Abstract
Antiphospholipid syndrome (APS) is an autoimmune prothrombotic disorder in association with autoantibodies to phospholipid (PL)–binding plasma proteins, such as β2-glycoprotein I (β2GPI). We have recently found that CD4+ T cells autoreactive to β2GPI in patients with APS preferentially recognize a cryptic peptide encompassing amino acid residues 276-290 (p276-290), which contains the major PL-binding site, in the context of DR53. However, it is not clear how previously cryptic p276-290 becomes visible to the immune system and elicits a pathogenics autoimmune response to β2GPI. Here we show that presentation of a disease-relevant cryptic T-cell determinant in β2GPI is induced as a direct consequence of antigen processing from β2GPI bound to anionic PL. Dendritic cells or macrophages pulsed with PL-bound β2GPI induced a response of p276-290–specific CD4+ T-cell lines generated from the patients in an HLA-DR–restricted and antigen-processing–dependent manner but those with β2GPI or PL alone did not. In addition, the p276-290–reactive T-cell response was primed by stimulating peripheral blood T cells from DR53-carrying healthy individuals with dendritic cells bearing PL-bound β2GPI in vitro. Our finding is the first demonstration of an in vitro mechanism eliciting pathogenic autoreactive T-cell responses to β2GPI and should be useful in clarifying the pathogenesis of APS.
Introduction
Antiphospholipid syndrome (APS) is characterized by arterial and venous thrombosis as well as recurrent intrauterine fetal loss in association with antiphospholipid antibodies.1 β2–glycoprotein I (β2GPI), a plasma protein that binds negatively charged substances including phospholipids (PLs), is the most common target for the antiphospholipid antibody associated with the clinical features of APS.2 Pathogenicity of the anti-β2GPI antibody has been demonstrated in animal models, including normal mice immunized with human β2GPI3 and severe combined immunodeficiency mice into which peripheral blood lymphocytes from patients with APS were transferred.4 We recently identified autoreactive CD4+ T cells to β2GPI that promote anti-β2GPI antibody production in patients with APS.5-7 β2GPI–specific CD4+ T cells recognize amino acid residues 276-290 (p276-290), which define the immunodominant β2GPI peptide, in the context of DRB4*0103 (DR53). This epitope is located on domain V and contains the major PL-binding site at amino acids 280-288.8 The p276-290–reactive T-cell clones did not respond to functional antigen-presenting cells (APCs) bearing native β2GPI but did to those bearing chemically reduced β2GPI or recombinant β2GPI fragments produced in bacteria.6 Given that β2GPI-reactive T cells are also detected in some healthy individuals,5 the p276-290 epitope defined by β2GPI-specific T cells is “cryptic,” since it is generated at a subthreshold level by the processing of native β2GPI under normal circumstances.9
There is increasing interest in the possibility that crypticity is an important characteristic of epitopes recognized by the autoreactive T cells and thus is relevant to autoimmune pathogenesis.10 T cells recognizing self-determinants generated in sufficient amounts in APCs undergo deletion in the thymus or anergy in the periphery. On the other hand, T cells specific for cryptic self-determinants are a component of the normal T-cell repertoire but normally do not encounter antigenic peptides in the periphery. These T cells might become activated and autoaggressive if the previously cryptic self-determinants were presented at a higher concentration. This concept represents the major hypothesis for the pathogenesis of autoimmune diseases, but the fundamental question is how epitopes that are normally cryptic become visible to the immune system and elicit a sustained pathogenic response. In this study, p276-290–specific T-cell lines generated from patients with APS were used to investigate the mechanisms that induce the efficient processing and presentation of cryptic p276-290 as a consequence of antigen processing.
Patients, materials, and methods
Study subjects
Peripheral blood T cells from 5 Japanese patients with APS were analyzed in this study. All patients fulfilled the preliminary classification criteria for APS proposed by the International Workshop.11 Primary APS was diagnosed in 3 of the patients, whereas the remaining 2 had secondary APS accompanied by systemic lupus erythematosus. At the time of blood examination, all the patients were on low-dose corticosteroids (< 10 mg/d) and aspirin. Samples from 6 healthy individuals possessing DRB4*0103 confirmed by polymerase chain reaction–based genotyping12 were used in experiments on priming the p276-290–specific T-cell response. All samples were obtained after the patients and control subjects gave their written informed consent in accordance with the Declaration of Helsinki, as approved by the Keio University Institutional Review Board (Tokyo, Japan).
Antigen preparations
Native β2GPI was purified from normal pooled human plasma as described elsewhere.13 Nicked and reduced β2GPI was prepared by treating β2GPI with plasmin14 and dithiothreitol,5 respectively. Fusion recombinant maltose-binding proteins (MalBPs) expressed in Escherichia coli included GP-F and GP3, which encoded the entire amino acid sequence (amino acids 1-326) and domains IV and V (amino acids 182-326), respectively, of human β2GPI.5 MalBP, a fusion partner, was also prepared as a control antigen. GP-F and GP3 lacking MalBP, GP-F/MalBP(–), and GP3/MalBP(–) were prepared by incubating MalBP fusion proteins with factor Xa followed by the removal of MalBP and factor Xa by passing the mixture through amylose resin and benzamidine-Sepharose columns, respectively. A recombinant polypeptide encoding domain V of β2GPI (amino acids 242-326; rDomain V) was expressed by a Pichia pastoris expression system.15 Peptides encompassing amino acids 276-290 and 306-320 of β2GPI (p276-290 and p306-320) were synthesized using a solid-phase multiple synthesizer (Advanced ChemTech, Louisville, KY) and purified by high-performance liquid chromatography. These peptides had potential DR53-binding anchor residues,16 but the binding capacity to the DR53 molecule was not examined. Capacity of individual antigen preparations to bind anionic PL was evaluated by competitive inhibition of an interaction between native β2GPI and immobilized cardiolipin.13 Briefly, cardiolipin-coated plates were incubated with native β2GPI in the presence of an excess amount of individual antigen preparations. The β2GPI-cardiolipin complex was detected by incubation with a monospecific APS serum positive for a high titer of anti-β2GPI antibodies.
Preparation of PL liposomes
Dipalmitoylphosphatidylserine (DPPS), phosphatidylserine from bovine brain (BBPS), and cardiolipin from bovine heart were purchased from Sigma Chemical (St Louis, MO); dilauroylphosphatidylserine (DLPS), dimyristoylphosphatidylserine (DMPS), dioleoylphosphatidylserine (DOPS), dioleoylphosphatidylcholine (DOPC), and monooleoylphosphatidylserine (MOPS) were from Avanti Polar Lipids (Alabaster, AL); and a lyso form of BBPS (lyso-BBPS) was from Doosan Serdary Research Laboratories (Englewood Cliffs, NJ). All chemicals were of reagent-grade quality. The fatty acid chains of bovine tissue–derived BBPS and cardiolipin were not well characterized, but all other PLs were chemically synthesized. Liposomes were prepared principally as described previously,17 with a lipid composition of DOPC at a molar ratio of 7:3 with the following PLs: DOPS, DPPS, DLPS, DMPS, MOPS, BBPS, lyso-BBPS, and cardiolipin. A mixture of the desired lipids in chloroform-methanol (1:1) was placed in a pear-shaped flask and solvent was removed in a rotary evaporator under reduced pressure. The dried lipids were dispersed with a vortex mixture in sterilized 0.3 M glucose solution. The liposome solutions were then sonicated for 1 minute at 70°C with a bath-type sonicator and adjusted to 1 μmol lipid/mL. These PL liposomes were preincubated with or without native β2GPI (100 μg/mL) for 30 minutes at room temperature before addition to the cultures. The capacity of PLs to bind β2GPI was evaluated by an assay as described13 with some modifications. Briefly, individual PLs were coated on microtiter plates and subsequently incubated with β2GPI. The PL-β2GPI complex was detected by anti-β2GPI monoclonal antibody (mAb) Cof-23.18
Culture media
All cultures were incubated in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 50 U/mL penicillin, and 50 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Before use in culture, the fetal bovine serum was depleted of bovine β2GPI using heparin-Sepharose as described previously5 to avoid its potential influence on the generation of the epitope peptide.
The p276-290–specific CD4+ T-cell lines
T-cell lines reactive with p276-290 were generated from peripheral blood T cells by the repeated stimulation with GP-F followed by limiting dilution as described previously.6 A total of 7 CD4+ T-cell lines established from patients with APS were used in this study. Four of them (KS3, OM2, OM7, and EY3) were confirmed to be clones based on the single functional T-cell receptor β-chain and were reported in detail previously.6,7 The clonality of the remaining 3 lines (OM-b, KY-a, KM-b) was not determined. All the T-cell lines recognized p276-290 in the context of HLA-DRB4*0103, had the T-helper 0 (Th0)/Th1 phenotype expressing interferon γ (IFN-γ), and had the capacity to induce anti-β2GPI antibody production from autologous B cells. T-cell lines were maintained by repetitive stimulation with GP-F, recombinant human interleukin 2 (IL-2; 100 U/mL), and irradiated autologous APCs at 7- to 10-day intervals.
Preparation of APCs
Epstein-Barr virus–transformed lymphoblastoid B-cell line cells (LBLs) were generated from all patients with APS. Circulating monocytes and B cells were isolated from peripheral blood mononuclear cells using anti-CD14 or anti-CD19 mAb-coupled magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) followed by magnetic-activated cell separation (MACS) column separation according to the manufacturer's protocol. Flow cytometric analysis revealed that purity of monocyte and B-cell fractions was greater than 98%. Macrophages and immature monocyte-derived dendritic cells (DCs) were obtained from plastic adherent peripheral blood mononuclear cells in the presence of macrophage-colony stimulating factor (R&D Systems, Minneapolis, MN) or granulocyte-macrophage-colony stimulating factor and IL-4 (PeproTech, Rocky Hill, NJ), respectively, according to previously published methods.19 Macrophages and immature DCs were pulsed with antigen and subsequently incubated with 50 ng/mL tumor necrosis factor α (TNF-α; PeproTech) for 24 hours to induce activation/maturation before being used in the assays. Flow cytometric analysis revealed that the activated macrophage fraction contained greater than 98% CD14+CD80+ cells, and the mature DC fraction contained greater than 95% CD83+HLA-DR+ cells. Allogeneic splenocytes were obtained from DR53-carrying patients with gastric cancer who required splenectomy as part of the dissection of tumor tissues.20 In some experiments, endosomal processing inhibitor chloroquine (0.1 μM; Sigma Chemical) or brefeldin A (1 μg/mL; Sigma Chemical) was added to the APC cultures 1 hour before the addition of antigen.
Assays for antigen-specific T-cell response
Antigen-specific proliferation of T-cell lines was determined principally as described previously.6 T-cell lines (2 × 104/well) were cultured with various combinations of irradiated APCs (2 × 104/well) and antigen. The APCs were autologous LBLs, monocytes, B cells, monocyte-derived DCs, macrophages, and allogeneic CD53-carrying splenocytes. Native β2GPI, nicked β2GPI, reduced β2GPI, GP-F, GP3, GP-F/MalBP(–), GP3/MalBP(–), rDomain V (10 μg/mL), and p276-290 (5 μg/mL) were used as antigens containing amino acids 276-290 of human β2GPI. Control antigens were MalBP (10 μg/mL) and p306-320 (5 μg/mL). PL liposomes that were preincubated with native β2GPI, GP3/MalBP(–), or rDomain V were used at a final concentration of 0.1 μmol lipid and 10 μg protein per 1 mL. A combination of immobilized anti-CD3 mAb (30 ng/mL) and phytohemagglutinin (1 μg/mL) was also used to exclude nonspecific unresponsiveness. LBLs were irradiated at 100 Gy and all other APCs at 40 Gy before being mixed with T-cell lines. After 60 hours of incubation with antigen, 0.5 μCi/well (0.0185 MBq) [3H]-thymidine was added to the cultures for 16 hours. The cells were then harvested and [3H]-thymidine incorporation was determined in a Top-Count microplate scintillation counter (Packard, Meriden, CT). The antigen-induced T-cell response was also evaluated from the production of IFN-γ as described previously.21 In some experiments, anti–HLA-DR (L243; immunoglobulin G2a [IgG2a]), anti–HLA-DQ (1a3; IgG2a), or an isotype-matched control mAb (1 μg/mL; Leinco Technologies, Ballwin, MO) were added at the initiation of the cultures. All experiments were carried out in duplicate or triplicate, and the values are the mean of multiple determinations.
In vitro priming of T cells responsive to p276-290 with β2GPI-PL liposome complex
Peripheral blood T cells (2 × 106) isolated from peripheral blood mononuclear cells using anti-CD3 mAb-coupled magnetic beads (Miltenyi Biotech) were cultured with autologous mature DCs or TNF-α–stimulated macrophages that were previously pulsed with a mixture of BBPS liposomes and β2GPI, BBPS liposomes, or β2GPI alone. On day 3, IL-2 (30 units/mL) was added to the cultures. On day 10, viable T cells were harvested and we examined the capacity to produce IFN-γ in response to antigenic stimulation with autologous LBLs pulsed with MalBP, GP-F, native β2GPI, p276-290, or p306-320. All culture experiments were carried out in duplicate, and all values represent the mean of duplicate determinations. Results were expressed after the background IFN-γ production was deducted.
Results
Conditions that induce the expression of p276-290 as a consequence of antigen processing
Various combinations of antigens and APCs were tested for their ability to stimulate p276-290–reactive CD4+ T-cell lines generated from patients with APS. In this in vitro assay system, a response of p276-290–reactive T-cell line can be used as an indicator for the efficient presentation of p276-290 by APCs as a consequence of antigen processing. First, various types of DR53-carrying APCs pulsed with native β2GPI were examined for their capacity to stimulate p276-290–reactive T-cell lines. Autologous LBLs, monocytes, B cells, mature DCs, activated macrophages, or allogeneic splenocytes bearing native β2GPI failed to induce a proliferative response of the T-cell lines OM7 and KS3 (Figure 1A). Identical results were obtained using all 7 T-cell lines. Next, autologous LBLs were pulsed with various forms of β2GPI and cultured with p276-290–reactive T-cell lines OM7 and KS3 (Figure 1B). A significant response was detected in the cultures with reduced β2GPI and recombinant fusion proteins expressed in a bacterial expression system (GP-F and GP3), as shown in our previous study.6 Interestingly, the capacity of GP-F and GP3 to stimulate p276-290–reactive T-cell lines was largely reduced when the fusion partner MalBP was removed from these recombinant fusion proteins. The addition of MalBP to these cultures did not reverse the response (data not shown), indicating that MalBP expressed as a fusion protein played a role in the T-cell response. In contrast, LBLs pulsed with native and nicked forms of β2GPI or with rDomain V expressed in the eukaryotic expression system did not induce the T-cell response. Analogous findings were obtained from all 7 T-cell lines examined.
Response of p276-290–reactive T-cell lines to various combinations of DR53-positive APCs and β2GPI preparations. (A) Various DR53-positive APCs were pulsed with or without native β2GPI (▪ and □, respectively) and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. All APCs except allogeneic splenocytes were autologous cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. A ▦ indicates a control T-cell line stimulated by a combination of anti-CD3 mAb and phytohemagglutinin (PHA). A representative result from at least 2 independent experiments is shown. (B) Autologous LBLs were pulsed with various β2GPI preparations and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. The capacity to bind β2GPI in individual preparations was assessed by inhibition of the interaction between native β2GPI and immobilized cardiolipin. Results are shown as mean and standard deviation. A representative result from 3 independent experiments is shown.
Response of p276-290–reactive T-cell lines to various combinations of DR53-positive APCs and β2GPI preparations. (A) Various DR53-positive APCs were pulsed with or without native β2GPI (▪ and □, respectively) and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. All APCs except allogeneic splenocytes were autologous cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. A ▦ indicates a control T-cell line stimulated by a combination of anti-CD3 mAb and phytohemagglutinin (PHA). A representative result from at least 2 independent experiments is shown. (B) Autologous LBLs were pulsed with various β2GPI preparations and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. The capacity to bind β2GPI in individual preparations was assessed by inhibition of the interaction between native β2GPI and immobilized cardiolipin. Results are shown as mean and standard deviation. A representative result from 3 independent experiments is shown.
None of the individual β2GPI preparations that stimulated p276-290–reactive T-cell lines were able to bind anionic PL (Figure 1B). Since β2GPI binds anionic PLs mainly through the major PL-binding site located on a surface-exposed turn,22 we hypothesized that the loss of the binding capacity in the β2GPI preparations was due to internalization of the major PL-binding site by a structural modification and that this feature is important for the subsequent presentation of p276-290 that occurs as a result of antigen processing. If this were the case, the β2GPI-PL complex, in which the major PL-binding site is covered by anionic PL, should stimulate p276-290–reactive T-cell lines. To test this possibility, immature DCs and macrophages were pulsed with DOPS liposomes that were preincubated with or without native β2GPI, induced to mature/activate by TNF-α treatment, and used to stimulate the p276-290–reactive T-cell lines KS3 and EY3 (Figure 2). DCs bearing DOPS-bound β2GPI induced a T-cell response, as was observed in those pulsed with GP-F or p276-290, but those bearing native β2GPI or DOPS liposomes alone did not. In contrast, macrophages pulsed with DOPS-bound β2GPI were less efficient in stimulating p276-290–reactive T-cell lines. Analogous findings were obtained from all 7 T-cell lines used in this study.
Response of p276-290–reactive T-cell lines in cultures of β2GPI and anionic PL. Autologous immature DCs and macrophages were pulsed with native β2GPI alone, DOPS liposomes, a mixture of native β2GPI and DOPS liposomes, MalBP, or GP-F; induced to maturate or activate; and used to stimulate the p276-290–reactive T-cell lines KS3 and EY3. Synthetic peptides were pulsed with APCs before their coculture with T cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. Results are shown as mean and standard deviation. A representative result from at least 2 independent experiments is shown.
Response of p276-290–reactive T-cell lines in cultures of β2GPI and anionic PL. Autologous immature DCs and macrophages were pulsed with native β2GPI alone, DOPS liposomes, a mixture of native β2GPI and DOPS liposomes, MalBP, or GP-F; induced to maturate or activate; and used to stimulate the p276-290–reactive T-cell lines KS3 and EY3. Synthetic peptides were pulsed with APCs before their coculture with T cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. Results are shown as mean and standard deviation. A representative result from at least 2 independent experiments is shown.
We tested various PL liposomes for their ability to stimulate the p276-290–reactive T-cell lines KS3, EY3, OM-b, and KM-b in the presence of β2GPI using DCs as APCs. All p276-290–reactive T-cell lines proliferated upon recognition of DCs preincubated with β2GPI and liposomes containing DOPS or BBPS (Figure 3A). There was a borderline response to a mixture of β2GPI and cardiolipin-containing liposomes, but all other PLs failed to induce a proliferation irrespective of the presence or absence of β2GPI. When T-cell response was evaluated by IFN-γ release in response to antigenic stimulation, the response of T-cell lines was specifically induced by liposomes containing DOPS or BBPS and to a lesser extent by liposomes containing cardiolipin (Figure 3B). All 4 T-cell lines represented similar findings, and the IFN-γ release assay appeared to be more sensitive than the proliferation assay. The assay to evaluate capacity of individual PLs to bind β2GPI revealed that DOPS, BBPS, and cardiolipin were able to bind β2GPI but the others were not (Figure 3C). These findings indicate that the capacity of individual PLs to induce a T-cell response was correlated with their β2GPI-binding capacity. We further tested whether β2GPI preparations with capacity to bind anionic PL (GP3/MalBP(–) and rDomain V) that were preincubated with PL liposomes induced a response of p276-290–reactive T-cell lines KS3 and EY3. DCs bearing DOPS liposomes preincubated with GP3/MalBP(–) or rDomain V induced a T-cell response, although these 2 β2GPI preparations alone failed to induce a response.
Binding of β2GPI to anionic PL facilitates the processing and presentation of p276-290, which activates p276-290–reactive T-cell lines. (A) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by [3H]thymidine incorporation. A representative result from 2 independent experiments is shown. (B) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by IFN-γ production. A representative result from 3 independent experiments is shown. (C) The capacity of individual PLs to bind β2GPI was evaluated by a solid-phase assay. A representative result from 2 independent experiments is shown. OD indicates optical density. (D) The p276-290–reactive T-cell line OM-b was cultured with autologous DCs bearing DOPS-bound β2GPI in the presence or absence of chloroquine, brefeldin A, anti–HLA-DR mAb, or control mAb. The antigen-induced T-cell response was evaluated by IFN-γ production. Results are shown as the mean and standard deviation. A representative result from 2 independent experiments is shown.
Binding of β2GPI to anionic PL facilitates the processing and presentation of p276-290, which activates p276-290–reactive T-cell lines. (A) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by [3H]thymidine incorporation. A representative result from 2 independent experiments is shown. (B) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by IFN-γ production. A representative result from 3 independent experiments is shown. (C) The capacity of individual PLs to bind β2GPI was evaluated by a solid-phase assay. A representative result from 2 independent experiments is shown. OD indicates optical density. (D) The p276-290–reactive T-cell line OM-b was cultured with autologous DCs bearing DOPS-bound β2GPI in the presence or absence of chloroquine, brefeldin A, anti–HLA-DR mAb, or control mAb. The antigen-induced T-cell response was evaluated by IFN-γ production. Results are shown as the mean and standard deviation. A representative result from 2 independent experiments is shown.
In addition, the T-cell response induced by BBPS-bound β2GPI was completely abolished by the pretreatment of antigen-captured DCs with chloroquine or brefeldin A, which impair the antigen-processing pathway (Figure 3D). Moreover, the T-cell response induced by BBPS-bound β2GPI was completely blocked by an anti–HLA-DR antibody during the DC–T-cell interaction. These findings indicate that the response of p276-290–reactive T-cell lines induced by β2GPI-PL complex–pulsed DCs is endosomal antigen-processing dependent and HLA class II dependent.
Induction of T-cell response to p276-290 by PL-bound β2GPI in peripheral blood T cells from healthy individuals
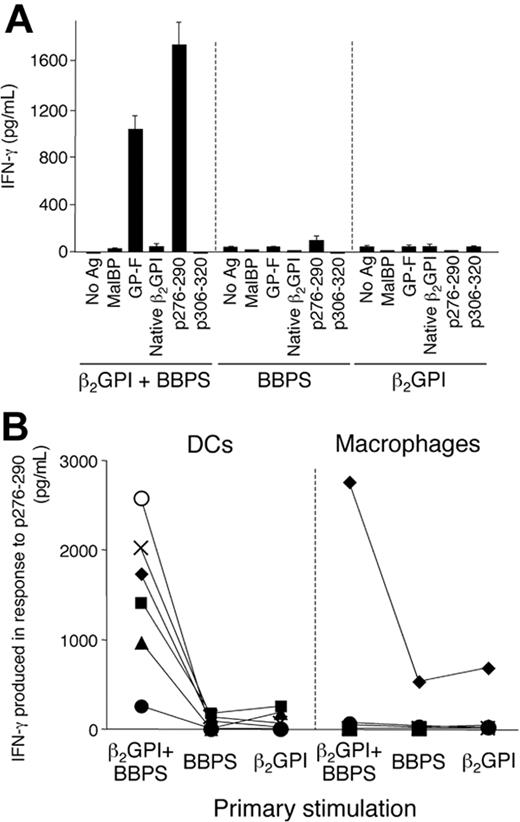
We further tested whether β2GPI bound to anionic PLs primes a T-cell response to p276-290 in healthy individuals in vitro. In this experiment, BBPS was used since liposomes containing this PL constantly induced a strong response of p276-290–reactive T-cell lines in the presence of β2GPI. Peripheral blood T cells from 6 healthy individuals carrying DR53 were stimulated once with autologous DCs or macrophages pulsed with BBPS-bound β2GPI, BBPS liposomes, or β2GPI alone, and the antigen-specific T-cell response was evaluated by IFN-γ production in response to various antigens, including p276-290. Figure 4A shows representative results obtained using DCs as APCs. T cells stimulated with BBPS-bound β2GPI showed profound responses to GP-F and p276-290, whereas those stimulated with BBPS liposomes or β2GPI alone did not. Successful priming of p276-290–reactive T cells was obtained in 5 of 6 individuals when DCs were used as APCs but detected in only one when macrophages were used instead (Figure 4B).
Induction of a T-cell response to p276-290 by BBPS-bound β2GPI in peripheral blood T cells from DR53+ healthy individuals in vitro. (A) Peripheral blood T cells from a representative DR53+ healthy individual were stimulated with DCs pulsed with BBPS-bound β2GPI, BBPS, or β2GPI alone, and the T-cell response to MalBP, GP-F, native β2GPI, p276-290, or p306-320 was measured by IFN-γ production. A representative result from 2 independent experiments is shown. (B) T-cell response to p276-290 after priming with autologous DCs or macrophages pulsed with BBPS-bound β2GPI, BBPS, or β2GPI alone in 6 DR53+ healthy individuals. The p276-290–specific T-cell response was evaluated by IFN-γ production. The results from each of 6 individuals are indicated by a different symbol. Results are shown as mean and standard deviation.
Induction of a T-cell response to p276-290 by BBPS-bound β2GPI in peripheral blood T cells from DR53+ healthy individuals in vitro. (A) Peripheral blood T cells from a representative DR53+ healthy individual were stimulated with DCs pulsed with BBPS-bound β2GPI, BBPS, or β2GPI alone, and the T-cell response to MalBP, GP-F, native β2GPI, p276-290, or p306-320 was measured by IFN-γ production. A representative result from 2 independent experiments is shown. (B) T-cell response to p276-290 after priming with autologous DCs or macrophages pulsed with BBPS-bound β2GPI, BBPS, or β2GPI alone in 6 DR53+ healthy individuals. The p276-290–specific T-cell response was evaluated by IFN-γ production. The results from each of 6 individuals are indicated by a different symbol. Results are shown as mean and standard deviation.
Discussion
The present study demonstrates that the binding of β2GPI to anionic PL surfaces renders the molecule highly immunogenic by an enhanced generation of the cryptic T-cell determinant as a direct consequence of antigen processing in functional APCs. To date, little information has been obtained about the mechanisms that induce the expression of cryptic self-peptides and elicit autoimmunity in human autoimmune diseases. Potential mechanisms that reveal cryptic self-determinants in APCs include modulation of antigen processing and/or increased antigen delivery to the processing compartment.10 One possible explanation is that the PL binding physically shields the p276-290 determinant from proteolytic attack in endocytic compartments. In this regard, Simitsek et al23 reported that antibody binding to the antigen suppresses the generation of some epitopes and boosts that of others. Remarkably, both suppressed and boosted epitopes were present within a protein domain that was “fingerprinted” by the antibody, whereas epitopes that lay outside this domain were not affected. Based on these findings, they speculated that the antibody, by binding and stabilizing a protein domain, might influence the accessibility of the site to proteases during antigen processing. In addition, processing of the thyroglobulin-autoantibody complex has been described to promote generation of cryptic pathogenic peptides in APCs in murine models for autoimmune thyroid disease.24 A similar mechanism can be proposed for the generation of p276-290 from the processing of PL-bound β2GPI. The major PL-binding site located on the surface of a β2GPI molecule22 should be easily accessed by proteases in endocytic compartments during antigen processing, and therefore, the peptides containing the intact major PL-binding site would not be generated from native β2GPI. In contrast, the binding of β2GPI to anionic surfaces may protect the major PL-binding site from protease attack by masking the site, resulting in the appearance of the previously cryptic peptide containing the entire major PL-binding site. Anionic PLs on apoptotic bodies and platelet microparticles were potentially present in our cultures, but p276-290–reactive T-cell lines did not respond to native β2GPI in the absence of PL liposomes. The precise reason for this phenomenon is unclear, but quantity and quality of the lipid vesicles may be important in inducing the presentation of the previously cryptic p276-290 in APCs.
The capacity of individual PLs to induce a response of the p276-290–reactive T-cell line was principally correlated with their β2GPI-binding capacity. However, β2GPI had a strong binding affinity to solid-phase cardiolipin, but the cardiolipin-β2GPI complex demonstrated only a weak T-cell stimulatory capacity. β2GPI interacts with lipid vesicles containing anionic PLs via the PL-binding patch (ie, a cluster of basic amino acid residues and a hydrophobic flexible loop in the domain V).22 In contrast to the β2GPI interaction with solid-phase cardiolipin, binding capacity of β2GPI to lipid vesicles depends not only on the strength of the negative charge but also on fluidity of the lipid vesicles. In general, the membrane fluidity is influenced by length of fatty acid chains, number of unsaturated double bonds, and/or phase transition temperature of each lipid composed in liposomes. Therefore, it is likely that such structural differences also contribute to the efficiency to reveal the cryptic epitope containing the intact major PL-binding site in APCs.
The in vitro priming experiment strongly suggested that an event inducing the presentation of p276-290 by DCs would activate β2GPI-reactive CD4+ T cells in the normal T-cell repertoire in genetically susceptible individuals. Therefore, exposure of p276-290 to the immune system might be a critical step for inducing APS by triggering the activation of disease-relevant β2GPI-reactive CD4+ T cells. Activated β2GPI-reactive T cells would subsequently stimulate B cells to produce pathogenic anti-β2GPI antibody through the expression of CD40 ligand and IL-6, as reported previously.6 Since β2GPI is a plasma protein abundant in the circulation (∼ 200 μg/mL), excessive exposure to anionic surfaces, such as microorganisms and apoptotic cells, may induce the formation of a large quantity of β2GPI bound to anionic surfaces in vivo. In this regard, associations between various types of infections and the production of antiphospholipid antibodies with or without APS manifestations have been reported,25 and infection is one of the major precipitating factors contributing to the development of catastrophic APS.26 Furthermore, an enhanced yield of cryptic determinants and T-cell stimulatory capacity can be achieved by highly potent DCs that have specialized mechanisms for antigen capture and increased expression of HLA class II and costimulatory and adhesion molecules. These 2 mechanisms may act synergistically to elicit the β2GPI-specific T-cell response, but additional factors, such as impaired regulatory function and nonspecific inflammation mediated by cytokines and toll-like receptor ligands, are apparently required to initiate the pathogenic autoimmune response. Once the T-cell response to p276-290 is primed, the specific T-cell response could be sustained and amplified by professional APCs that have taken up the β2GPI complexed with anionic surfaces that are normally present in a small quantity in vivo, such as apoptotic cells, platelet microparticles, and oxidized low-density lipoprotein.27 In addition, this response can be further boosted by the formation of immune complexes consisting of anti-β2GPI antibodies and β2GPI bound to anionic surfaces.
In summary, our finding is the first demonstration of a mechanism that elicits pathogenic autoreactive T-cell responses in APS. Further studies examining anionic surfaces that bind to β2GPI and induce the presentation of the cryptic peptide of β2GPI in vivo would be useful in clarifying the pathogenesis of APS. In addition, it is likely that modulation of antigen processing is an inevitable consequence of the high-affinity binding and influence processing of autoantigens that are bound by high-affinity ligands. This theory encourages further research examining the possibility that the unveiling of cryptic self-determinants by the altered processing of autoantigens complexed with certain ligands is a major mechanism of the initiation of the autoimmune spiral in other autoimmune diseases.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-08-3145.
Supported by a grant from the Japanese Ministry of Health, Welfare and Labor and by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Takahide Arai and Kazue Yoshida for helpful discussions.

![Figure 1. Response of p276-290–reactive T-cell lines to various combinations of DR53-positive APCs and β2GPI preparations. (A) Various DR53-positive APCs were pulsed with or without native β2GPI (▪ and □, respectively) and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. All APCs except allogeneic splenocytes were autologous cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. A ▦ indicates a control T-cell line stimulated by a combination of anti-CD3 mAb and phytohemagglutinin (PHA). A representative result from at least 2 independent experiments is shown. (B) Autologous LBLs were pulsed with various β2GPI preparations and subsequently cocultured with the p276-290–reactive T-cell lines OM7 and KS3. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. The capacity to bind β2GPI in individual preparations was assessed by inhibition of the interaction between native β2GPI and immobilized cardiolipin. Results are shown as mean and standard deviation. A representative result from 3 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-08-3145/6/m_zh80040574080001.jpeg?Expires=1769238459&Signature=Ru7QnKOXNmv5gm~JqHenMcyiUpr2hD0ZDRh4GZ6ooiW1JP6tcWeGaiTNrTcUqTZE0KietdTeIEtlkgKrAK619~6ffLS4Kas71L51zW5g5tDf4~1NZkQGjGHhEVJx5TSz8QRu0BQFVO-H3sWRn3j18eB8wdrE3D~ZRlE7OX~sB5xeYB7SPsHcOHCxQpXCzqXaXr-aUARMUqgXRiHfkfJlM0K6BETTf83ZH90JIIEB0E02ICpJEtg2Jic-TyhANASsxRWeWbgKBNohVRdsNbIx7blaZNEh-3Az7WcKLqoz5Xd5U5r1g5tBlaYmwFRChwS66x5WCxwEyycBstpSpDRNdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Response of p276-290–reactive T-cell lines in cultures of β2GPI and anionic PL. Autologous immature DCs and macrophages were pulsed with native β2GPI alone, DOPS liposomes, a mixture of native β2GPI and DOPS liposomes, MalBP, or GP-F; induced to maturate or activate; and used to stimulate the p276-290–reactive T-cell lines KS3 and EY3. Synthetic peptides were pulsed with APCs before their coculture with T cells. The antigen-induced T-cell response was measured by [3H]thymidine incorporation. Results are shown as mean and standard deviation. A representative result from at least 2 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-08-3145/6/m_zh80040574080002.jpeg?Expires=1769238459&Signature=GRVjQgW3XfklWpcDDvBrmuc8l3p3c1LLwMo9HYRGsvwHA-9JJRwcsOL1OORyt48LeC28Q36ppYhy1U7KM2yuU0Sn2TjfMcCM6cqTWltkPks8-nJyT-PAyDoZ86p3Avv3GxOKsB0v0MV-~Q8Z4SVyJen09oe-wqzbX8x-ls7txa2aTtKh0Nfb7ZIdfH6z8frnbA5~uja6TUW~MWVJR4sBrv2RBZsPbbQhvpwg0oIzsoW4xMU6h1KK-ghbuFgnFU72b83VOwEwuR8e4~3C6Tu0ZWQ1Ca3BePtPyERenKVUvQ~3oZ-p-rsyT0J9NDxdYBHmH2GIsdSJE8j8tKZoaUw9Tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Binding of β2GPI to anionic PL facilitates the processing and presentation of p276-290, which activates p276-290–reactive T-cell lines. (A) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by [3H]thymidine incorporation. A representative result from 2 independent experiments is shown. (B) Autologous immature DCs were pulsed with or without a mixture of native β2GPI and various PL liposomes (▪ and □, respectively), induced to mature, and used to stimulate the p276-290–reactive T-cell line KS3. The antigen-induced T-cell response was evaluated by IFN-γ production. A representative result from 3 independent experiments is shown. (C) The capacity of individual PLs to bind β2GPI was evaluated by a solid-phase assay. A representative result from 2 independent experiments is shown. OD indicates optical density. (D) The p276-290–reactive T-cell line OM-b was cultured with autologous DCs bearing DOPS-bound β2GPI in the presence or absence of chloroquine, brefeldin A, anti–HLA-DR mAb, or control mAb. The antigen-induced T-cell response was evaluated by IFN-γ production. Results are shown as the mean and standard deviation. A representative result from 2 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-08-3145/6/m_zh80040574080003.jpeg?Expires=1769238459&Signature=QJQ0kBrCqdYumb9RrdGJM2CLYdThYHDOkKg8pGU-2Dl68PHfxulckUhLhQ6QUpcnB~U9~cQQ7BCTUHLbr8RO6eu9MPRR1zasUOJ-6ec0TT5v~7P1r2nktxXL~j5THUZbzNW7sxKeMDUZXjaiw4t20nsXRmtVC1x0Vw6KT5k9MBTY4R4J28VvF3N6Oz0BrFEjjUQnfiDcD3RvUkcvTQdRkgooNP8wZW2b32GJo0qxV8fODRUqZYaxnixzhDEgXevNUcfg-XO4kNSfQHwVjTzO0FXaGDi9d72OqeFIqxzJ-kPwoeCxxWKUq0rl5yAR8cOtZPrxsH56WRsa9~hVBcG5~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal