGF-β could inhibit restimulation of CTL activity in vitro. PBLs were cultured with irradiated HLA-matched LCL stimulators in the presence or absence of TGF-β1 for 5 days. CTL activity was assessed using standard CTL assays. Figure 1A shows that PBLs from individuals with the A/A or A/T IFNG genotype had an impaired CTL response if TGF-β was added to the restimulation cultures. TGF-β–treated cultures for these donors had 25% to 70% inhibition of cytolysis compared to control cultures. In contrast, TGF-β had no effect on CTL restimulation of the PBLs with T/T genotype. Data are shown as the mean percent control lysis, determined using LUs. The difference between the A/A plus T/A genotype cultures and the T/T genotype cultures was significant (P = .015). In Figure 1B we verified the inhibition of CTL restimulation using 2-week LCL growth inhibition assays, similar to those described by Wilson et al.44 Growth inhibition assays assess the ability of a set number of restimulated CTLs to lyse a titrated number of LCLs under more stringent conditions than regular CTL assays. LCLs not killed by the CTLs will proliferate and detectable differences in metabolic activity are seen after 2 weeks. Figure 1B shows that CTLs inhibited long-term growth of matched but not mismatched LCLs and that A/A or A/T genotype CTLs (n = 3 donors) restimulated in the presence of TGF-β did not inhibit growth of their matched LCL targets. In contrast, T/T genotype CTLs restimulated in the presence of TGF-β (n = 3 donors) inhibited LCL growth similarly to control CTLs. Thus, TGF-β can inhibit CTL restimulation in A/A or A/T genotype PBLs but not T/T genotype PBLs. Given that A/A and A/T genotype PBLs are more likely to produce LPD in hu PBL-SCID mice, we next examined the influence of TGF-β in the in vivo system.
Activity of CTLs. (A) IFNG genotype associates with TGF-β–mediated inhibition of CTL activity. PBLs were cultured with HLA-A/-B–matched LCLs in the absence or presence of 10 ng/mL TGF-β for 5 days. Viable cells were washed 3 times to remove any exogenous TGF-β and CTL activity assessed using standard lysis assays as described in “Materials and methods.” Data are shown as percent control lysis of PBLs cultured with LCLs in the absence of TGF-β. For each donor, multiple effector-to-target ratios were tested in triplicate, and LUs determined from the linear portions of the curves. The percent inhibition was calculated using LUs from control versus TGF-β–treated cultures. The results shown are the mean and SD for the triplicates from representative experiments for each donor. When analyzed by the t test, the CTL activity in A/A and T/A PBLs restimulated in the presence of TGF-β is significantly different from either control CTL activity or the CTL activity in T/T PBLs after culture with TGF-β (P = .015). (B) The ability of CTLs to prevent matched LCL growth is inhibited by CTL restimulation in the presence of TGF-β. CTLs were restimulated in the presence or absence of 10 ng/mL TGF-β. At the end of 5 days, CTL activity was assessed by standard CTL assays as in panel A. In addition, a portion of the restimulated cells (104/well) were cultured with titrated numbers of HLA-A/-B–matched or mismatched LCLs for 2 weeks. Data are shown as the mean percent LCL growth ± SD in wells containing both CTLs and LCLs compared to growth in wells containing only LCLs as determined by Alamar blue. Data are combined for 3 donors of each genotype at an 8: 1 effector-to-target ratio; ▪, control CTLs restimulated in the absence of TGF-β; □, CTLs restimulated in the presence of TGF-β.
Activity of CTLs. (A) IFNG genotype associates with TGF-β–mediated inhibition of CTL activity. PBLs were cultured with HLA-A/-B–matched LCLs in the absence or presence of 10 ng/mL TGF-β for 5 days. Viable cells were washed 3 times to remove any exogenous TGF-β and CTL activity assessed using standard lysis assays as described in “Materials and methods.” Data are shown as percent control lysis of PBLs cultured with LCLs in the absence of TGF-β. For each donor, multiple effector-to-target ratios were tested in triplicate, and LUs determined from the linear portions of the curves. The percent inhibition was calculated using LUs from control versus TGF-β–treated cultures. The results shown are the mean and SD for the triplicates from representative experiments for each donor. When analyzed by the t test, the CTL activity in A/A and T/A PBLs restimulated in the presence of TGF-β is significantly different from either control CTL activity or the CTL activity in T/T PBLs after culture with TGF-β (P = .015). (B) The ability of CTLs to prevent matched LCL growth is inhibited by CTL restimulation in the presence of TGF-β. CTLs were restimulated in the presence or absence of 10 ng/mL TGF-β. At the end of 5 days, CTL activity was assessed by standard CTL assays as in panel A. In addition, a portion of the restimulated cells (104/well) were cultured with titrated numbers of HLA-A/-B–matched or mismatched LCLs for 2 weeks. Data are shown as the mean percent LCL growth ± SD in wells containing both CTLs and LCLs compared to growth in wells containing only LCLs as determined by Alamar blue. Data are combined for 3 donors of each genotype at an 8: 1 effector-to-target ratio; ▪, control CTLs restimulated in the absence of TGF-β; □, CTLs restimulated in the presence of TGF-β.
In vivo treatment with anti–TGF-β improves survival of hu PBL-SCID mice
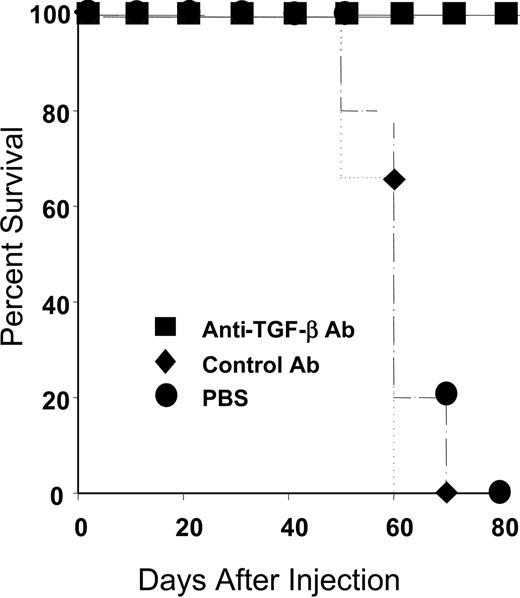
Like the majority of the general population, all of the rapid LPD donors exhibited genotypes linked to high TGF-β production. Given our in vitro data indicating that TGF-β could inhibit CTL restimulation, we investigated whether treatment with anti–TGF-β would prolong survival of hu PBL-SCID mice. The hu PBL-SCID mice were injected intraperitoneally with 100 μg PBS, isotype control antibody, or a commercially available anti–TGF-β antibody (Genzyme) 3 times per week for the duration of the experiment. All animals were engrafted, as evidenced by more than 750 μg/mL human IgG in the sera at 4 weeks after injection (not shown). As shown in Figure 2, animals treated with either PBS or isotype control antibody had a mean survival of 60 days. In contrast, animals treated with anti–TGF-β survived more than 80 days. Thus, anti–TGF-β treatment significantly enhanced survival of hu PBL-SCID mice (P < .002).
In vivo treatment with anti–TGF-β prevents death from LPD. SCID mice were injected with 50 million PBLs as described in “Materials and methods.” Animals received either PBS (•; n = 3), isotype 100 μg control antibody (♦; n = 5), or 100 μg anti–TGF-β (▪; n = 5) every other day for the duration of the experiment. Animals were confirmed to be engrafted by the presence of more than 750 μg/mL human IgG in their sera and were monitored for LPD development. Survival time was determined for each group. When animals died or became moribund, flow cytometry was performed to confirm the development of LPD. As shown, all control animals (PBS or isotype control antibody) died within 70 days, whereas animals treated with anti–TGF-β antibody survived more than 80 days. The differences in survival were highly significant (P = .004 for PBS versus anti–TGF-β and P = .002 for isotype control versus anti–TGF-β).
In vivo treatment with anti–TGF-β prevents death from LPD. SCID mice were injected with 50 million PBLs as described in “Materials and methods.” Animals received either PBS (•; n = 3), isotype 100 μg control antibody (♦; n = 5), or 100 μg anti–TGF-β (▪; n = 5) every other day for the duration of the experiment. Animals were confirmed to be engrafted by the presence of more than 750 μg/mL human IgG in their sera and were monitored for LPD development. Survival time was determined for each group. When animals died or became moribund, flow cytometry was performed to confirm the development of LPD. As shown, all control animals (PBS or isotype control antibody) died within 70 days, whereas animals treated with anti–TGF-β antibody survived more than 80 days. The differences in survival were highly significant (P = .004 for PBS versus anti–TGF-β and P = .002 for isotype control versus anti–TGF-β).
In vivo neutralization of TGF-β reduces LPD and results in CD8+ expansion and activation
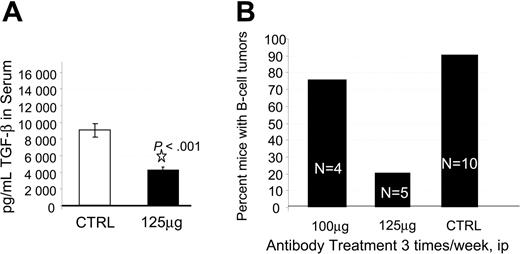
To investigate the mechanism by which in vivo treatment with anti–TGF-β antibody prolonged survival and to assess the utility of anti–TGF-β treatment, we performed a second experiment using the A411 anti–TGF-β antibody and a second PBL donor. In the second trial we assessed neutralization of TGF-β, LPD development, and CD8 T-cell expansion. We initially injected hu PBL-SCID mice with anti–TGF-β antibody 3 times per week and monitored human immunoglobulin levels, serum TGF-β, and LDP development. All animals were engrafted, as evidenced by more than 750 μg/mL human IgG in the sera at 4 weeks after injection (not shown). Also at week 4, hu PBL-SCID mice routinely exhibited circulating levels of 9000 pg/mL TGF-β. Treatment of the animals with anti–TGF-β significantly reduced that level to less than 4000 pg/mL (P < .001; Figure 3A). Animals were killed at 9 weeks, at which point 100% of the control animals had developed human B-cell tumors. In contrast, only 20% (1 of 5) of the animals receiving 125 μg anti–TGF-β developed LPD (Figure 3B). Flow cytometric analysis of the spleens and tumors indicated that human CD8+ cells had dramatically expanded in the anti–TGF-β–treated mice. Control mice had a median of 0% CD8+ cells in their spleens. These mice rarely had human non-B cells in the spleens. In contrast, animals receiving 125 μg anti–TGF-β had a median of 17.5% CD8+ cells in their spleens. The one treated animal that developed a B-cell tumor had significant numbers of B cells in the spleen (25%), as well as significant numbers of CD8+ cells (25%). Importantly, CD8+ T cells were also expanded in the tumor of the one tumor-positive anti–TGF-β–treated animal.
Effect of anti–TGF-β on TGF-β and incidence of LPD. (A) Anti-TGF-β neutralizes TGF-β in vivo. The hu PBL-SCID mice were given injections of 125 μg anti–TGF-β antibody (A411) or PBS 3 times per week. Serum samples were tested at week 6 for the presence of TGF-β by ELISA. Data are shown as mean pg/mL TGF-β derived from triplicate determinations, 5 mice/group. Error bars indicate standard deviation. (B) Anti-TGF-β reduces incidence of LPD in a dose-dependent manner. The hu PBL-SCID mice were treated with 100 μg or 125 μg anti–TGF-β antibody A411 or mouse IgG 3 times per week for 9 weeks. At harvest, the presence of B-cell tumors was assessed visually and confirmed by flow cytometry. (ip indicates intraperitoneally.)
Effect of anti–TGF-β on TGF-β and incidence of LPD. (A) Anti-TGF-β neutralizes TGF-β in vivo. The hu PBL-SCID mice were given injections of 125 μg anti–TGF-β antibody (A411) or PBS 3 times per week. Serum samples were tested at week 6 for the presence of TGF-β by ELISA. Data are shown as mean pg/mL TGF-β derived from triplicate determinations, 5 mice/group. Error bars indicate standard deviation. (B) Anti-TGF-β reduces incidence of LPD in a dose-dependent manner. The hu PBL-SCID mice were treated with 100 μg or 125 μg anti–TGF-β antibody A411 or mouse IgG 3 times per week for 9 weeks. At harvest, the presence of B-cell tumors was assessed visually and confirmed by flow cytometry. (ip indicates intraperitoneally.)
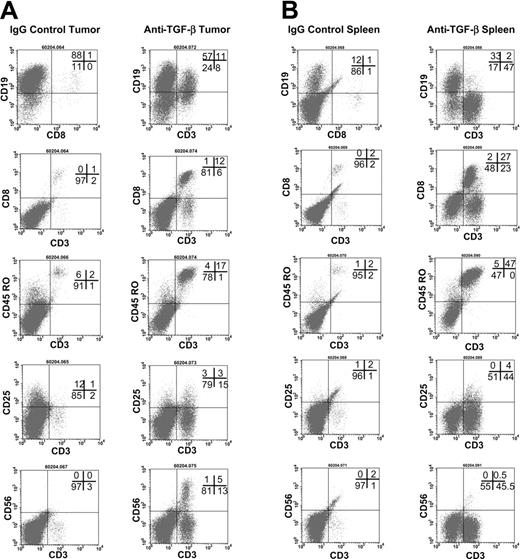
To further examine the effects of anti–TGF-β, an additional study using a third donor was performed. The hu PBL-SCID mice were treated with 100 μg anti–TGF-β antibody every other day for 9 weeks (Figure 4). Anti-TGF-β treatment effectively neutralized TGF-β in the sera of these animals (not shown). Flow cytometry was used to assess the expansion human cells in the tumors (Figure 4A) and spleens (Figure 4B). Tumors from control IgG-treated mice contained human B cells and very few CD3+CD8+ T cells. Likewise, spleens from these animals contained B cells but very few, if any, T cells. In contrast, tumors and spleens from anti–TGF-β–treated mice exhibited large numbers of CD3+ T cells that were memory cells expressing CD45RO. In the tumors, 20% of the CD3+ cells also expressed CD25, indicating that they were activated. However, only 9% of the CD3+ cells in the spleens expressed CD25.
Flow cytometric analyses of tumors and spleens. (A) Flow cytometric analysis of tumors in anti–TGF-β (right column)–and control-treated (left column) hu PBL-SCID mice. The hu PBL-SCID mice were injected with 100 μg anti–TGF-β (A411) or mouse IgG every other day for 9 weeks. At harvest, tumors were analyzed by flow cytometry for the presence of human B cells and T-cell expansion and activation. Data are shown from a representative animal in each group (n = 5 mice/group). (B) Flow cytometric analysis of spleens from anti–TGF-β (right column)–and control-treated (left column) hu PBL-SCID mice. The hu PBL-SCID mice were given injections of 100 μg anti–TGF-β (A411) or mouse IgG every other day for 9 weeks. At harvest, spleens were analyzed by flow cytometry for the presence of human B cells and T-cell expansion and activation. Data are shown from a representative animal in each group (n = 5 mice/group).
Flow cytometric analyses of tumors and spleens. (A) Flow cytometric analysis of tumors in anti–TGF-β (right column)–and control-treated (left column) hu PBL-SCID mice. The hu PBL-SCID mice were injected with 100 μg anti–TGF-β (A411) or mouse IgG every other day for 9 weeks. At harvest, tumors were analyzed by flow cytometry for the presence of human B cells and T-cell expansion and activation. Data are shown from a representative animal in each group (n = 5 mice/group). (B) Flow cytometric analysis of spleens from anti–TGF-β (right column)–and control-treated (left column) hu PBL-SCID mice. The hu PBL-SCID mice were given injections of 100 μg anti–TGF-β (A411) or mouse IgG every other day for 9 weeks. At harvest, spleens were analyzed by flow cytometry for the presence of human B cells and T-cell expansion and activation. Data are shown from a representative animal in each group (n = 5 mice/group).
Discussion
PTLD can be a devastating complication following transplantation, even in adults with previously established anti-EBV immunity. Known risk factors include the type of transplanted organ, EBV viral load, and the type and duration of immunosuppression administered to prevent graft rejection.1 Although most patients receiving transplants at a particular transplant center receive similar immunosuppressive regimens, only a portion of these patients develop PTLD. This suggests that the inherent immune capacity of a patient receiving a transplant contributes to the development of PTLD. Our original observation with 9 PTLD patients at the Ohio State University Medical Center suggested an impressive skewing of the IFNG genotype distributions, with 80% of PTLD patients exhibiting the A/A genotype, compared to 27% of 135 non-PTLD patients who received renal transplants.19 We have since extended our study to include 12 PTLD patients. The proportion of patients with the A/A genotype for the IFNG gene is higher in PTLD patients than in 135 non-PTLD transplant patients at the same transplant center (58% versus 27%, P = .02). When we assessed the genotype distributions for TGFB, IL-6, IL-10, and TNFA, we did not observe any statistically significant differences between PTLD and non-PTLD patients.
Our preliminary clinical observation is strengthened by the current prospective study using the hu PBL-SCID mouse model of spontaneous EBV-LPD. Fifty-three percent of the EBV-seropositive donors produced LPD in the hu PBL-SCID mice within 6 months. Of donors producing LPD, 12 rapidly produced LPD (median time to LPD, 8 weeks) with high penetrance (median 100%). The other LPD producer phenotype developed LPD later (median time, 12 weeks) and with lower penetrance (median 55%). Although the heterogeneity of LPD production among PBL donors in this animal model is well established,39,46,47 the factors leading to this heterogeneity have not been extensively studied. EBV strain46 and atopic status47 have been implicated as factors. Our prior work had identified the IFNG A/A genotype as a risk factor in PTLD. Therefore, we performed a more complete evaluation of the relationship between cytokine genotype and LPD development in the hu PBL-SCID mouse model using 49 donors. We did not collect data on either the EBV strain infecting the different donors, or the donors' atopic status, and so cannot address these issues in our donor pool. Murine NK cells are also known to influence LPD development,40,48 as are murine macrophages,49 and it is possible that differential ability to activate murine NK cells could account for some heterogeneity in LPD development. We purposefully did not deplete or neutralize NK cells in our study, reasoning that this made the model more stringent. Thus, any observed association of cytokine polymorphism and LPD would be more likely to be a strong association.
Importantly, we observed a striking association of the A allele for IFNG at base + 874 with LPD production. Of the rapid, high-penetrance LPD donors, 58% were homozygous for the A allele (A/A), whereas 42% were heterozygous (T/A). None of the rapid, high-frequency LPD producers were homozygous for the T allele. In contrast, all genotypes were represented in the groups of donors who produced LPD late or not at all. The frequency of the A/A genotype among the rapid LPD producers was significantly different compared to the intermediate/late LPD producers, and the no LPD donors (P = .0144). Also significant (P = .0257) is the presence of the A allele in rapid LPD producers compared to the other 2 LPD groups. These data mirror our clinical observations, suggesting that the IFNG genotype association with LPD production in hu PBL-SCID mice may have clinical relevance.
The A/A, T/A, and T/T IFNG genotypes for base + 874 have been reported to correspond to low, intermediate, and high in vitro cytokine production, respectively.13-15 We observed a clear-cut association of genotype with cytokine production only when HLA-A/-B–matched donors were tested using the same EBV-LCL, thereby providing the same antigenic stimulus. Of the 4 donors that met these criteria, the A/A genotype donor produced the least IFN-γ, with the 2 A/T genotype donors producing an intermediate amount of cytokine and the one T/T genotype donor producing the most IFN-γ (data not shown). It is important to remember that gene polymorphisms are only one of several mechanisms by which IFN-γ production could be influenced. In addition, our genotyping analysis of base + 874 does not account for any other polymorphisms within the IFNG gene or for any posttranslational mechanisms that may influence IFN-γ production.
IFN-γ is a critical regulatory cytokine in cellular immunity that is important in immune surveillance of EBV-related LPD. TGF-β is antagonistic to IFN-γ and has been implicated in EBV reactivation,23-26 suppressing T-cell effector function,22 or otherwise inhibiting immune surveillance (for a review, see Gold21 ). Strong cellular immunity and CTL activity are crucial to preventing or clearing PTLD5-8 or LPD in hu PBL-SCID mice.40,50-52 Our data indicate that the low-producer IFNG genotype is associated with both PTLD in patients and LPD in hu PBL-SCID mice. In trying to understand the relationship between IFNG genotype and TGF-β in LPD development, we focused on whether TGF-β could differentially influence CTL restimulation in vitro. Detecting CTL activity against EBV antigens requires a 5- to 12-day restimulation culture.53 We determined that CTLs were restimulated efficiently in vitro regardless of the IFNG genotype (not shown), indicating that a lack of CTL precursors or a generalized defect in CTL restimulation could not explain the association of the A/A genotype with LPD development. However, when TGF-β was present, CTL restimulation was significantly reduced in A/A or A/T, but not T/T, genotype PBLs.
Given that all of the donors whose cells produced LPD rapidly and with high penetrance in hu PBL-SCID mice exhibited genotypes consistent with high TGF-β production, and that TGF-β could inhibit CTL restimulation in vitro, we then examined whether reducing TGF-β in hu PBL-SCID mice would prolong survival of hu PBL-SCID mice. A survival trial using a commercially available anti–TGF-β antibody resulted in 100% survival of more than 80 days in the anti–TGF-β–treated mice. In contrast, all control animals died within 70 days. These data support an important role of TGF-β in LPD development.
To determine the mechanism by which anti–TGF-β prolonged survival, additional experiments were performed with both a different antibody and using additional PBL donors. Antibody treatment resulted in a significant reduction of circulating TGF-β. Serum TGF-β levels can be variable and often correlate with platelet numbers,54,55 as platelets both release latent TGF-β56 and activate it.57 Although we were unable to control for platelet activation in our samples, sera from anti–TGF-β–treated animals consistently had less TGF-β than control-treated animals within the same experiment. Control-treated mice had B-cell tumors with very few (< 5%) infiltrating CD8+ T cells. Spleens of these animals had B-cell infiltration but no CD8+ T-cell infiltration. In contrast, anti–TGF-β treatment resulted in a dramatic expansion of human CD3+CD8+ cells in the tumors. These CD3+ cells were CD45RO and 20% also expressed CD25+, indicating they were activated memory cells. A large number of CD45RO+CD3+ T cells also infiltrated the spleens of these mice, but fewer expressed CD25.
Whether the CD8+ T-cell expansion is limited to EBV-reactive T cells or is nonspecific will be tested in future experiments. It is possible that in anti–TGF-β–treated mice, CD8+ cells could be expanded nonspecifically and result in GVHD. GVHD in the hu PBL-SCID model can present with CD8+ cell infiltration in the spleen.58 Although we observed general increases in CD8+ cells in the spleens of anti–TGF-β–treated animals, only one animal in the 9-week trial and none of the survival trial animals, exhibited overt GVHD with cachexia.
In summary, we demonstrate a significant association of the A/A genotype for IFNG with rapid LPD production in hu PBL-SCID mice. In contrast, the T/T genotype is absent in rapid LPD-producing donors and is uncommon among PTLD patients. We hypothesize the following mechanism by which genotype could influence LPD development. In A/A and T/A genotype individuals, TGF-β acts to inhibit memory CTL restimulation, allowing EBV transformation of B cells and uncontrolled growth of the transformed cells. Strong cellular immunity is critical for the control of EBV LPD,5-8 and IFN-γ is an important cytokine in cellular immunity. TGF-β is important in EBV-associated disease27,28,59,60 and can contribute to viral activation.23-26 Further, TGF-β can inhibit T-cell and APC functions.20,22 Our data show an association of IFNG cytokine gene polymorphisms with both LPD development and functional differences in CTL restimulation cultures in response to TGF-β. In addition, our data demonstrate that antibody neutralization of TGF-β can prevent LPD and result in expansion of activated, memory CD8+ cells in hu PBL-SCID mice. Thus, we propose that the association of the A/A genotype with LPD production in hu PBL-SCID mice and with PTLD in humans may reflect sensitivity in these individuals to TGF-β–mediated inhibition of CTL restimulation. The subsequent reduction in CTL activity could then predispose these individuals to EBV-driven LPD.
Prepublished online as Blood First Edition Paper, October 21, 2004; DOI 10.1182/blood-2003-07-2476.
Supported in part by grants from the Roche Organ Transplant Research Foundation, the Ohio State University Comprehensive Cancer Center-R.J. Solove Research Institute Seed Grant program, the Department of Surgery Medical Research Development Fund and the National Institutes of Health: P30 CA16058 (R.A.B., C.F.E., M.A.C., A.M.V.), R29 AI-40909 and R03 AI-54383 (A.M.V.), and K08 CA93518 (C.F.E.) and T32 CA 09338 (R.A.B.). A.M.V. is the 2002 recipient of the American Society for Transplantation Women's and Minority Faculty Grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs Burlingham, Ledbetter, Moeschberger, Orosz, and Porcu for helpful discussions. Tyler Hoppes, Kelly Johnson, Vanita Malik, and Navneet Cheema provided excellent technical assistance, and Donna Bucci provided expert administrative assistance. The authors wish to express their appreciation to the members of the OSU Tissue Typing Laboratory for assistance with blood draws and HLA typing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal