Abstract
Growth impairment and growth hormone (GH) deficiency are complications after total body irradiation (TBI) and hematopoietic cell transplantation (HCT). To determine the impact of GH therapy on growth, the final heights of 90 GH-deficient children who underwent fractionated TBI and HCT for malignancy were evaluated. Changes in height standard deviation (SD) from the diagnosis of GH deficiency to the achievement of final height were compared among 42 who did and 48 who did not receive GH therapy. At HCT, GH-treated patients were younger (P = .001), more likely to have undergone central nervous system irradiation (P = .007), and shorter (P = .005) than patients who did not receive GH therapy. After HCT, GH deficiency was diagnosed at 1.5 years (range, 0.8-9.5 years) for GH-treated and 1.2 years (range, 0.9-8.8 years) for nontreated patients. GH therapy was associated with significantly improved final height in children younger than 10 years at HCT (P = .0001), but GH therapy did not impact the growth of older children. Girls (P = .0001) and children diagnosed with acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), or myelodysplastic syndromes (MDS) (compared with acute lymphoblastic leukemia [ALL] or non-Hodgkin lymphoma [NHL]; P = .02) also showed more rapid growth than their counterparts. These data demonstrate that GH therapy improves the final height of young children after fractionated TBI.
Introduction
Growth impairment and growth hormone (GH) deficiency are frequent complications of the use of total body irradiation (TBI) in children.1-12 The incidence of GH deficiency after hematopoietic cell transplantation (HCT) varies widely, from 20% to 85%; the differences are likely caused by variations in the time of testing, differences in the preparative regimens received, the inclusion of patients with and without cranial irradiation, and inconsistencies in the GH testing methods used.1,13-19 Decreased growth velocity occurs after single-exposure and fractionated-exposure TBI.3,7,13 Posttransplantation growth rates vary, with some observers reporting the poorest growth rates during the first year after TBI whereas others report the greatest losses in height several years after TBI.7,8,11-14 The poorest growth rates occur among very young children and those who previously underwent central nervous system (CNS) irradiation.1,8,9,11,14 Preparative regimens including TBI also have greater effects on reducing height standard deviation (SD) than preparative regimens composed of chemotherapy only.11
Even though GH deficiency has been observed in up to 85% of children after HCT, fewer than half received GH therapy.8,9,11,20 Retrospective studies have found final height SD of HCT children to be significantly lower than that of their height at HCT.11 These same studies also reported that the HCT children's final heights were significantly lower than their predicted genetic heights based on corrected mid-parental heights.9 Both studies concluded that GH therapy was not needed because final height SD was not more than 2 SD below the mean or because GH-treated children did not have significantly improved growth. The conclusion from a third retrospective study is open to question because most of those patients received less than 1 year of GH therapy.10
Results to date clearly demonstrate growth and GH deficiency are significant problems after HCT, but none of the studies prospectively evaluates the impact of GH therapy on growth rates, and none adequately evaluates GH impact on final height. The present study evaluates the final height achieved among 90 GH-deficient children, 42 of whom received GH therapy, and 48 of whom did not receive GH therapy. Results indicate improved growth rates for those who received GH therapy.
Patients and methods
Study design
This study is based on an analysis of height data and GH testing data collected prospectively on all patients younger than 18 years of age at the time of HCT at the Fred Hutchinson Cancer Research Center (FHCRC, Seattle, WA). These children were entered in a protocol evaluating endocrine function beginning 1 year after HCT and continuing annually until they were 17 or final height was achieved and gonadal maturity was reached, whichever occurred first. Serum was obtained for thyroid function, growth hormone and insulinlike growth factor (IGF), and gonadotropin levels. Radiographic examination of bone age was requested. Height measurements were taken with the Harpenden stadiometer, and Tanner Developmental Scores were estimated for all patients. Informed consent was obtained from the patients and their parents or responsible guardians for study participation using FHCRC Institutional Review Board (IRB)–approved protocol and consent forms.
Patients
Final heights were evaluated of children with malignant diseases who underwent HCT after fractionated TBI at the FHCRC from July 1978 through July 2000. The study was restricted to those who survived more than 2 years and had reached more than 16 years of age. Evaluations were performed in March 2004. Of the 183 children who met protocol entry criteria, 76 were excluded because GH testing could not be performed given the refusal on the part of a parent or a consulting endocrinologist to participate in the study. Among the 107 who underwent GH testing, 90 (84%) had GH deficiency. Characteristics of the 90 children with GH deficiency are shown in Table 1. Treatment before referral for HCT varied with the referring institution. Thirty-two patients underwent CNS irradiation. Those given 900 to 1080 cGy CNS irradiation received it immediately before TBI, whereas those given 1500 to 2400 cGy CNS irradiation received it months to years before TBI administration.
Patient characteristics
GH therapy . | Yes . | No . | P . |
|---|---|---|---|
| No. patients | 42 | 48 | — |
| Age at HCT, y, median (range) | 7.9 (1.0-14) | 11 (2.9-15) | .001* |
| Sex (female/male) | 13:29 | 22:26 | — |
| Cranial irradiation (%) | |||
| Yes | 21 (50) | 11 (23) | .007† |
| No | 21 | 37 | |
| Diagnosis at HCT, (%) | |||
| ALL | 28 (67) | 17 (35) | .001† |
| NHL | 2 (5) | 1 (2) | — |
| AML | 8 (19) | 15 (31) | — |
| MDS | 1 | 3 | — |
| CML | 3 (7) | 12 (25) | — |
| TBI regimen, (%) | |||
| 1200 cGy | 5 (12) | 12 (25) | — |
| 1400-1575 cGy | 37 (88) | 36 (75) | — |
| Type of HCT, (%) | |||
| Allogeneic identical sibling | 22 (52) | 19 (40) | — |
| Allogeneic unrelated donor | 6 (14) | 11 (23) | — |
| Allogeneic mismatched family | 12 (29) | 17 (35) | — |
| Autologous | 2 (5) | 1 (2) | — |
| GVHD, (%) | |||
| Acute | |||
| Grades 0-1 | 15 | 20 | — |
| Grades 2-4 | 27 (64) | 28 (58) | — |
| Chronic, extensive | 19 (45) | 21 (44) | — |
| Extensive steroid use, (%) | |||
| No | 39 (93) | 44 (92) | — |
| Yes | 3 (7) | 4 (8) | — |
| Follow-up, y, median (range) | 11.0 (3.2-23) | 11.2 (2.7-20.3) | — |
| Height SD at HCT, median (range) | –0.6 (–3.3-1.0) | 0 (–2.6-3.1) | .005* |
GH therapy . | Yes . | No . | P . |
|---|---|---|---|
| No. patients | 42 | 48 | — |
| Age at HCT, y, median (range) | 7.9 (1.0-14) | 11 (2.9-15) | .001* |
| Sex (female/male) | 13:29 | 22:26 | — |
| Cranial irradiation (%) | |||
| Yes | 21 (50) | 11 (23) | .007† |
| No | 21 | 37 | |
| Diagnosis at HCT, (%) | |||
| ALL | 28 (67) | 17 (35) | .001† |
| NHL | 2 (5) | 1 (2) | — |
| AML | 8 (19) | 15 (31) | — |
| MDS | 1 | 3 | — |
| CML | 3 (7) | 12 (25) | — |
| TBI regimen, (%) | |||
| 1200 cGy | 5 (12) | 12 (25) | — |
| 1400-1575 cGy | 37 (88) | 36 (75) | — |
| Type of HCT, (%) | |||
| Allogeneic identical sibling | 22 (52) | 19 (40) | — |
| Allogeneic unrelated donor | 6 (14) | 11 (23) | — |
| Allogeneic mismatched family | 12 (29) | 17 (35) | — |
| Autologous | 2 (5) | 1 (2) | — |
| GVHD, (%) | |||
| Acute | |||
| Grades 0-1 | 15 | 20 | — |
| Grades 2-4 | 27 (64) | 28 (58) | — |
| Chronic, extensive | 19 (45) | 21 (44) | — |
| Extensive steroid use, (%) | |||
| No | 39 (93) | 44 (92) | — |
| Yes | 3 (7) | 4 (8) | — |
| Follow-up, y, median (range) | 11.0 (3.2-23) | 11.2 (2.7-20.3) | — |
| Height SD at HCT, median (range) | –0.6 (–3.3-1.0) | 0 (–2.6-3.1) | .005* |
AML indicates acute myelogenous leukemia; MDS, myelodysplastic syndromes; CML, chronic myelogenous leukemia.
Wilcoxon rank sum test
χ2 test
Preparative regimens depended on protocols in use at the time of transplantation, the phase of patient disease, and donor type. TBI was administered from dual cobalt-60 sources at a dose rate of 4.5 cGy/min given as 2.0 Gy/d for 6 consecutive days (n = 17), 14.0 Gy given as 2.0 Gy/d for 7 consecutive days (n = 3), 14.4 Gy given as 1.2 Gy 3 times a day for a total of 4 consecutive days (n = 33), or 2.25 Gy/d for 7 consecutive days (n = 37), as has been previously described.21-26 The 11 boys with acute lymphoblastic leukemia (ALL) received 400 cGy testicular boost irradiation at the time of TBI. In addition, 9 boys had previously received 1200 to 2400 cGy testicular irradiation as treatment for testicular leukemia. All patients received cyclophosphamide (CY) at 60 mg/kg per day for 2 consecutive days before TBI administration.
Acute and chronic graft-versus-host disease (GVHD) was graded as previously described,27-30 and treatment was administered with glucocorticoid or according to GVHD therapy protocols active at the time.31-33 Glucocorticoid therapy for acute or chronic GVHD was administered to 66 patients. Cyclosporin (CSP) or tacrolimus was administered to 69 patients for a median of 9.5 (range, 1.9-135.2) months as prophylaxis of acute GVHD or as treatment of acute or chronic GVHD. Thirty of the 42 (71.4%) GH therapy recipients were given CSP for a median of 11.1 (range, 1.9-92.2) months, and 39 of 48 (81.2%) who did not receive GH therapy were given CSP for a median of 9.5 (range, 2.8-135.2) months. Sex hormone replacement therapy was administered to 37 patients (18 male, 19 female) starting at 14.3 years (range, 12.1-20.3 years) of age in males and at 14.6 years (range, 10.6-18.2 years) of age in females.
Growth and growth hormone testing
Height was measured before transplantation and annually thereafter using a Harpenden stadiometer (Holtain Ltd, United Kingdom). Height was expressed as SD score calculated as height minus mean height for age and sex divided by the SD of height for age and sex. In general, all patients underwent 2 tests to measure GH production; they included spontaneous production of GH, performed with 12-hour sampling every 20 minutes, and clonidine stimulation.34-36 Normal results for spontaneous production of GH included more than 3 spontaneous peaks, with at least 1 spontaneous peak greater than 18 ng/dL, and a cumulative GH production level greater than 3 ng/dL. For growth to be considered normal, 2 of the 3 spontaneous production parameters had to be in the normal range. Clonidine stimulation results at 60 and 90 minutes after stimulation had to be greater than 8.6 ng/dL to be considered normal. Both GH tests had to demonstrate decreased GH production before a diagnosis of biochemical GH deficiency could be made. In general, these patients also had reduced growth rates. It was suggested to referring physicians that patients who had GH deficiency, as demonstrated by biochemical testing, and who had growth failure receive supplemental GH therapy. The decision to treat or not treat patients for GH deficiency and clinical growth failure was made by each patient's local pediatric endocrinologist. The 42 patients who received GH were treated with recombinant human growth hormone at 0.4 to 0.6 U/kg per week, administered as subcutaneous daily injection at least 5 to 6 days each week. A pediatric endocrinologist in their home community treated all patients.
Statistical analysis
Characteristics of patients categorized by receipt of GH therapy were compared using the t test for continuous variables and the χ2 test for categorical factors. Growth in these patients in the absence of GH therapy was quantified by plotting the mean height SD scores at 3 meaningful time points: HCT, GH deficiency diagnosis, and last height measurement without GH therapy. The last time point represents the beginning of GH therapy for those who were treated and the final height for patients not treated with GH. Final height was defined as the height measured closest to age 16 years. Height SD scores were compared with those of a normal SD of 0 using 1-sample t tests and were compared across patient subgroups using 2-sample t tests.
To assess the impact of GH therapy on growth, the change in height SD score from GH deficiency diagnosis to final height was evaluated. Factors other than GH therapy that might have contributed to this change in height were also examined using multiple linear regression models. These factors included age at HCT, sex, primary diagnosis, exposure to cranial irradiation, height SD score at HCT, and extensive steroid use. The effect of GH therapy on growth was then tested, after adjusting for patient and transplantation characteristics. Interactive effects with GH therapy were also investigated.
Two-sided P values from fitted regression models were derived from the Wald test. No adjustments were made for multiple comparisons. P less than .05 was considered statistically significant.
Results
Although the 42 patients who did and the 48 patients who did not receive GH therapy were not part of a randomized study, all participated in the prospective study of endocrine function after transplantation, all had GH deficiency that was diagnosed during the same time period, and all were evaluated at FHCRC and by a local pediatric endocrinologist. The characteristics of these patients are shown in Table 1. Bone age results were available for only 78% of patients, so age 16 years or height measurement closest to age 16 years was selected as the time for determination of final height. The patients treated with GH were significantly younger at HCT, were more likely to have received CNS irradiation, were more likely to have undergone transplantation for ALL or non-Hodgkin lymphoma (NHL), and were shorter at HCT than those who did not receive GH therapy. Similar proportions of patients in the GH therapy group and in the no GH therapy group received higher doses of TBI, and similar numbers of patients had prolonged steroid therapy as treatment for chronic GVHD. Follow-up for both groups was 11 years after HCT. All patients treated with GH received a median of 4.5 years (range, 1.0-9.6 years) of GH therapy, for a total of 269 patient-years of GH therapy.
Patients were diagnosed with GH deficiency at a median of 1.3 years (range, 0.8-9.5 years) after HCT. Time to diagnosis of GH deficiency did not differ by eventual GH therapy status (medians, 1.5 years in GH-treated and 1.2 years in nontreated patients). Median age at GH deficiency diagnosis was 11.7 years (range, 6.0-15.9 years). Among the patients who were treated, the median time from GH deficiency diagnosis to start of GH therapy was 0.9 years (range, 0-5.9 years). For untreated patients, the median time from GH deficiency diagnosis to final height was 3.1 years (range, 0-12.1 years). Age at final height ranged from 15 to 17 years, except for 3 patients for whom the final height measurements were available only at ages 18, 21, and 22 years.
At the time patients were diagnosed with GH deficiency, 36 of the 66 given glucocorticoid therapy for chronic GVHD were off therapy, and 30 were still receiving glucocorticoid therapy. Among the 30 patients receiving glucocorticoids at diagnosis of GH deficiency, the dose for 23 was 0.5 mg/kg or less every other day for 0.4 years (range, 0.1-1.8 years). Of these 23 patients, the duration of therapy was less than 1 year for 10 who received GH therapy and for 8 who did not receive GH therapy and less than 2 years for 5 patients. The dose for the remaining 7 patients ranged from 0.6 to 1.0 mg/kg every other day for 1.4 to 4.8 years for 4 patients not given GH. One patient given GH received an average of 1 mg/kg glucocorticoid therapy every day for 2 years, and 2 patients given GH therapy received 0.6 mg/kg glucocorticoid therapy every other day for 1.3 and 2.0 years.
At the time patients were diagnosed with GH deficiency, bone age was assessed in 29 of 42 (69%) patients who received GH and in 41 of 48 (85%) patients who did not receive GH. Among the 29 given GH, chronological age was 9.8 years (range, 6.0-14.8 years) and bone age was 8.8 years (range, 4.0-14.0 years), whereas among the 48 not given GH, chronological age was 12.6 years (range, 6.4-15.9 years) and bone age was 12.0 years (range, 4.0-16.5 years). Bone age was within 2 years of chronological age for all except 6 patients given GH and 8 patients not given GH.
Thyroid function was assessed using thyroid-stimulating hormone (TSH) and thyroxine (T4) serum levels for all patients. At diagnosis of GH deficiency, 36 of 42 (86%) of those given GH therapy had normal thyroid function, 4 (9.5%) had compensated hypothyroidism with elevated TSH levels and normal T4 levels, and 2 had hypothyroidism with elevated TSH and low T4. These latter 2 patients were treated with thyroid hormone therapy. Among the 48 not given GH, 40 (83%) had normal thyroid function and 8 (17%) had compensated hypothyroidism. The course of thyroid function during the time of GH therapy for both groups of patients is shown in Table 2.
Characteristics of patient thyroid and gonadal function at age 16 years
. | GH therapy . | No GH therapy . |
|---|---|---|
| No. patients | 42 | 48 |
| Thyroid function | ||
| Normal | 23 (55) | 41 (85) |
| Hypothyroid, treated | 16 (38) | 5 (10) |
| Compensated, treated | 0 | 2 |
| Compensated, untreated | 2 | 0 |
| Hyperthyroid, treated | 1 | 0 |
| Gonadal function | ||
| Girls | ||
| Normal | 4 (31) | 9 (41) |
| Ovarian failure, treated | 8 | 11 |
| Ovarian failure, untreated | 1 | 2 |
| Boys | ||
| Normal | 5 (17) | 7 (27) |
| Testicular failure (normal testosterone, untreated) | 5 | 6 |
| Testicular failure (normal testosterone, treated) | 1 | 0 |
| Testicular failure (low testosterone, untreated) | 3 | 2 |
| Testicular failure (low testosterone, treated) | 12 | 5 |
| Not evaluable | 3 | 6 |
. | GH therapy . | No GH therapy . |
|---|---|---|
| No. patients | 42 | 48 |
| Thyroid function | ||
| Normal | 23 (55) | 41 (85) |
| Hypothyroid, treated | 16 (38) | 5 (10) |
| Compensated, treated | 0 | 2 |
| Compensated, untreated | 2 | 0 |
| Hyperthyroid, treated | 1 | 0 |
| Gonadal function | ||
| Girls | ||
| Normal | 4 (31) | 9 (41) |
| Ovarian failure, treated | 8 | 11 |
| Ovarian failure, untreated | 1 | 2 |
| Boys | ||
| Normal | 5 (17) | 7 (27) |
| Testicular failure (normal testosterone, untreated) | 5 | 6 |
| Testicular failure (normal testosterone, treated) | 1 | 0 |
| Testicular failure (low testosterone, untreated) | 3 | 2 |
| Testicular failure (low testosterone, treated) | 12 | 5 |
| Not evaluable | 3 | 6 |
Numbers in parentheses indicate percentage.
Gonadal function was evaluated with luteinizing hormone (LH), follicle-stimulating hormone (FSH), and either estradiol or testosterone in all patients 10 years and older at the time of GH deficiency diagnosis. Three of the 5 girls who received GH therapy had normal ovarian function, and 2 had primary ovarian failure. Among 20 girls not treated with GH therapy, 19 (95%) had primary ovarian failure, but only 3 received hormone therapy. Among the boys given GH therapy, 8 of 17 (47%) had normal gonadal function, but only 3 of the remaining 9 with testicular failure received therapy. Among the boys not given GH therapy, 10 of 19 (53%) had normal testicular function, one was not evaluable, and 8 had primary gonadal failure. The course of gonadal function through GH therapy for treated and untreated girls and boys is shown in Table 2. These data show that nearly all patients with primary gonadal failure were treated with appropriate sex hormone therapy.
Growth after HCT
Table 3 shows the mean height SD scores over time for all patients. At the time of transplantation, patient heights were significantly below average (mean, -0.4 SD; 95% confidence interval (CI), -0.6, -0.1 SD; P = .002). Height SD scores continued to decrease to an average of -1.0 SD (95% CI, -1.0, -0.7 SD; P < .0001) at GH deficiency diagnosis and to -1.4 SD (95% CI, -1.7, -1.2 SD, P < .0001) at the last untreated measurement.
Mean height SD scores over time by patient characteristics
. | N . | HCT . | GH deficiency . | Last before therapy . |
|---|---|---|---|---|
| All patients | 90 | –0.36 | –0.99 | –1.41 |
| Age at HCT | ||||
| Younger than 10 y | 56 | –0.39 | –1.09 | –1.57 |
| 10 y and older | 34 | –0.30 | –0.83 | –1.13 |
| Cranial irradiation | ||||
| No | 58 | –0.27 | –0.78 | –1.27 |
| Yes | 32 | –0.51 | –1.38* | –1.65 |
| Sex | ||||
| Female | 35 | –0.32 | –1.05 | –1.27 |
| Male | 55 | –0.38 | –0.96 | –1.49 |
| Diagnosis | ||||
| ALL or NHL | 48 | –0.25 | –0.80 | –1.20 |
| Other | 42 | –0.44 | –1.16 | –1.59 |
. | N . | HCT . | GH deficiency . | Last before therapy . |
|---|---|---|---|---|
| All patients | 90 | –0.36 | –0.99 | –1.41 |
| Age at HCT | ||||
| Younger than 10 y | 56 | –0.39 | –1.09 | –1.57 |
| 10 y and older | 34 | –0.30 | –0.83 | –1.13 |
| Cranial irradiation | ||||
| No | 58 | –0.27 | –0.78 | –1.27 |
| Yes | 32 | –0.51 | –1.38* | –1.65 |
| Sex | ||||
| Female | 35 | –0.32 | –1.05 | –1.27 |
| Male | 55 | –0.38 | –0.96 | –1.49 |
| Diagnosis | ||||
| ALL or NHL | 48 | –0.25 | –0.80 | –1.20 |
| Other | 42 | –0.44 | –1.16 | –1.59 |
P < .05 for differences between patient subgroups at specific time points
Table 3 also shows average height SD scores grouped by the patient characteristics: age at HCT, exposure to cranial irradiation, sex, and primary diagnosis. The age of 10 years was selected as a cut point given that these children were clearly prepubertal. Patients younger than 10 years tended to have lower height SD scores at HCT than children who were 10 at HCT. This pattern persisted across time, though the differences in average SD scores were not statistically significant. Patients who received cranial irradiation showed consistently lower height SD scores than those without such exposure, with a statistically significant difference at GH deficiency diagnosis (P = .04). Boys had slightly lower average height SD scores than girls, which follows from the above data because the boys were younger than the girls on average. Patients who underwent transplantation for ALL or NHL had lower average height SD scores over time than patients with other diseases. This trend follows from the fact that significantly more patients in the ALL/NHL group received cranial irradiation than did those with other diagnoses.
Figure 1 shows the mean height SD scores over time for all patients, grouped by whether they received GH therapy. Figure 2 shows average height SD scores by age at HCT and GH therapy group. Figure 3 displays average height SD scores by exposure to cranial irradiation and GH therapy group. On average, patients in the GH therapy group had lower height SD scores than those in the untreated group. All patient subgroups experienced declining height over time compared with their age-sex counterparts.
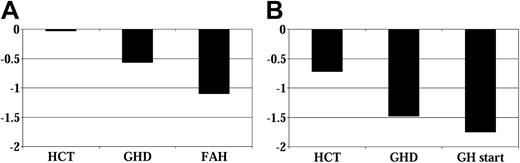
Height SD score before GH therapy. (A) No GH therapy group (n = 48). (B) GH therapy group (n = 42). GHD indicates growth hormone deficiency diagnosis; GH, growth hormone therapy; FAH, final adult height.
Height SD score before GH therapy. (A) No GH therapy group (n = 48). (B) GH therapy group (n = 42). GHD indicates growth hormone deficiency diagnosis; GH, growth hormone therapy; FAH, final adult height.
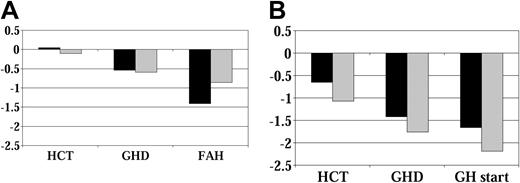
Height SD score by age at HCT before GH therapy. (A) No GH therapy group. (B) GH therapy group (younger than 10 years; n = 21;10 years, n = 27) (younger than 10 years, n = 35; 10 years, n = 7). Abbreviations are as in Figure 1.
Height SD score by age at HCT before GH therapy. (A) No GH therapy group. (B) GH therapy group (younger than 10 years; n = 21;10 years, n = 27) (younger than 10 years, n = 35; 10 years, n = 7). Abbreviations are as in Figure 1.
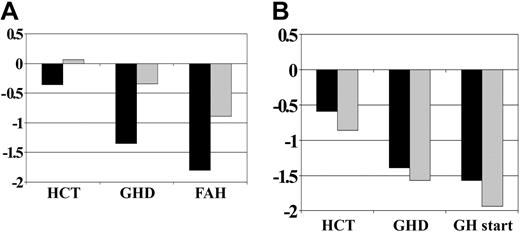
Height SD score by exposure to cranial irradiation before GH therapy. (A) No GH therapy group (CXRT, n = 11; no CXRT, n = 37). (B) GH therapy group (CXRT, n = 21; no CXRT, n = 21). CXRT indicates cranial irradiation therapy; remaining abbreviations are as in Figure 1.
Height SD score by exposure to cranial irradiation before GH therapy. (A) No GH therapy group (CXRT, n = 11; no CXRT, n = 37). (B) GH therapy group (CXRT, n = 21; no CXRT, n = 21). CXRT indicates cranial irradiation therapy; remaining abbreviations are as in Figure 1.
Impact of GH therapy
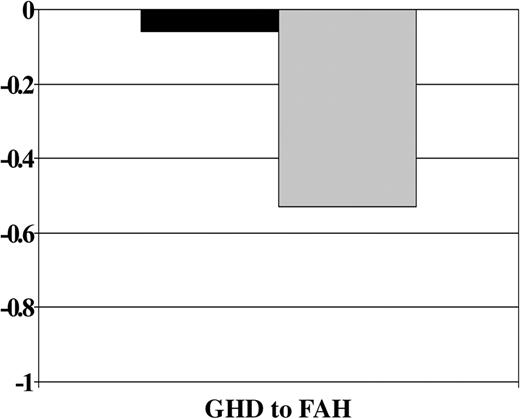
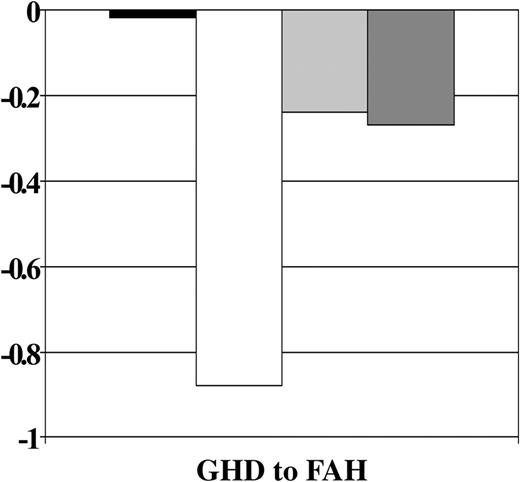
GH therapy was significantly associated with improved growth from GH deficiency diagnosis to final height after all other covariates were accounted for (P = .0004). A significant interaction of the GH treatment effect with age at HCT was also observed (P = .04). Figure 4 shows that, on average, GH-treated patients lost .06 SD and those not treated lost 0.53 SD from GH deficiency diagnosis to final height. Figure 5 shows that the treatment effect was stronger in the younger group. Among patients younger than 10 years, those who received GH therapy had significantly smaller losses in height SD than those who did not receive therapy, whereas there appeared to be no difference in height SD score change between GH-treated and nontreated subjects who were 10 years at HCT.
Change in height SD score by treatment with GH therapy. Abbreviations are as in Figure 1.
Change in height SD score by treatment with GH therapy. Abbreviations are as in Figure 1.
Change in SD score by age at HCT and use of GH therapy. Abbreviations are as in Figure 1.
Change in SD score by age at HCT and use of GH therapy. Abbreviations are as in Figure 1.
Adjusted GH therapy effects for each of the 2 age groups are described in Table 4. Young GH-treated patients were expected to gain an additional 0.86 SD compared with similarly young, untreated patients (P = .0001). There was no significant effect of GH therapy on older patients (P = .6).
Effect of patient characteristics on change in height SD score from diagnosis of GH deficiency to final height
. | Coefficient . | 95% CI . | P . |
|---|---|---|---|
| GH therapy | |||
| Younger than 10 y at HCT | 0.86 | 0.46, 1.25 | .0001 |
| 10 y and older at HCT | 0.15 | –0.43, 0.72 | .6 |
| Sex (male vs female) | –0.64 | –0.94, –0.34 | .0001 |
| Diagnosis (ALL/NHL vs other) | –0.42 | –0.76, –0.08 | .02 |
| Height SD score at HCT | –0.12 | –0.25, 0.02 | .09 |
| Extensive steroid use | –0.47 | –1.02, 0.07 | .09 |
| Cranial irradiation | 0.25 | –0.10, 0.59 | .2 |
. | Coefficient . | 95% CI . | P . |
|---|---|---|---|
| GH therapy | |||
| Younger than 10 y at HCT | 0.86 | 0.46, 1.25 | .0001 |
| 10 y and older at HCT | 0.15 | –0.43, 0.72 | .6 |
| Sex (male vs female) | –0.64 | –0.94, –0.34 | .0001 |
| Diagnosis (ALL/NHL vs other) | –0.42 | –0.76, –0.08 | .02 |
| Height SD score at HCT | –0.12 | –0.25, 0.02 | .09 |
| Extensive steroid use | –0.47 | –1.02, 0.07 | .09 |
| Cranial irradiation | 0.25 | –0.10, 0.59 | .2 |
Coefficient refers to growth difference in SD.
Table 4 also shows the effects of the patient and transplantation characteristics after adjusting for GH treatment. Boys were expected to lose 0.64 SD of height more than girls (P = .0001), given the other factors in the model. In addition, diagnoses of ALL or NHL were associated with an expected loss of 0.42 SD more than the other diagnoses from GH deficiency diagnosis to final height (P = .02). Height SD score at HCT, extensive steroid use, and previous cranial irradiation were not significantly associated with outcome after adjusting for all other factors (Table 4).
Toxicity of GH therapy
None of the GH-treated patients developed recurrent leukemia, but 1 of the non–GH-treated patients had a relapse (Table 5). A second neoplasm developed in 4 patients during GH treatment and in 2 patients after GH treatment had been discontinued for 3.3 and 9.0 years. Among the 48 patients not receiving GH therapy, a second neoplasm developed in 8. Other complications of or late effects after transplantation included diabetes, hypothyroidism, osteochondroma, and exostosis, as presented in Table 5. There were significantly higher incidences of hypothyroidism (P = .05) and osteochondroma/exostosis (P = .03) in GH-treated than in untreated patients. Osteochondroma/exostosis occurred at a median of 8.2 years (range, 6.0-12.7 years) after transplantation among the 12 GH recipients who had HCT at a median of 5.6 years (range, 1.0-9.9 years) of age. Osteochondroma/exostosis occurred at a median of 6.4 years (range, 3.5-9.2 years) after HCT in the 5 children not given GH who had HCT at 8.2 years (range, 5.3-9.1 years) of age.
Complications occurring after transplantation
GH therapy . | Yes . | No . | P . |
|---|---|---|---|
| No. patients | 42 | 48 | — |
| Recurrent leukemia | 0 | 1 | .9 |
| Diabetes | 3 | 1 | .3 |
| Hypothyroidism | 14 | 7 | .05 |
| Osteochondroma/exostosis | 12 | 5 | .03 |
| Secondary malignancy | 6 | 8 | .8 |
| Brain tumor | 2 | 1 | — |
| Basal cell carcinoma | 1 | 3 | — |
| Sarcoma | 1 | 1 | — |
| Squamous carcinoma | 1 | 1 | — |
| Thyroid carcinoma | 1 | 1 | — |
| Lymphoma | 0 | 1 | — |
GH therapy . | Yes . | No . | P . |
|---|---|---|---|
| No. patients | 42 | 48 | — |
| Recurrent leukemia | 0 | 1 | .9 |
| Diabetes | 3 | 1 | .3 |
| Hypothyroidism | 14 | 7 | .05 |
| Osteochondroma/exostosis | 12 | 5 | .03 |
| Secondary malignancy | 6 | 8 | .8 |
| Brain tumor | 2 | 1 | — |
| Basal cell carcinoma | 1 | 3 | — |
| Sarcoma | 1 | 1 | — |
| Squamous carcinoma | 1 | 1 | — |
| Thyroid carcinoma | 1 | 1 | — |
| Lymphoma | 0 | 1 | — |
Discussion
Linear growth is a continuous and finely regulated process that is largely regulated in infancy by nutritional and metabolic factors, in childhood by GH, and in puberty by the synergistic action of GH and sex steroids. The impact of GH deficiency on subsequent growth and final height after HCT is difficult to determine because limited observations have been reported and no randomized studies comparing patient final height by GH treatment have been conducted. In fact, some may consider such a study unethical to perform. However, the present study is the only study that permits comparison of a reasonably sized group of contemporary patients who did or did not receive GH based on parent or treating endocrinologist recommendation. Although there are some differences between the 2 groups of patients, the data from the present study provide evidence that children reap significant benefit from GH therapy.
CNS irradiation has been associated with the development of GH deficiency, which appears to be related to the child's age at the time of irradiation, the irradiation dose received, and the length of time lapsed after completion of irradiation.37 Many children, especially those with ALL, referred for HCT receive 18 to 24 Gy CNS irradiation as part of the initial treatment before referral for HCT. When TBI is included in the preparative regimen, the total CNS irradiation dose usually exceeds 30 Gy, the estimated threshold for the development of GH deficiency.38 GH deficiency may be expected to develop in most patients who receive this total dose of CNS irradiation 2 to 3 years after initial irradiation. In the present study, height at GH deficiency diagnosis of those who underwent cranial irradiation was significantly less than it was in those who had not received CNS irradiation. Having received CNS irradiation, however, did not significantly influence whether response to GH therapy occurred.
Patient age at transplantation has been found to be a significant factor in predicting final height.10,39-41 Children younger than 10 years at the time of transplantation were at greatest risk for growth failure and significantly decreased adult height. The present study shows that children who did not receive GH therapy and who were younger than 10 years of age did have the shortest final height. However, the study also demonstrates that younger children show the best response to GH therapy and could be expected to gain an additional 0.86 SD height (P = .0001) compared with young untreated children. This height gain resulted in a significant benefit to their final height such that the young children who received GH had a relative final height similar to their relative height at HCT.
The present study showed that girls had better height growth than boys. These data confirm the observation of Cohen et al,9 who found that loss in height SD was more profound in boys than in girls. The reasons for this difference in height growth between girls and boys are not readily explained, suggesting that additional specific studies are needed.
Survivors of childhood transplantation are known to be at risk for secondary cancers.42 Data from the Childhood Cancer Survivor Study cohort of 13 539 survivors of childhood cancer evaluated the impact of GH therapy on disease recurrence and development of secondary malignancies. Results reassuringly showed that the administration of GH to 361 childhood cancer survivors was not associated with an increased risk for relapse of the original malignancy.43 Among those 361 GH-treated cancer survivors, however, 15 secondary malignancies developed, primarily among the leukemia survivors. This number was significantly higher than expected and was significantly associated with the administration of GH. This study analyzed only childhood cancer survivors who received GH and did not evaluate survivors with GH deficiency who were not given GH. In the present study, no differences were observed in the number or types of secondary malignancies among those who received GH and those who did not. The types of malignancies observed were similar to those observed after childhood transplantation.42 These data suggest that GH treatment of GH-deficient children after HCT does not appear to result in an excess of secondary malignancies or in an increase of recurrent leukemias.
Radiation-induced osteochondromas occur at an incidence of 10% to 12% after radiotherapy and an incidence of 10% to 24% after TBI.44-47 Younger children are at greatest risk because of their greater growth potential. Chemotherapy and radiotherapy can arrest growth and cause epiphyses to remain open longer than usual, permitting osteochondromas to develop. Children receiving GH develop osteochondromas earlier and at a shorter time after transplantation than children not receiving GH.45-47 These data suggest that in this population of children given TBI, use of GH may stimulate radiotherapy-disturbed epiphyses resulting in an increased incidence of osteochondromas.45,47 GH recipients in the present study developed osteochondromas longer after transplantation but were younger at HCT than the non–GH-treated patients. Malignant degeneration of the osteochondromas has been rare.46 All TBI recipients should be monitored carefully for the development of secondary malignancies, including those who develop osteochondromas. None of the children in the present report who have been receiving GH have experienced malignant degeneration related to osteochondroma, and GH therapy has not been discontinued because of the osteochondroma.
Interactions between GH therapy and the hypothalamic-pituitary-thyroid axis are complex. Children with idiopathic GH deficiency have clinically insignificant decreases in T4 levels, but children with central hypothyroidism develop clinically significant decreases in thyroid function while on GH therapy.48 Long-term GH therapy significantly decreases free T4 levels and causes T3 levels to increase. Most investigators agree that GH therapy for GH-deficient children does not induce hypothyroidism, but GH reveals previously unrecognized hypothyroidism.48,49 Others, however, suggest that changes in thyroid function during the first year of GH therapy are transient and that thyroid hormone supplementation is not needed unless the patient still has hypothyroidism after more than 1 year of GH therapy.50 The children found to be hypothyroid in the present study had peripheral (end-organ) hypothyroidism, with elevated TSH and low T4 serum levels, and had received more than 1 year of GH therapy at the time decreased thyroid function was discovered. All patients should be monitored after transplantation for the development of hypothyroidism or compensated hypothyroidism because this is also a known complication of TBI.1
The current study demonstrates a measurable improvement in final height for the GH-deficient child after HCT who has been administered GH. Factors influencing final height include patient age, underlying diagnosis, and sex. Best growth may be anticipated in girls younger than 10 not treated for ALL or NHL. Future studies must address improving final height by decreasing the length of time between the diagnosis of GH deficiency and the initiation of GH therapy.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-07-2528.
Supported in part by National Institutes of Health grants CA18029, CA18221, and CA36444.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal