Abstract
B-cell lymphoma 2 (Bcl-2) is a pivotal regulator of apoptotic cell death and it is overexpressed in many cancers. Consequently, the Bcl-2 protein is an attractive target for drug design, and Bcl-2–specific antisense oligonucleotides or small-molecule Bcl-2 inhibitors have shown broad anticancer activities in preclinical models and are currently in several clinical trials. The clinical application of immunotherapy against cancer is rapidly moving forward in multiple areas, including the adoptive transfer of anti–tumor-reactive T cells and the use of “therapeutic” vaccines. The overexpression of Bcl-2 in cancer and the fact that immune escape by down-regulation or loss of expression of this protein would impair sustained tumor growth makes Bcl-2 a very attractive target for anticancer immunotherapy. Herein, we describe spontaneous T-cell reactivity against Bcl-2 in peripheral blood from patients suffering from unrelated tumor types (ie, pancreatic cancer, breast cancer, acute myeloid leukemia [AML], and chronic lymphocytic leukemia [CLL]). Additionally, we show that these Bcl-2–reactive T cells are indeed peptide-specific, cytotoxic effector cells. Thus, Bcl-2 may serve as an important and widely applicable target for anticancer immunotherapeutic strategies (eg, in the combination with conventional radiotherapy and chemotherapy).
Introduction
In recent years, research into the interplay between cancer cells and cells of the immune system has conveyed a detailed understanding of the capacity of the immune system to recognize and destroy cancer cells. To this end, a large number of peptide antigens derived from tumor-associated antigens (TAAs) have been applied in immunotherapeutic trials for the treatment of various malignancies (eg, melanoma; cancers of the breast, prostate, and kidney; in addition to hematologic cancers).1 In some cases the response rates have been impressive and no adverse autoimmunity has been observed.2 A major strategic difficulty associated with the abovementioned trials relates to the choice of the best-suited peptide antigens. Many of the peptide epitopes used in vaccination trials are melanocyte specific and these peptides can only be applied for melanoma.3 More important, the vast majority of the antigens described thus far are not vital for survival and growth of the tumor cells, and immunoselection of antigen-loss variants may therefore prove to be an additional obstacle for the clinical applicability of most of the known peptide epitopes. In this respect, the development of acquired antigen loss during immunotherapy has been demonstrated in several cases.4,5 Obviously, the development of loss-variant tumor cells implies that these cells acquire a pronounced growth advantage and are left unaffected by further treatment. The development of peptide vaccines with a broader immunotherapeutic potential and with a low probability of acquisition of immune escape over the course of treatment therefore awaits the molecular characterization of more appropriate antigens. The identification of tumor antigens, which are essential for the survival of tumor cells, represents a new avenue to prevent antigen loss variants emerging due to selection, particularly during therapy.
Resistance to a wide variety of chemotherapeutic agents poses a major obstacle in the treatment of cancer. To this end, intrinsic or acquired drug resistance represents a general characteristic observed in virtually every type of tumor (with few exceptions), with every known type of anticancer chemotherapeutic drug. The mechanisms contributing include drug inactivation and extrusion of the drug by cell membrane pumps, mutations of drug targets, and failure to initiate apoptosis.6-9 The latter may result from a variety of conditions (eg, overexpression of the antiapoptotic molecules Bcl-2 and survivin).10,11 The Bcl-2 family comprises several key players in the regulation of apoptosis and includes both proapoptotic as well as antiapoptotic molecules.12-14 Although a precise understanding of how Bcl-2 exerts its antiapoptotic effects remains to be elucidated, Bcl-2 is overexpressed in many cancers including lung, colorectal, prostate, and breast, as well as in leukemias and lymphomas.15-20 Bcl-2 protects cells from a wide range of cytotoxic insults, including cytokine deprivation, UV- and γ-irradiation, and chemotherapeutic drugs.21 Hence, Bcl-2 is a critical cellular factor contributing to the pathogenesis and progression of cancer. Consequently, Bcl-2 has become a very attractive target for the design of new anticancer drugs,22-24 and antisense oligonucleotides and small-molecule Bcl-2 inhibitors are currently being evaluated in clinical trials in different cancers.25 Additionally, this suggests Bcl-2 as a suitable target for anticancer immunotherapy. In the present study, we examined the natural cellular immunogenicity of Bcl-2 in patients with cancer.
Patients, materials, and methods
Patients
Peripheral blood lymphocytes (PBLs) were isolated using Lymphoprep separation, HLA typed (Department of Clinical Immunology, University Hospital, Copenhagen, Denmark), and frozen in fetal calf serum (FCS) with 10% dimethyl sulfoxide (DMSO). None of the patients received immunotherapy prior to sampling of blood. Informed consent was obtained from the patients and controls prior to any of theses measures according to the Declaration of Helsinki. This study was approved by the Danish Ethical Committee.
PBLs were collected from 13 HLA-A2–positive patients with breast cancer presenting with progressive disease with distant metastases defining stage IV disease; the majority of patients had more than 1 tumor location (8/13 patients). Prior treatment included chemotherapy, endocrine therapy, and radiation therapy. Eight patients were previously treated with chemotherapy, whereas 5 patients had only received endocrine therapy and no chemotherapy prior to study inclusion. Furthermore, 12 HLA-A2–positive patients with localized operable breast cancer were included and blood samples were collected prior to primary surgery and chemotherapy. Additionally, PBLs were collected from 2 HLA-A2–positive pancreatic patients with cancer presenting with progressive disease with distant metastases defining stage IV disease. Finally, PBLs from 10 HLA-A2 patients with newly diagnosed chronic lymphocytic leukemia (CLL) and 3 patients with acute myeloid leukemia (AML) were collected prior to therapy. PBLs from 12 HLA-A2–positive healthy individuals served as controls.
Assembly assay for peptide binding to MHC class I molecules
The binding affinity of the synthetic peptides (Invitrogen, Carlsbad, CA) to HLA-A2 molecules, metabolically labeled with [35S]-methionine, was measured in the assembly assay, as described previously.26 The assay is based on stabilization of the class I molecule after loading of different concentrations of peptide to the transporter associated with antigen processing (TAP)–deficient cell line T2. Stably folded HLA molecules were immune-precipitated using the HLA class I–specific, conformation-dependent monoclonal antibody (mAb) W6/32 and separated by isoelectric focusing (IEF) gel electrophoresis. Major histocompatibility complex (MHC) heavy-chain bands were quantified using the ImageGauge Phosphorimager program (FUJI Photo Film, Carrollton, TX). The intensity of the band is directly related to the amount of peptide-bound class I MHC complex recovered during the assay. Subsequently, the extent of stabilization of HLA-A2 is directly related to the binding affinity of the added peptide. The recovery of HLA-A2 was measured in the presence of 100, 10, 1, 0.1, and 0.01 μM of the relevant peptide. The C50 value was calculated for each peptide as the peptide concentration sufficient for half-maximal stabilization.
Antigen stimulation of PBLs
To extend the sensitivity of the enzyme-linked immunospot (ELISPOT) assay, PBLs were stimulated once in vitro prior to analysis.27,28 At day 0, PBLs were thawed and plated in 2 mL/well at a concentration of 2 × 106 cells in 24-well plates (Nunc, Roskilde, Denmark) in X-vivo medium (Bio Whittaker, Walkersville, MD) with 5% heat-inactivated human serum (HS) in the presence of 10 μM of peptide. Two days later, 20 IU/mL recombinant interleukin-2 (IL-2; Chiron, Ratingen, Germany) was added to the cultures. The cultured cells were tested for reactivity in the ELISPOT on day 10.
IFN-γ ELISPOT assay
The ELISPOT assay was used to quantify peptide epitope–specific interferon γ (IFN-γ)–releasing effector cells as described previously.29 Briefly, nitrocellulose-bottomed 96-well plates (MultiScreen MAIP N45; Millipore, Hedehusene, Denmark) were coated with anti–IFN-γ antibody (1-D1K; Mabtech, Nacka, Sweden). The wells were washed and blocked by X-vivo medium before adding 104 stimulator T2 cells (with or without 10 μM peptide) and effector cells at different concentrations. The plates were incubated overnight. The following day, medium was discarded and the wells were washed prior to addition of biotinylated secondary antibody (7-B6-1-Biotin; Mabtech). The plates were incubated for 2 hours, washed, and avidin-enzyme conjugate (AP-Avidin; Calbiochem, Life Technologies, San Diego, CA) was added to each well. Plates were incubated at room temperature (RT) for 1 hour and the enzyme substrate NBT/BCIP (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate; Gibco, Life Technologies, Carlsbad, CA) was added to each well and incubated at RT for 5 to 10 minutes. The reaction was terminated by washing with tap water upon the emergency of dark purple spots. The spots were counted using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers, Cleveland, OH) and the peptide-specific cytotoxic T lymphocyte (CTL) frequency could be calculated from the numbers of spot-forming cells. All assays were performed in triplicate for each peptide antigen.
Granzyme B ELISPOT
The granzyme B (GrB) ELISPOT assay was used for measuring antigenspecific CTL cytotoxicity as described.30 Briefly, nitrocellulose-bottomed 96-well plates (MultiScreen MAIP N45; Millipore) were coated with GrB capture antibody (BD Biosciences, Brondby, Denmark). The wells were washed and blocked by X-vivo medium with 5% HS. The cells were added at different cell concentrations. T2 cells and peptides were then added to each well and the plates were incubated 4 hours, medium was discarded, and the wells were washed prior to addition of GrB detection antibody (BD Biosciences). The plates were incubated for 2 hours, washed, and avidin horseradish peroxidase (BD Biosciences) was added to each well. Plates were incubated at RT for 1 hour and 3-amino-9-ethylcarbazole (AEC) substrate reagent (BD Biosciences) was added to each well and incubated at RT for 5 to 10 minutes. The reaction was terminated by washing with tap water upon the emergency of red spots. The spots were counted and the peptide-specific CTL frequency was calculated like for the IFN-γ ELISPOT. All assays were performed in duplicate or triplicate for each peptide antigen.
Isolation of peptide-specific T cells
Antigen-specific cells were isolated by means of Bcl208/HLA-A2–coated magnetic beads as previously described.31 Biotinylated monomers (ProImmune, Oxford, United Kingdom) were coupled to streptavidin-coated magnetic beads (Dynabeads M-280; Dynal A/S, Oslo, Norway) by incubating 2.5-μg monomers with 5 × 106 beads in 40 μL phosphate-buffered saline (PBS) for 20 minutes at RT. The magnetic complexes were washed 3 times in PBS in a magnetic field (Dynal A/S) and subsequently mixed with PBLs at a ratio of 1:10 in PBS with 5% bovine serum albumin (BSA) and rotated very gently for 1 hour. Antigen-specific CD8+ T cells associating with the magnetic complexes were gently washed 3 times. Isolated cells were resuspended numerous times in X-vivo with 5% HS and incubated for 2 hours before the magnetic beads were released and removed from the cell suspension. The isolated cells were cultured in a 48-well plate in X-vivo, 5% HS, and 106 anti-CD28–coated and anti-CD3–coated artificial cell–based antigen-presenting cells (K32/41BBL) that express 4-1BB ligand (4-1BBL; kindly provided by Dr Carl H. June, Department of Pathology and Laboratory Medicine, University of Pennsylvania).32 One day after isolation, 20 units/mL IL-2 was added, and on day 5 the capacity of these cells to kill target cells was tested in standard 51Cr-release assays.
Cloning by limiting dilution
CTL clones were established from the isolated cultures by limiting dilution in 96-well plates using irradiated peripheral blood mononuclear cells (PBMCs) as feeder cells in the presence of 40 IU/mL IL-2 and 1 μg/mL phytohemagglutinin (PHA) in X-vivo with 5% HS. Fresh medium and IL-2 were added to the clones every 3 to 4 days.
Cytotoxicity assay
Conventional [51Cr]-release assays for CTL-mediated cytotoxicity were carried out as described elsewhere.33 Target cells were T2 cells with or without the relevant peptide, the HLA-A2–positive breast cancer cell line MDA-MB-231, and the HLA-A2–negative breast cancer cell line ZR75-1. Both breast cancer cell lines expressed Bcl-2 as examined by reverse transcription–polymerase chain reaction (PCR) (data not shown).
Results
Binding of Bcl-2–derived peptides to HLA-A2
The amino acid sequence of the Bcl-2 protein was screened for the most probable HLA-A2 nonamer and decamer peptide epitopes, using the main HLA-A2–specific anchor residues.34 Thirteen Bcl-2–deduced peptides were synthesized and examined for binding to HLA-A2 by comparison with the HLA-A2 high-affinity positive control epitope from HIV-1 pol476-484 (ILKEPVHGV) by the assembly assay. The peptide concentration required for half-maximal recovery of class I MHC molecules (C50 value) was 0.7 μM for the HIV-1 pol476-484 (Table 1). Six Bcl-2–derived peptides bound with almost similar high affinity as the positive control: bcl224, bcl85, bcl222, bcl218, bcl220, and bcl124 (C50 = 0.7, 1, 1, 2, 1, and 1 μM, respectively; Table 1). The peptides bcl80, bcl208, and bcl180 bound only with intermediate affinity (C50 = 36, 7, and 10 μM, respectively), whereas the 2 peptides bcl214 and bcl172 only bound very weakly (C50 > 100 μM). bcl216 and bcl200 did not show any measurable binding to HLA-A2 (Table 1).
Peptides examined in this study
Protein* . | Sequence . | C50, μM† . |
|---|---|---|
| HIV-1 pol476 | ILKEPVHGV | 0.7 |
| Bcl224 | A L V G A C I T L | 0.7 |
| Bcl85 | A L S P V P P V V | 1 |
| bcl222 | S L A L V G A C I | 1 |
| bcl218 | K T L L S L A L V | 2 |
| bcl220 | L L S L A L V G A | 1 |
| bcl214 | W L S L K T L L S L | > 100 |
| bcl80 | A A A G P A L S P V | 36 |
| bcl216 | S L K T L L S L A L | Not binding |
| bcl208 | P L F D F S W L S L | 7 |
| bcl124 | F T A R G R F A T V | 1 |
| bcl180 | Y L N R H L H T W I | 10 |
| bcl172 | N I A L W M T E Y L | > 100 |
| bcl200 | E L Y G P S M R P L | Not binding |
Protein* . | Sequence . | C50, μM† . |
|---|---|---|
| HIV-1 pol476 | ILKEPVHGV | 0.7 |
| Bcl224 | A L V G A C I T L | 0.7 |
| Bcl85 | A L S P V P P V V | 1 |
| bcl222 | S L A L V G A C I | 1 |
| bcl218 | K T L L S L A L V | 2 |
| bcl220 | L L S L A L V G A | 1 |
| bcl214 | W L S L K T L L S L | > 100 |
| bcl80 | A A A G P A L S P V | 36 |
| bcl216 | S L K T L L S L A L | Not binding |
| bcl208 | P L F D F S W L S L | 7 |
| bcl124 | F T A R G R F A T V | 1 |
| bcl180 | Y L N R H L H T W I | 10 |
| bcl172 | N I A L W M T E Y L | > 100 |
| bcl200 | E L Y G P S M R P L | Not binding |
The value range listed in subscript indicates the position of the first amino acid in the sequence
The C50 value is the concentration of the peptide required for half-maximal binding to HLA-A2
CTL responses against Bcl-2–derived peptides in patients with cancer and healthy individuals
Using the ELISPOT IFN-γ secretion assay, we examined peripheral blood T cells from different patients with cancer and healthy individuals for the presence of specific T-cell responses against the Bcl-2–derived peptides. This method has previously been highly effective for identifying tumor-specific CTLs in patients with cancer.35-37
PBLs from 13 HLA-A2–positive, late-stage patients with breast cancer were stimulated once in vitro before examination by ELISPOT. This procedure was chosen to extend the sensitivity of the ELISPOT as described.35,38 Since many described CTL epitopes are in fact low-affinity peptides, we included all HLA-A2–binding Bcl-2–deduced peptides in the first line of experiments. Responses were detected against bcl172, bcl180, bcl208, and bcl214 (Figure 1), whereas we could not detect a response against any of the high-affinity peptides bcl85, bcl218, bcl220, bcl222, bcl224, or bcl124 (Figure 2). Spontaneous CTL responses were detected against bcl180 in PBLs from 4 of the patients (≈31%; Figure 1B). However, more frequent responses were detected against bcl172, bcl208, and bcl214, since 8 of the patients (≈62%) showed a bcl172 response (Figure 1A), 10 (≈77%) of the patients hosted a CTL response against bcl208 (Figure 1C), and 9 of the patients (≈69%) hosted a response against the bcl214 peptide (Figure 1D). Furthermore, the PBLs from this group of late-stage patients with breast cancer were tested against the irrelevant peptide HIV-1 pol476-484. Data from these experiments showed highly comparable results when testing unpulsed T2 cells versus T2 cells pulsed with HIV-1 pol476-484, even after a 10-day stimulation of the PBLs with the HIV-1 pol476-484 peptide (data not shown).
T-cell responses against Bcl-2 as measured by IFN-γ ELISPOT. PBLs from 12 healthy individuals, 13 patients with primary breast cancer, 13 patients with late-stage breast cancer, and 2 patients with late-stage pancreatic cancer were analyzed. All individuals were HLA-A2 positive. The peptides Bcl172 (A), Bcl180 (B), Bcl208 (C), and Bcl214 (D) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
T-cell responses against Bcl-2 as measured by IFN-γ ELISPOT. PBLs from 12 healthy individuals, 13 patients with primary breast cancer, 13 patients with late-stage breast cancer, and 2 patients with late-stage pancreatic cancer were analyzed. All individuals were HLA-A2 positive. The peptides Bcl172 (A), Bcl180 (B), Bcl208 (C), and Bcl214 (D) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
Lack of spontaneous T-cell responses against high-affinity HLA-A2–binding peptides. PBLs from 8 healthy individuals and 8 patients with late-stage breast cancer were analyzed. All individuals were HLA-A2 positive. The peptides bcl85 (A), bcl218 (B), bcl220 (C), bcl222 (D), bcl224 (E), and bcl124 (F) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). All individuals were nonresponding. Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation < 25 per 105 lymphocytes) are indicated by  , whereas
, whereas  indicates nonresponding individuals.
indicates nonresponding individuals.
Lack of spontaneous T-cell responses against high-affinity HLA-A2–binding peptides. PBLs from 8 healthy individuals and 8 patients with late-stage breast cancer were analyzed. All individuals were HLA-A2 positive. The peptides bcl85 (A), bcl218 (B), bcl220 (C), bcl222 (D), bcl224 (E), and bcl124 (F) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). All individuals were nonresponding. Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation < 25 per 105 lymphocytes) are indicated by  , whereas
, whereas  indicates nonresponding individuals.
indicates nonresponding individuals.
Additionally, PBLs from 13 HLA-A2–positive patients with primary localized breast cancer were examined in a similar matter by the ELISPOT. However, either no or very weak responses were detected (Figure 1). Furthermore, we examined PBLs from 2 pancreatic cancers and identified that both patients hosted a CTL response against the bcl208 and bcl214 peptides (Figure 1C-D). Similarly, PBLs from 12 healthy HLA-A2–positive individuals were examined (Figure 1). Surprisingly, a weak CTL response was detected against the bcl208 peptide in one of the healthy individuals (Figure 1C).
CTL responses against Bcl-2–derived peptides in patients with leukemia
To examine whether Bcl-2–specific T cells were also present in PBLs from patients with leukemia, we examined PBLs from 10 HLA-A2–positive patients with CLL and 3 patients with AML for reactivity against the 2 peptides bcl208 and bcl214. Bcl-2 responses were present in 5 of the patients with CLL and 2 of the patients with AML (Figure 3).
T-cell responses against Bcl-2 as measured by IFN-γ ELISPOT. PBLs from 10 HLA-A2–positive patients with CLL and 3 HLA-A2–positive patients with AML were analyzed. The peptides Bcl208 (A) and Bcl214 (B) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
T-cell responses against Bcl-2 as measured by IFN-γ ELISPOT. PBLs from 10 HLA-A2–positive patients with CLL and 3 HLA-A2–positive patients with AML were analyzed. The peptides Bcl208 (A) and Bcl214 (B) were examined. T lymphocytes were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide. The average number of peptide-specific spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
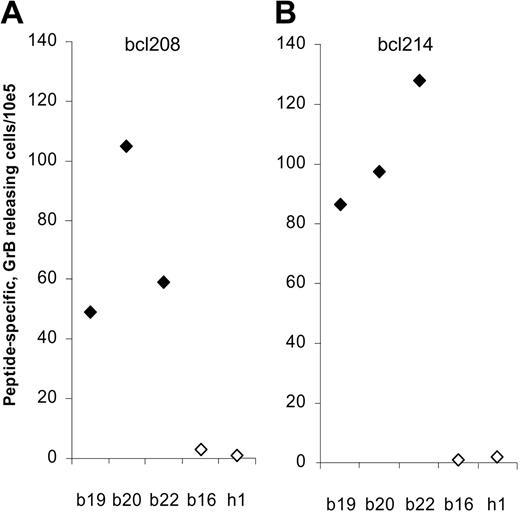
Bcl-2–specific GrB release in PBLs
Using the GrB ELISPOT we assessed whether the bcl-2–specific T cells detected in PBLs exhibit cytotoxic function. Thus, PBLs from 3 of the bcl-2–reactive patients with breast cancer (patient nos. 19, 20, and 22) were analyzed for reactivity against the 2 epitopes bcl208 and bcl214 (Figure 4). In all 3 patients, responses against both peptides could be detected with a frequency at about 50 to 140 peptide-specific CTLs per 105 PBLs. As controls we included a patient (patient no. 16) in which we could only detect a response against bcl172 but not against bcl208 or bcl214 in the IFN-γ ELISPOT and a healthy control (h1). As expected, no GrB release was detected against bcl208 or bcl214 in either the breast cancer patient no. 16 or the healthy control.
Detection of Bcl-2–specific CTLs by granzyme B ELISPOT. T lymphocytes from 4 different late-stage patients with breast cancer (b19, b20, b22, b16) and a healthy control (h1) were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide Bcl208 (A) or Bcl214 (B). The average number of peptide-specific granzyme B spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
Detection of Bcl-2–specific CTLs by granzyme B ELISPOT. T lymphocytes from 4 different late-stage patients with breast cancer (b19, b20, b22, b16) and a healthy control (h1) were stimulated once with peptide before being plated at 105 cells per well in triplicates either without or with peptide Bcl208 (A) or Bcl214 (B). The average number of peptide-specific granzyme B spots (after subtraction of spots without added peptide) was calculated for each patient using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzers). Responders (defined as average number of antigen-specific spots ± 1/2 standard deviation > 25 per 105 lymphocytes) are marked as  , whereas nonresponding individuals are marked as ⋄.
, whereas nonresponding individuals are marked as ⋄.
The functional capacity of Bcl-2–reactive CTLs
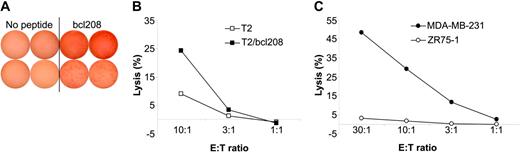
To further characterize the functional capacity of Bcl-2–reactive CTLs, these cells were enriched by means of magnetic beads coated with HLA-A2/bcl208 complexes as described.39-41 Cells were stimulated once with peptide in vitro prior to isolation. A small fraction of the isolated cells were cloned by limiting dilution. The expanding cultures were examined for recognition of T2 cells either without peptide or pulsed with bcl208 in a GrB ELISPOT. Several of these clones showed specific recognition of bcl208-pulsed T2 cells. Two examples are given in Figure 5A; however, unfortunately, we were not able to expand these clones for further analysis.
Cytolytic capacity of Bcl-2–specific CTLs. bcl208-reactive CTLs were isolated from PBLs from a breast cancer patient using HLA-A2/bcl208-coated magnetic beads. (A) A small fraction of these cells was cloned by limiting dilution and expanded. Response of 2 such clones (top and bottom rows) as measured in a GrB ELISPOT against no peptide or the bcl208 peptide. Each experiment was performed in duplicate with 103 effector cells/well. (B) The isolated bulk culture was analyzed for specific lysis of T2 cells with (▪) or without (□) bcl208 peptide. (C) Lysis by bcl208-isolated T cells of the HLA-A2–positive breast cancer cell line MDA-MB-231 (•) and the HLA-A2–negative breast cancer cell line ZR75-1 (○). E/T indicates effector-target ratio.
Cytolytic capacity of Bcl-2–specific CTLs. bcl208-reactive CTLs were isolated from PBLs from a breast cancer patient using HLA-A2/bcl208-coated magnetic beads. (A) A small fraction of these cells was cloned by limiting dilution and expanded. Response of 2 such clones (top and bottom rows) as measured in a GrB ELISPOT against no peptide or the bcl208 peptide. Each experiment was performed in duplicate with 103 effector cells/well. (B) The isolated bulk culture was analyzed for specific lysis of T2 cells with (▪) or without (□) bcl208 peptide. (C) Lysis by bcl208-isolated T cells of the HLA-A2–positive breast cancer cell line MDA-MB-231 (•) and the HLA-A2–negative breast cancer cell line ZR75-1 (○). E/T indicates effector-target ratio.
One day after isolation, IL-2 was added to the remaining cells, and on day 5 the capacity of the cells to kill peptide-loaded T2 cells was tested in standard 51Cr-release assays. To this end, either unloaded T2 cells or T2 cells loaded with bcl208 peptide served as targets. This assay revealed that only T2 cells pulsed with bcl208 were killed (Figure 5B). These enriched and in vitro–stimulated bcl208-reactive T cells were further used to test the capacity to kill the HLA-A2–positive, Bcl-2–expressing breast cancer cell line MDA-MB-231. The enriched T cells efficiently lysed the MDA-MB-231 cells, whereas, in contrast, no cytotoxicity was observed against the Bcl-2–expressing, HLA-A2–negative breast cancer cell line ZR75-1 (Figure 5C).
Discussion
Ideal targets for immunotherapy are gene products silenced in normal tissues, overexpressed in cancer cells, and directly involved in tumor cell survival and progression. The overexpression of Bcl-2 in cancers of different origin implies that immune escape by down-regulation or loss of expression of this protein would impair sustained tumor cell growth. These characteristics highlight Bcl-2 as a very attractive target for immunotherapy of cancer. To sustain the hypothesis of Bcl-2 as a broadly expressed T-cell target, we initially followed a “reverse immunology” approach. We scanned the Bcl-2 protein for the presence of HLA-A2 peptide–binding motifs and used these to search for specific T-cell responses in patients with cancer. To this end, spontaneous T-cell reactivity was detected against Bcl-2 in patients suffering from unrelated tumor types (ie, pancreatic cancer, breast cancer, AML, and CLL). These data indicate that CTL-defined Bcl-2 epitopes might be broadly applicable in therapeutic vaccinations against cancer and are therefore of substantial immunotherapeutic value.
We identified strong CTL responses against 4 peptide epitopes (ie, the intermediate HLA-A2–binding peptides bcl172 and bcl208 and the weaker HLA-A2–binding peptides bcl214 and bcl180). Taken together, 11 of the patients with breast cancer possessed Bcl-2–specific CTLs, 8 of these patients were previously treated with at least one type of chemotherapy. In 2 patients (patient nos. 14 and 17), no CTL responses to the 4 different Bcl-2 peptides were detectable. Both patients had previously received antihormonal therapy but no chemotherapy. Similarly, we were not able to detect any responses in patients with primary localized breast cancer prior to chemotherapy. Thus, in patients with breast cancer, responses were only detected in the patients who had received chemotherapy. Although, tumor load may play an important role, this might indicate that the immune responses are introduced or increased as a consequence of the treatment-induced increase of Bcl-2 expression. Although this notion needs to be substantiated in large-scale studies in which the clinical and prognostic significance of anti–Bcl-2 CTL responses is scrutinized, it could point to a scenario in which the combination of a Bcl-2–based immunotherapy with cytotoxic chemotherapy might in a synergy improve current response rates.
The IFN-γ ELISPOT assay is one of the most useful techniques for immunologic monitoring of CTL responses and has gained increased application as a measure of specific T-cell activation. However, it does not assess cell-mediated cytotoxicity directly as IFN-γ secretion is not limited to only cytolytic cells. Thus, although it has been shown that IFN-γ ELISPOT reactivity in most cases correlates with the capacity to exhibit cytotoxic function, the formal proof for this notion can only be obtained directly. GrB is a key mediator of target cell death via the granule-mediated pathway and the GrB ELISPOT assay was recently demonstrated to provide an estimation of cytotoxic effector cell frequency.42 Additionally, unlike the IFN-γ ELISPOT assay, the GrB ELISPOT directly measures the release of a cytolytic protein. In the present study we took advantage of the GrB ELISPOT assay to demonstrate that the Bcl-2–specific CTLs in the patients' PBLs are indeed cytotoxic effector cells. To further prove this notion, we enriched Bcl-2–reactive T cells from patient PBLs and showed that the resulting T-cell line was able to lyse peptide-pulsed T2 cells in a conventional 51Cr-release assay. Moreover, this Bcl-2–reactive T-cell line was capable of killing an HLA-matched breast cancer cell line, whereas HLA-A2–negative target cells were not killed. These findings strongly indicate that cancer cells indeed process and present the Bcl-2 peptide in the context of the HLA-A2 molecule. Finally, we were able to clone these isolated cells and showed that they reacted highly specifically against the Bcl-2 peptide epitope.
Surprisingly, a weak response against one of the Bcl-2 epitopes was detected in a healthy donor. The significance of this finding is unclear, since none of the donors included in the present study showed any signs of autoimmunity. In that regard, it is well established that circulating CTL precursors (CTLp's) against melanocyte differentiation antigens like melanoma antigen recognized by T cells 1 (MART-1), glycoprotein 100 (gp100), and tyrosinase can be detected not only in patients with melanoma but also in some healthy donors.43-45 Available data along this line of research suggests that antitumor CTLp's in healthy donors display a naive phenotype, whereas such cells are antigen experienced in patients with cancer.43 Unfortunately, we were not able to address this question in the present study.
The frequency of Bcl-2–reactive CTLs in PBLs from patients with cancer is low compared with known viral responses (eg, Epstein-Barr virus [EBV] or influenza) and may be insufficient to generate any clinically significant immune response. However, the frequency of Bcl-2–specific CTLs might increase due to vaccination therapy. Since a number of human cancers express high levels of Bcl-2, immunotherapeutic strategies targeting this antigen may have broad clinical applications. The major concern of such an approach would be the induction of autoreactive immune responses. Thus, the future of Bcl-2–based vaccination will depend on both the therapeutic efficacy and on the type of side effects that may follow immunization. When peptides derived from melanocyte differentiation antigens were first used to treat patients with stage IV melanoma, it was envisioned that this might lead to pronounced destruction of melanocytes, which in turn would manifest clinically (ie, vitiligo or retinitis). However, clinical experience demonstrated that the incidence of vitiligo in patients receiving vaccinations was not significantly higher than the incidence of melanoma-associated hypopigmentation in patients receiving other forms of therapy. Additionally, no serious side effects have been reported in various vaccination trials against selfantigens.1 Nevertheless, the safety of therapeutic vaccination against cancer through targeting of Bcl-2 awaits the initiation of clinical trials, which in addition is the only way to determine if Bcl-2 truly is a tumor rejection antigen (ie, if the cellular immune response against Bcl-2 may be protective or therapeutic in patients with cancer).
Bcl-2 is only one member of a family of proteins that regulates apoptosis,13,14 hence it seems likely that cellular immune responses also exist or can be introduced against other members of the Bcl-2 protein family (eg, myeloid cell factor–1 [Mcl-1] or B-cell lymphoma XL [Bcl-XL], which also is related to drug resistance).12-14
Tanaka et al46 described that the presence of another inhibitor-ofapoptosis protein survivin in breast carcinoma was strongly associated with expression of Bcl-2 and with a reduced apoptotic index (AI) and poor overall survival. A similar association between survivin and Bcl-2 has been described in neuroblastoma,47 gastric cancer,48 colorectal cancer,49 and high-grade non-Hodgkin lymphoma.46 Thus, in most human cancers, inhibition of apoptosis is a general feature, and expression of antiapoptotic proteins (eg, survivin and/or Bcl-2) may cause more pronounced antiapoptotic effects, as reflected in reduced AI. Recently, we and others described that survivin is the target for spontaneous T-cell reactivity in patients with various cancers.31,35,41,50-53 The safety and potential efficacy of survivin-derived peptides in therapeutic vaccinations against cancer is currently being investigated in phase 1/2 clinical trials (Dr Andreas Eggert, Würzburg University Hospital; oral communication, April 4, 2004). Thus, an exciting immunotherapeutic strategy would be to target both Bcl-2 and survivin, especially since they execute their antiapoptotic function through different cellular pathways.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-07-2548.
Supported by grants from the Danish Medical Research Council, The Novo Nordisk Foundation, The Danish Cancer Society, The John and Birthe Meyer Foundation, Danish Cancer Research Foundation, and Christian og Ottilia Brorsons rejselegat.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Merete Jonassen for excellent technical assistance. We further extend our thanks to all the patients who donated blood to perform these studies.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal