Abstract
Interleukin 15 (IL-15) is a critical factor for the proliferation and activation of natural killer (NK) and CD8 T cells. Recently, we demonstrated that IL-15Rα expressed on monocytes/dendritic cells captures and presents IL-15 to neighboring cells in trans (trans-presentation of IL-15) through cell-cell contact. In the current study, we provide evidence that the IL-15 presented in trans, but not soluble IL-15 at physiologic concentrations, augments the killing activity mediated by NK cells in vitro. In addition, transfection of IL-15Rα into a colon carcinoma cell line (MC38) enabled these cells to present IL-15 in trans to NK cells and augmented their killing activity, resulting in the efficient lysis of MC38 cells by NK cells in vitro. Furthermore, these transfected MC38 cells no longer form fatal pulmonary metastases in mice. It was also shown that NK cells play an important role in the rejection of MC38 cells under these circumstances. These results collectively suggest that the IL-15 trans-presentation mechanism operates in vivo to augment the tumor immune surveillance mechanism. Furthermore, our observation provides the scientific basis for a novel strategy to prevent cancer development/metastasis.
Introduction
Interleukin 15 (IL-15) is a pivotal factor for memory CD8+ T1-5 and natural killer (NK) cells6-8 as IL-15-/-9 or IL-15Rα-/-10 mice have profoundly reduced numbers of these cells. IL-15 activates the killing activity of NK cells in vitro6,7 and in vivo.11 Thus, IL-15 could contribute to tumor surveillance mechanisms in vivo. Attempts to prevent tumor growth in mice by a bolus administration of IL-15 proved effective,12-15 although the high doses of IL-15 used to achieve meaningful effects in these experiments made the interpretation rather difficult in the physiologic context. We believe that the mode of the IL-15 action in vivo remains unclear as we made several observations inconsistent with the prevailing paradigms. First, IL-15 cannot be detected as a protein in biologic fluids, although the relevance of IL-15 in vivo was obviously demonstrated9,10 by the phenotypes manifested by IL-15-/- or IL-15Rα-/- mice. Second, the IL-15Rα receptor subunit alone bound IL-15 with high affinity (dissociation constant [Kd], 10-100 pM),16 suggesting that IL-15Rα binds IL-15 under physiologic conditions. IL-15Rα may transduce its own signal,17 but this conclusion requires confirmation in vivo. Third, no convincing demonstration has been made to determine whether the hetero-trimeric IL-15R (the receptor composed of α, β, and γc subunits) is indispensable for IL-15 function in vivo.
Ma and colleagues (Lodolce et al,18 Koka et al,19 and Burkett et al20 ) recently demonstrated that the expression of IL-15Rα on NK or CD8 memory T cells is dispensable for their development and survival in vivo. Simultaneously, we demonstrated that IL-15 and IL-15Rα form stable complexes on monocytes/dendritic cells under physiologic conditions21 and that the resultant complex could stimulate the proliferation of neighboring IL-2/IL-15Rβ-γc+ cells in trans even in the absence of their expression of IL-15Rα.22 The corollaries of the work are: 1) IL-15 may predominantly exist as a membrane factor in vivo, which explains why it is difficult to detect soluble IL-15 protein; 2) the trans-presentation mechanism eliminates the need for the IL-15Rα expression on the Taud NK (T/NK) cells in vivo, which explains the observations made by Lodolce et al.18
Here, we demonstrate that ex vivo–expanded NK cells do not express IL-15Rα so that even sub-nanomolar concentrations of soluble IL-15 cannot activate them. However, such NK cells could be led to proliferation by presentation of IL-15 in trans. Moreover, these NK cells efficiently killed tumor cells in vitro following the IL-15 presentation between cells. We then reasoned that a forced expression of the IL-15Rα molecule on engraftable tumor cells might enable them to capture and present IL-15 in trans to NK or cytotoxic T lymphocyte (CTL) cells and thereby induce efficient killer cells. As a result, we anticipated efficient lysis of the IL-15Rα–transfected tumor cells and suppression or elimination of the tumor growth in vivo. Indeed, murine colon carcinoma cells (MC3823 ) transfected with IL-15Rα no longer form metastases in wild-type (wt) mice, although they killed the IL-15-/- mice due to metastases. These data collectively demonstrate the relevance of IL-15 trans-presentation in the activation of NK cells and suggest that IL-15 presented in trans may play a major role in augmenting the host's immunosurveillance mechanism against tumor cells.
Materials and methods
Cells
MC38 is a metastatic murine colon cancer cell line syngenic to the C57BL6 strain of mice.23 PT-18 is a murine mast cell line.24,25 To establish IL-15Rα–positive clones, cells were electroporated with an expression construct containing murine IL-15Rα cDNA in the pEF-Neo vector26 (pEF-IL-15Rα-Neo), and the positive clones were selected by G418 selection. The truncated mutant of IL-15Rα was constructed by polymerase chain reaction (PCR), and the resultant expression construct in the pEF-Neo vector directed the expression of an IL-15Rα mutant, which has only the 3 initial amino acids (of 41 amino acids) of the cytoplasmic tail. The expression levels of IL-15Rα were confirmed by flow cytometry, using goat anti–mouse IL-15Rα antibody (N-19; Santa Cruz Biotech, Santa Cruz, CA). The same antibody was used for all flow cytometry experiments. Ex vivo NK cells were obtained by culturing bone marrow precursor cells with 20 to 70 nM IL-15 for 10 to 14 days. Mature dendritic cells were grown from bone marrow precursors in the presence of 100 UmL-1 granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ) for 8 to 10 days and were then stimulated with 1 μgmL-1 lipopolysaccharide (Calbiochem, La Jolla, CA) to augment the expression of the IL-15Rα molecule.
In vivo tumor growth studies
IL-15 transgenic (Tg) mouse was described elsewhere.11 These mice are on a C57BL6 background. Control C57BL6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). IL-15-/- mice9 were purchased from Taconic Farms (Germantown, NY). Age- and sex-matched C57 BL6 and IL-15 transgenic mice (8-10 weeks old) were injected with 2 million MC38 cells (or MC38 cells transfected with the mock pEF-Neo vector in some experiments) intravenously via the tail vein. The end point of the observation was determined either by the death of the mouse or a visible tumor mass of more than 1 cm in diameter. All experiments were conducted based on an animal experiment protocol preapproved by the National Institutes of Health (NIH) animal care and use committee.
In vivo depletion of NK or CD8 T cells
Rabbit polyclonal anti-asialo GM1 antibody (Wako Chemicals, Richmond, VA), reconstituted in 1 mL sterile water according to the manufacturer's instructions, was injected intraperitoneally into mice (in 30 μL volume) every 3 days, starting 9 days prior to the injection of MC38 cells and lasting throughout the course of the experiment. Similarly, 200 μg anti-CD8 antibody (clone 2.43; PharMingen, San Diego, CA) was repeatedly injected into mice (once per week) to remove CD8 T cells. The cell depletion was occasionally monitored by analyzing peripheral blood mononuclear cells by flow cytometry using the NK1.1 or CD8 marker.
Proliferation assay
Fifty thousand MC38 (or its clone) cells were cultured in the presence or absence of 10 nM IL-15 for 18 hours, followed by the addition of 1 μCi (0.037 MBq) 3H-thymidine for 6 additional hours. For the NK cell trans-proliferation assay, the PT-18/IL-15Rα+ clone cells received 50 Gy of γ-ray irradiation on the previous day of the experiment. They were incubated with serially diluted recombinant IL-15 for 1 hour and washed extensively to remove any residual soluble IL-15. Fifty thousand of these cells were then incubated with ex vivo–expanded NK cells (> 90% NK1.1 positive by flow cytometry, data not shown) in the absence of any soluble cytokine. Following 18 hours of incubation to allow the IL-15 presented in trans to NK cells, the culture was pulsed with 1 μCi (0.037 MBq) 3H-thymidine for 6 additional hours.
Lytic assay by ex vivo NK cells
Fifty thousand MC38 (or IL-15Rα+ clones) cells were radiolabeled with 0.1μCi (0.0037 MBq) Na 51CrO4 and simultaneously loaded with or without IL-15 (0.7 nM) for 6 hours. The radiolabeled IL-15 preloaded cells were extensively washed to remove any residual soluble IL-15 and were incubated with various numbers of NK cells (E:T ratio ranging from 10:1 to 0.3:1) for 6 hours before the 51Cr release was assessed. In some experiments, the lytic assay was shortened to 2 hours without significantly losing the effective cytotoxicity.
Micromagnetic resonance imaging
To visualize tumor growth in mice, micromagnetic resonance imaging (MRI) was conducted using a 1.5-T Signa LX superconductive magnetic unit (General Electric Medical Systems, Milwaukee, WI) equipped with a custom-made coil. Mice were anesthetized before the procedure. The T1-weighted 3D fast-spoiled gradient echo technique was used for data acquisition.
Results
IL-15 Tg mice rejected MC38 tumor cells in vivo
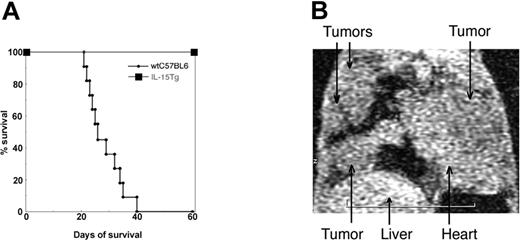
Previous reports described that administration of some members of the γc-user cytokines [ie, IL-2, -4, -7, -9, -15, and -21] effectively suppressed tumor growth in vivo.27 In line with this observation, we first determined whether mice constitutively expressing IL-15 (IL-15 Tg mice11 ) displayed an augmented resistance to tumor growth. We chose MC38 colon carcinoma cells,23 which always form fatal pulmonary metastases in syngenic C57BL/6 mice. Wild-type C57BL6 mice, following intravenous injection of MC38 cells, died within 6 weeks (n = 11, between 23 and 41 days). However, IL-15 Tg mice (n = 8) survived more than 8 months (Figure 1A). Pathologic examinations (data not shown) revealed no pulmonary metastasis in IL-15 Tg mice injected with MC38 cells, indicating that the overexpression of IL-15 helped mice effectively suppress the growth of MC38 cells in vivo. In the experiments that follow, we assessed the death or survival of the studied mice at the 60-day point after injection of tumor cells. In some experiments we confirmed the actual tumor development in live mice using micro-MRI imaging (Figure 1B).
(A) Survival of IL-15 Tg mice following injection with MC38 cells. (A) Survival curve describing MC-38–injected IL-15 Tg mice. Two million MC38 cells,23 a murine colon carcinoma cell line, were injected via the tail vain into C57BL/6 mice or IL-15 Tg mice11 (n = 7), and their survival was monitored over 8 months. (B) MC38 cells formed lung metastases in the IL-15 Tg mouse following the NK-cell depletion. Anti-asialo GM1 antibody was injected into IL-15 Tg mice every 3 days (from day -9 to day 45) to deplete NK cells, which were occasionally monitored by analyzing the peripheral blood cells from mice by flow cytometry. Micro-MR (magnetic resonance) images were collected using a 1.5-Tesla superconductive magnet unit (Signa LX, WI). The arrows indicate the location of metastatic MC38 tumor masses in the lungs of an NK-depleted IL-15 Tg mouse.
(A) Survival of IL-15 Tg mice following injection with MC38 cells. (A) Survival curve describing MC-38–injected IL-15 Tg mice. Two million MC38 cells,23 a murine colon carcinoma cell line, were injected via the tail vain into C57BL/6 mice or IL-15 Tg mice11 (n = 7), and their survival was monitored over 8 months. (B) MC38 cells formed lung metastases in the IL-15 Tg mouse following the NK-cell depletion. Anti-asialo GM1 antibody was injected into IL-15 Tg mice every 3 days (from day -9 to day 45) to deplete NK cells, which were occasionally monitored by analyzing the peripheral blood cells from mice by flow cytometry. Micro-MR (magnetic resonance) images were collected using a 1.5-Tesla superconductive magnet unit (Signa LX, WI). The arrows indicate the location of metastatic MC38 tumor masses in the lungs of an NK-depleted IL-15 Tg mouse.
NK cells played a major role in the rejection of MC38 cells in IL-15 Tg mice
Constitutive expression of IL-15 in mice results in the activation of NK cells and the expansion of CD8 T cells.11,28,29 We thus tried to determine which of these cell types contributed to the suppression of the MC38 growth in IL-15 Tg mice. Wt mice were injected with parental MC38 cells, and lymphocytes collected from spleen, liver, and peripheral blood compartments were analyzed by flow cytometry. Numbers of NK and CD8 T cells did not meaningfully change in tumor-bearing mice. In addition, expression of NK cell markers (asialo GM-1, shown; NK1.1, not shown) and a T-cell activation marker (CD69) on CD8 T cells were analyzed to assess the activation status of these cells. As shown in Figure 2, there was no meaningful induction of these markers on CD8 T cells during the tumor development, suggesting that MC38 tumor cells do not strongly activate the host T-cell–mediated immune system during their development into tumor in mice. To functionally address this issue, anti-asialo GM1 antibody was repeatedly injected (every 3 days for 9 weeks) into IL-15 Tg mice. Total depletion of NK cells was observed, as confirmed by periodical flow cytometric analyses (data not shown). Interestingly, this treatment rendered IL-15 Tg mice susceptible to MC38 tumor cell growth, as MC38 cells formed pulmonary metastases (Figure 1B) as well as lymph node metastases in NK-depleted IL-15 Tg mice. In contrast, depletion of CD8 T cells using anti-CD8 antibody from IL-15 Tg mice did not allow the growth of MC38 cells in these mice (data not shown), suggesting that NK cells rather than antigen-specific CTLs played a major role in the rejection of MC38 cells in IL-15 Tg mice.
Flow cytometry analyses of CD8 T cells from mice challenged by parental MC38 and its IL-15Rα–transfected clone. Lymphocytes were separated (day 1, day 4, day 7, and day 14 after injection) from spleens of wild-type mice challenged by MC38 cells or their IL-15Ra transfected clone (mIL-15Rα+ MC38) and were analyzed for their expression of surface markers (CD8 versus CD69 or CD8 versus Asialo GM1) by flow cytometry.
Flow cytometry analyses of CD8 T cells from mice challenged by parental MC38 and its IL-15Rα–transfected clone. Lymphocytes were separated (day 1, day 4, day 7, and day 14 after injection) from spleens of wild-type mice challenged by MC38 cells or their IL-15Ra transfected clone (mIL-15Rα+ MC38) and were analyzed for their expression of surface markers (CD8 versus CD69 or CD8 versus Asialo GM1) by flow cytometry.
IL-15 did not directly modulate the growth of MC38 cells
To rule out that IL-15 acted on MC38 cells and directly suppressing their growth in IL-15 Tg mice, rather than indirectly suppressed their growth by enhancing the host immune system, we first examined the expression of IL-15R components on MC38 cells. Reverse transcription (RT)–PCR (Figure 3A) failed to detect either any isoform of IL-15Rα or IL-2/15Rβ mRNA expression in MC38 cells, suggesting that IL-15 transduced no signal in MC38 cells. Furthermore, addition of recombinant IL-15 to MC38 cells in vitro did not modulate the proliferative responses of these cells (Figure 3B), even after an expression construct containing the human or murine IL-15Rα molecule was transfected into MC38 cells. Collectively, IL-15 does not seem to inhibit the growth of MC38 or that of IL-15Rα transfected MC38 cells in vitro or in vivo.
Evidence that IL-15 does not directly modulate the proliferation of MC38 cells. (A) No expression of IL-15Rα or IL-2/IL-15Rβ in MC38 cells. Expression of these molecules in MC38 cells was assessed by RT-PCR. Lanes: DNA marker (1-kb ladder; Invitrogen, Carlsbad, CA), MC38, CTLL2 (from left to right). Marker band size (from top to bottom) was 517/506 (doublet), 396, 344, 298, 220/201 (doublet). Due to the presence of multiple splice variants of the IL-15Rα transcript expressed by CTLL2 cells, at least 3 amplicons were detectable. In contrast, no band was amplified from MC38 mRNA. Amplification of the β-actin fragment from cDNA of MC38 or CTLL2 transcripts was confirmed as a loading control. Primer sets were IL-15Rα: SN, 5′-ATGGCCTCGCCGCAGCTCCGG-3′, AS, 5′-TAGAGATGGCCACTTTCGTCATTTTGG-3′; IL-2/IL-15Rβ: SN, 5′-CTT GGA GAT GCT GAT CCC TAG TAC-3′, AS, 5′-GGG AGC TCA GCT GGG AGA AGA ACT-3′. (B) Proliferative responses of MC38 cells and their IL-15Rα–transfected clones to IL-15. No significant proliferative responses were observed in the presence or absence of IL-15 with parental MC38 cells and their IL-15Rα–transfected clones.
Evidence that IL-15 does not directly modulate the proliferation of MC38 cells. (A) No expression of IL-15Rα or IL-2/IL-15Rβ in MC38 cells. Expression of these molecules in MC38 cells was assessed by RT-PCR. Lanes: DNA marker (1-kb ladder; Invitrogen, Carlsbad, CA), MC38, CTLL2 (from left to right). Marker band size (from top to bottom) was 517/506 (doublet), 396, 344, 298, 220/201 (doublet). Due to the presence of multiple splice variants of the IL-15Rα transcript expressed by CTLL2 cells, at least 3 amplicons were detectable. In contrast, no band was amplified from MC38 mRNA. Amplification of the β-actin fragment from cDNA of MC38 or CTLL2 transcripts was confirmed as a loading control. Primer sets were IL-15Rα: SN, 5′-ATGGCCTCGCCGCAGCTCCGG-3′, AS, 5′-TAGAGATGGCCACTTTCGTCATTTTGG-3′; IL-2/IL-15Rβ: SN, 5′-CTT GGA GAT GCT GAT CCC TAG TAC-3′, AS, 5′-GGG AGC TCA GCT GGG AGA AGA ACT-3′. (B) Proliferative responses of MC38 cells and their IL-15Rα–transfected clones to IL-15. No significant proliferative responses were observed in the presence or absence of IL-15 with parental MC38 cells and their IL-15Rα–transfected clones.
IL-15 trans-presentation augmented NK cell–mediated cytotoxicity against MC38 cells in vitro
Previously, we demonstrated that IL-15Rα expressed on activated monocytes can capture IL-15, which is then presented in trans to neighboring T cells.22 This concept led us to speculate that the expression of IL-15Rα on MC38 cells may enable these cells to present IL-15 to NK cells, thereby resulting in the augmented lysis of those MC38 cells through the activation of NK cells. To test this hypothesis, we first studied the expression of IL-15Rα on ex vivo–cultured NK cells. Flow cytometric analysis failed to detect IL-15Rα protein on these NK cells, whereas activated mature dendritic cells were strongly positive (Figure 4A). Consistently, these homogenous NK cells did not respond to picomolar concentrations of IL-15 (ie, concentrations of IL-15 needed to saturate the high-affinity IL-15Rαβγ hetero-trimer complex, if it existed) in vitro, although they did respond well to 10 pM IL-2 (Figure 4B), demonstrating that physiologic doses of soluble IL-15 should not activate these NK cells in vivo. Then, we determined whether the IL-15Rα+ donor cells can stimulate the proliferation of IL-15Rα- NK cells. Indeed, preloading of IL-15 to donor cells (PT-18/IL-15Rα) by incubating those cells with 0.5 nM IL-15, followed by extensive washing to remove any free IL-15, successfully rendered these donor cells capable of stimulating the proliferation of ex vivo IL-15Rα- NK cells (Figure 4C). To test whether the IL-15 trans-presentation augments NK-mediated cytotoxicity as well, MC38 cells transfected with or without murine IL-15Rα were preloaded with IL-15 by incubating them with soluble IL-15 (50 pM) during the 51Cr labeling procedure. These cells were then incubated with NK cells in the absence of any exogenous IL-15. As shown in Figure 4D, IL-15Rα+ MC38 cells preloaded with IL-15 were far more efficiently killed by NK cells than the parental MC38 or IL-15Rα+ MC38 cells without IL-15 preloading. We also tested different transfection clones of MC38 cells that expressed lower levels of IL-15Rα and confirmed that the expression levels of IL-15Rα correlated with the NK-mediated killing of these cells (Figure 4D). These results indicate that the cytotoxicity as well as proliferation of NK cells can be activated in trans by the membrane-bound IL-15 on the IL-15Rα+ MC38 cells. Furthermore, the obvious lack of IL-15Rα expression on NK cells and their inability, when isolated, to respond to picomolar concentrations of soluble IL-15 suggests that NK cells require IL-15 to be presented in trans for their proliferation and development into efficient killer cells in vivo.
Lack of IL-15Rα expression on NK cells. (A) Flow cytometry of IL-15Rα expression on ex vivo NK cells. NK cells were expanded from bone marrow precursors from C57BL/6 mice in the presence of 70 nM IL-15 for 10 days. The cells were stripped of any bound cytokine by treating briefly in acetic acid–phosphate-buffered saline (PBS; pH 3)22 and then stained using anti–IL-15Rα polyclonal antisera (Santa Cruz), followed by a staining with phycoerythrin (PE) anti–goat IgG (Sigma, St Louis, MO). As a positive control, dendritic cells were expanded from bone marrow precursors in the presence of 1 nM GM-CSF (PeproTech) and were stimulated by lipopolysaccharide (LPS; 1μgmL-1) and interferon-γ (10 nM) for 24 hours to augment their expression of IL-15Rα. (B) Lack of proliferative responses of NK cells to low doses of IL-15. NK cells expanded by IL-15 as described in panel A (> 90% were NK1.1+, data not shown) were incubated with the indicated concentrations of IL-15 for 20 hours and pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 4 hours to measure their DNA synthesis. More than 10 nM IL-15 was needed to induce meaningful proliferative responses in these NK cells, although less than 100 pM IL-2 induced an almost maximum proliferative response from these cells. (C) Induction of NK proliferation by the IL-15trans-presentation. PT-18 cells24,25 were transfected with a human IL-15Rα expression construct, and clones expressing this molecule were selected using G418. The IL-15Rα+ PT-18 cells were first irradiated by 50 Gy of x-ray on the previous day of the experiment and then incubated with indicated concentrations of soluble IL-15 for 60 minutes at 37°C. After an extensive wash with PBS, 100 000 of these IL-15–bearing PT-18 cells were added to equal numbers of NK cells and incubated for 20 hours, followed by a pulse with 1μCi (0.037 MBq)3[H] thymidine for 4 hours. A similar experiment was undertaken by incubating IL-15Rα+ MC38 cells with effector NK cells, but those MC38 cells were lysed almost instantaneously, so that we could not observe NK cell proliferation (data not shown). (D) Strong induction of NK-cell mediated killing of IL-15Rα+ MC38 cells. IL-15Rα+ or IL-15Rα- (parental) MC38 cells were incubated with 0.5 nM IL-15 for 2 hours concurrent with the 51Cr labeling prior to the cytotoxicity assay. The cells were then extensively washed in PBS to remove any free IL-15. Twenty-five thousand labeled (with IL-15 and 51Cr) cells were mixed with various numbers of effector NK cells for 6 hours, and the cell-bound and released radioactivity were measured.
Lack of IL-15Rα expression on NK cells. (A) Flow cytometry of IL-15Rα expression on ex vivo NK cells. NK cells were expanded from bone marrow precursors from C57BL/6 mice in the presence of 70 nM IL-15 for 10 days. The cells were stripped of any bound cytokine by treating briefly in acetic acid–phosphate-buffered saline (PBS; pH 3)22 and then stained using anti–IL-15Rα polyclonal antisera (Santa Cruz), followed by a staining with phycoerythrin (PE) anti–goat IgG (Sigma, St Louis, MO). As a positive control, dendritic cells were expanded from bone marrow precursors in the presence of 1 nM GM-CSF (PeproTech) and were stimulated by lipopolysaccharide (LPS; 1μgmL-1) and interferon-γ (10 nM) for 24 hours to augment their expression of IL-15Rα. (B) Lack of proliferative responses of NK cells to low doses of IL-15. NK cells expanded by IL-15 as described in panel A (> 90% were NK1.1+, data not shown) were incubated with the indicated concentrations of IL-15 for 20 hours and pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 4 hours to measure their DNA synthesis. More than 10 nM IL-15 was needed to induce meaningful proliferative responses in these NK cells, although less than 100 pM IL-2 induced an almost maximum proliferative response from these cells. (C) Induction of NK proliferation by the IL-15trans-presentation. PT-18 cells24,25 were transfected with a human IL-15Rα expression construct, and clones expressing this molecule were selected using G418. The IL-15Rα+ PT-18 cells were first irradiated by 50 Gy of x-ray on the previous day of the experiment and then incubated with indicated concentrations of soluble IL-15 for 60 minutes at 37°C. After an extensive wash with PBS, 100 000 of these IL-15–bearing PT-18 cells were added to equal numbers of NK cells and incubated for 20 hours, followed by a pulse with 1μCi (0.037 MBq)3[H] thymidine for 4 hours. A similar experiment was undertaken by incubating IL-15Rα+ MC38 cells with effector NK cells, but those MC38 cells were lysed almost instantaneously, so that we could not observe NK cell proliferation (data not shown). (D) Strong induction of NK-cell mediated killing of IL-15Rα+ MC38 cells. IL-15Rα+ or IL-15Rα- (parental) MC38 cells were incubated with 0.5 nM IL-15 for 2 hours concurrent with the 51Cr labeling prior to the cytotoxicity assay. The cells were then extensively washed in PBS to remove any free IL-15. Twenty-five thousand labeled (with IL-15 and 51Cr) cells were mixed with various numbers of effector NK cells for 6 hours, and the cell-bound and released radioactivity were measured.
Transfection of IL-15Rα to MC38 cells rendered these cells unable to form metastases in wild-type C57BL6 mice
These in vitro observations led us to postulate that the expression of IL-15Rα on MC38 cells might render them unable to grow efficiently in wild-type C57BL6 mice since these transfected tumor cells are capable of efficiently stimulating NK cells (and CTLs) through IL-15 trans-presentation. To test this hypothesis, wild-type C57BL6 mice were injected with IL-15Rα+ MC38 transfectant clones (2 clones, 1 expressing high levels and another with moderate levels of IL-15Rα; Figure 5A). Parental (IL-15Rα-) MC38 cells showed fatal growth in wt mice in 6 weeks (Figure 1), whereas the majority (6 of 7) mice injected with IL-15Rα+ (high) MC38 cells survived throughout the period of observation (60 days). Furthermore, nearly half (3 of 7) of the mice injected with IL-15Rα+ (moderate) MC38 cells survived for more than 60 days (Figure 5B). Thus, the transfection of IL-15Rα into MC38 cells led to a significant prolongation in the survival of mice that received these cells (P < .001). It should be noted that we observed a correlation between the level of IL-15Rα expression on MC38 and the duration of the survival of mice injected with these tumor cells. These results imply that the expression of IL-15Rα on the MC38 cancer cell could dose-dependently delay or prevent the formation of fatal metastases in vivo.
Survival curves of wt or NK-depleted C57BL/6 mice injected with parental and IL-15Rα-transfected MC38 cells. (A) Expression levels of IL-15Rα on MC38 transfectants. Flow cytometry data showing the expression levels of mouse IL-15Rα on MC38 transfectants. (Left) Parental MC38/isotype control staining of all MC38 clones; (middle) MC38-mouse IL-15Rα (moderate); (right) MC38-mouse IL-15Rα (high). (B) Survival of wt mice challenged by IL-15Rα–transfected MC38 cells. Two million parental or IL-15Rα (moderate or high expression)–transfected MC38cells were injected via the tail vain into C57BL/6 mice (n = 11 for parental MC38 injected mice, and n = 6 for mice injected with IL-15Rα+[moderate] or IL-15Rα+MC38 cells, n = 4 for NK-depleted wt mice injected with IL-15Rα+MC38), and their survival was monitored for 60 days. The survival of mice injected with IL-15Rα+ (high) MC38 cells was significantly longer (P < .001) than that of mice injected with the parental MC38 cells. The left panel shows the levels of IL-15Rα expression on these MC38 transfectants as assessed by flow cytometry. When the IL-15Rα+ MC 38 cells were injected into mice that had been depleted of either NK cells or CD8 T cells, only NK-depleted mice allowed the development of IL-15Rα+ (high) fatal MC38 cell tumors.
Survival curves of wt or NK-depleted C57BL/6 mice injected with parental and IL-15Rα-transfected MC38 cells. (A) Expression levels of IL-15Rα on MC38 transfectants. Flow cytometry data showing the expression levels of mouse IL-15Rα on MC38 transfectants. (Left) Parental MC38/isotype control staining of all MC38 clones; (middle) MC38-mouse IL-15Rα (moderate); (right) MC38-mouse IL-15Rα (high). (B) Survival of wt mice challenged by IL-15Rα–transfected MC38 cells. Two million parental or IL-15Rα (moderate or high expression)–transfected MC38cells were injected via the tail vain into C57BL/6 mice (n = 11 for parental MC38 injected mice, and n = 6 for mice injected with IL-15Rα+[moderate] or IL-15Rα+MC38 cells, n = 4 for NK-depleted wt mice injected with IL-15Rα+MC38), and their survival was monitored for 60 days. The survival of mice injected with IL-15Rα+ (high) MC38 cells was significantly longer (P < .001) than that of mice injected with the parental MC38 cells. The left panel shows the levels of IL-15Rα expression on these MC38 transfectants as assessed by flow cytometry. When the IL-15Rα+ MC 38 cells were injected into mice that had been depleted of either NK cells or CD8 T cells, only NK-depleted mice allowed the development of IL-15Rα+ (high) fatal MC38 cell tumors.
To determine whether the transfection of IL-15Rα on MC38 cells resulted in the loss of fatal pulmonary metastasis formation, we examined lungs of those mice by MRI. While all mice (n = 11) that received wild-type MC38 displayed massive growth of MC38 cells in their lungs (Figure 6A), no apparent pulmonary metastasis were detectable in mice injected with both high and moderate IL-15Rα+ MC38 cells (Figure 6B-C). Curiously, mice (n = 5) that were injected with IL-15Rα+ (moderate) MC38 cells developed palpable metastatic tumors in the peripheral and internal lymph nodes (see “Discussion”), which may be responsible for the death of nearly half of the mice that received the injection of IL-15Rα+ (moderate) MC38 cells. These results indicate that the expression of IL-15Rα on MC38 cells efficiently prevented fatal pulmonary metastases of MC38 cells in immune-competent wild-type C57BL6 mice, leading to the prolonged survival of the host. Overall, the in vivo observations seem to correlate well with the highly augmented killing activity of NK cells as a consequence of the IL-15 trans-presentation by IL-15Rα+ MC38 cells in vitro.
No pulmonary metastasis in C57BL/6 mice injected with IL-15Rα–transfected MC38 cells. Micro-MR images of lungs of C57/BL6 mice injected with parental or IL-15Rα–transfected (high or moderate expression) MC38 cells are shown. Parental MC38 cells formed fatal pulmonary metastases and almost filled the lungs of all of the injected mice (n = 11) (left). In contrast, no visible metastatic masses were observed in lungs of all mice (n = 6) injected with IL-15Rα+MC38 (images were taken at 28 days after injection; T indicates tumor).
No pulmonary metastasis in C57BL/6 mice injected with IL-15Rα–transfected MC38 cells. Micro-MR images of lungs of C57/BL6 mice injected with parental or IL-15Rα–transfected (high or moderate expression) MC38 cells are shown. Parental MC38 cells formed fatal pulmonary metastases and almost filled the lungs of all of the injected mice (n = 11) (left). In contrast, no visible metastatic masses were observed in lungs of all mice (n = 6) injected with IL-15Rα+MC38 (images were taken at 28 days after injection; T indicates tumor).
Expression of IL-15Rα on MC38 cells did not alter the tumor-forming capacity of these cells in vivo in the absence of IL-15
The efficient rejection of IL-15Rα+ MC38 cells in wt C57BL6 mice could be accounted for by the clonal differences of the tumorigenic capabilities between IL-15Rα- and IL-15Rα+ MC38 cells. The survival of mice and the expression levels of IL-15Rα on the injected MC38 cells showed a good correlation (Figure 5B), which argues against the clonal difference explanation. Nonetheless, we examined the tumorigenic capabilities of IL-15Rα+ and IL-15Rα- MC38 cells in IL-15-/- mice9 (C57BL6 background) that totally lack functional NK and CTL cells. We observed a more rapid growth of MC38 cells in IL-15-/- mice than in wt cohorts (P < .001), which implies that IL-15-/- mice lack the components which suppressed the growth of IL-15Rα+ MC38 cells in wt mice. In addition, IL-15Rα+ and IL-15Rα- MC38 cells showed similar rates of growth in IL-15-/- mice, suggesting that the bona fide tumorigenic capacity of MC38 cells was not altered by the introduction of IL-15Rα. These results are consistent with other observations made by us, and collectively suggest that the rejection of IL-15Rα+ MC38 cells in mice is dependent on in vivo activation of NK cells caused by the IL-15 trans-presentation. Thus, the cell surface–associated IL-15 and its subsequent presentation in trans seems to contribute to an enhanced tumor immunosurveillance through the activation of NK cells.
Depletion of NK cells from mice restored the tumor-forming capability of IL-15Rα+ MC38 cells
We then asked whether the depletion of NK cells from mice would allow IL-15Rα+ MC38 cells to form tumors in wt mice. NK cells or CD8 T cells were depleted separately from mice by repeatedly injecting either anti-asialo–GM1 or anti-CD8 antibodies. The anti-CD8 treatment showed no effects because IL-15Ra+ MC38 cells failed to form fatal lung metastases in wt mice. In contrast, these IL-15Rα+ MC38 cells formed pulmonary metastases and killed wt mice (Figure 5B) following the depletion of NK cells (confirmed by flow cytometry, data not shown). As shown in Figure 2, we did not observe strong activation of CD8 T cells in mice bearing IL-15Rα+ MC38 tumors. Collectively, these results suggest that the engineered expression of IL-15Rα on MC38 cells may have allowed these cells to capture endogenously produced IL-15 and then present it to NK cells in their neighborhood, thereby activating those NK cells into lymphokine-activated killer (LAK), which may account for the non-occurrence of fatal pulmonary metastases of IL-15Rα+ MC38 cells in untreated wt mice.
Discussion
We previously proposed a novel mechanism by which physiologic concentrations of IL-15 can activate target lymphoid cells that express the intermediate affinity IL-15R22 (IL-2/IL-15Rβ-γc heterodimer complex in the absence of IL-15Rα). Soluble IL-15 cannot activate these cells in vivo, but those cells can be fully activated if IL-15 is presented in trans by neighboring IL-15Rα+ cells.22 In this study, we elaborated on the relevance of the IL-15 trans-presentation to NK cell activation and to the in vivo antitumor surveillance mechanism. NK cells expanded ex vivo do not seem to express IL-15Rα, nor do they respond to picomolar concentrations of IL-15. Thus, only trans-presented IL-15, but not soluble IL-15, could stimulate the proliferation of these NK cells at physiologic concentrations, similar to what we reported using a T-cell line.22 More importantly, trans-presentation of IL-15 to NK cells efficiently augmented the cytotoxic function of these cells against tumor cells in vitro. IL-15Rα+ MC38 cells form metastases in IL-15-/- mice, but not in wt mice, suggesting the involvement of both IL-15 and NK cells in the rejection of IL-15Rα+ MC38 cells in wt mice. Furthermore, only IL-15Rα+, but not IL-15Rα MC38 cells were rejected from wt mice, implying that IL-15 trans-presentation may account for the effective rejection of MC38 cells that express IL-15Rα.
The relevance of the IL-15Rα expression on NK or memory CD8 T cells remains elusive despite the accumulating evidence that IL-15 is indispensable for the in vivo propagation and survival of these cells.1-5,9,10 As described here and by a recent publication,30 NK cells in general do not seem to express IL-15Rα. In contrast, CD8 T cells express IL-15Rα upon stimulation, but the functional requirement of this expression was challenged by a series of experiments performed by Ma and colleagues (Lodolce et al,18 Koka et al,19 and Burkett et al20 ). These observations argue against the presumption that the expression of the αβγ hetero-trimer IL-15R complex on target cells is indispensable for IL-15 function in vivo. As we showed previously,22 the IL-15–trans-presentation mechanism enables even IL-15Rα–negative T or NK cells to respond to physiologic concentrations of IL-15. In the scenario we propose, monocytes or dendritic cells expressing IL-15Rα capture soluble IL-15 under physiologic conditions and present it to NK/T cells in trans. This explains the survival of IL-15Rα- NK and CD8 T cells in mice if environmental cells express IL-15Rα.18-20 Collectively, these results led us to speculate that the trans-presentation of IL-15 can be at least an alternative, possibly a dominant, mechanism to account for the in vivo actions of IL-15. In this context, the IL-15Rα molecule seems to function as a trans-membrane anchorage component of the membrane IL-15 complex, rather than as a receptor for IL-15. It is important to note, however, that IL-15Rα induced by activation on T cells may be advantageous to their functional differentiation or to their survival under certain conditions. Gett et al31 have recently demonstrated that a stronger antigenic stimulation directs CD8 T cells to express higher levels of IL-15Rα to reach a particular “fitness” status, which could become more resistant to death by neglect and survive as memory CD8 T cells. It is likely that IL-15, through evolution, has become a unique cytokine which functions in 2 alternative modes (ie, soluble versus membrane-associated) depending on the initial immunological challenge.
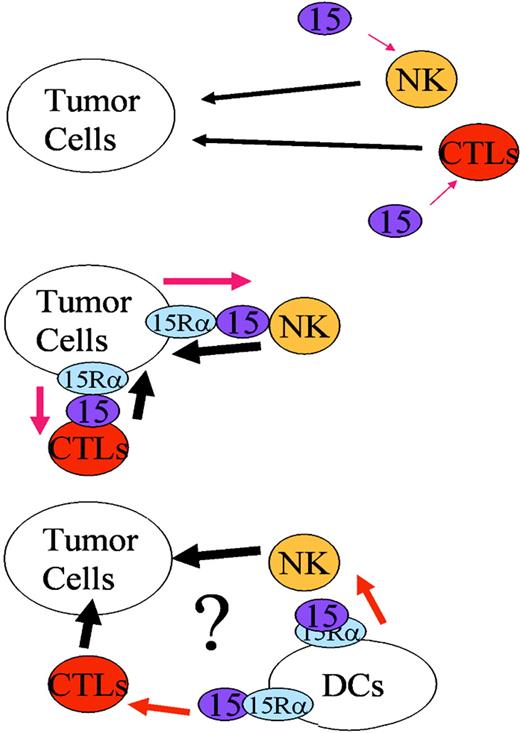
The MC38 cells23 we used in this study do not seem to evoke a potent T-cell–mediated immunity. In light of previous publication,32 activated CTLs are known to express NK markers, an observation which may require an alternative interpretation of our depletion data. In our system, however, we did not observe induction of NK markers on CD8 T cells during MC38 tumor development in mice. In addition, depletion of CD8 T cells using anti-CD8 antibodies did not allow IL-15Rα+ or wt MC38 cells to develop tumor in wt or IL-15 Tg mice. Collectively, we believe that NK cells, rather than antigen-specific CD8 T cells, play an important role in rejecting the MC38 tumor in mice. Nevertheless, CD8 T cells may constitute the second defense phase. Adoptive transfer of CD8 T cells from MC38-bearing mice to a naive host will help us address this issue. As shown in Figure 4D, we observed a strong enhancement of the killing toward MC38 cells by ex vivo NK cells only when IL-15 was presented in trans. Therefore, we propose that the inability of IL-15Rα+ MC38 cells to metastasize in normal mice can be explained by the up-regulation of the immune surveillance mechanism as the consequence of the IL-15 trans-presentation by IL-15Rα+ MC38 to NK/CTL cells (Figure 7). As described by many investigators (reviewed by Waldmann and Tagaya33 and Fehniger et al15 ), IL-15 protein can be constitutively produced in small amounts in vivo and can be picked up by the IL-15Rα+ MC38 cells.
A schematic representation of the MC38-mediatedtrans-activation of NK cells. In IL-15 Tg mice, the constitutive presence of IL-15 activates NK (and CTL) cells to enhance cytokine-induced lysis of MC38 cells, resulting in the efficient rejection of these tumor cells (top). If the MC38 cells express IL-15Rα on their surface, the endogenously produced IL-15 in mice can be captured and be presented intrans to NK/CTL cells in the neighborhood (left). This scenario explains the reduced growth or lack of growth of MC38/IL-15Rα transfectants in normal mice. Hypothetically, it is plausible that administration of dendritic cells that have been engineered or activated to express IL-15Rα may activate NK/CTLs, and thus leads to activation of immune-surveillance mechanism against tumor cell development in vivo.
A schematic representation of the MC38-mediatedtrans-activation of NK cells. In IL-15 Tg mice, the constitutive presence of IL-15 activates NK (and CTL) cells to enhance cytokine-induced lysis of MC38 cells, resulting in the efficient rejection of these tumor cells (top). If the MC38 cells express IL-15Rα on their surface, the endogenously produced IL-15 in mice can be captured and be presented intrans to NK/CTL cells in the neighborhood (left). This scenario explains the reduced growth or lack of growth of MC38/IL-15Rα transfectants in normal mice. Hypothetically, it is plausible that administration of dendritic cells that have been engineered or activated to express IL-15Rα may activate NK/CTLs, and thus leads to activation of immune-surveillance mechanism against tumor cell development in vivo.
The IL-15Rα+ (moderate) MC38 cells did not form lung metastases in C57BL6 mice, but some of those mice manifested lymph node metastases and were eventually killed. We cannot precisely determine the cause of these observations, although it is likely that these IL-15Rα+ (moderate) MC38 cells express homogenous levels of IL-15Rα but were heterogenous with regard to their metastatic capability. Thus, some clones of IL-15Rα+ (moderate) MC38 cells bearing stronger metastatic potential could survive in mice by overcoming the relatively weak tumor immunosurveillance in these mice, as compared with that in mice injected with IL-15Rα+ (high) MC38 cells. This may explain the delayed death of mice injected with IL-15Rα+ (moderate) MC38 cells. Nonetheless, combination of IL-15 trans-presentation and specific cancer vaccinization34 can be evaluated as a therapeutic strategy against various types of neoplasms. As we propose in Figure 7, engineered expression of IL-15Rα on dendritic cells may augment the efficacy of these cells to activate immune cells and thus can be integrated into the existing dendritic cell–mediated vaccinization strategy. In fact, our preliminary data suggest that dendritic cells from IL-15Rα transgenic mice, constitutively expressing IL-15Rα, more efficiently present antigens to CD8 T cells than those from wt mice (N.S. and Y.T., unpublished observation, November 2003). In the same context, it is noteworthy that some tumors are known as poor activators of the acquired host immunity/CTL activities.35 Conventional therapeutic strategies aiming to enhance CTL activity do not work well with these type of tumors, and our strategy may provide an alternative option against such cases, or recurrent tumors observed following conventional CTL-mediated therapy.
Our demonstration has other clinical ramifications as well. IL-15 has been used as a promising therapeutic agent in mouse models of autoimmune, immunodeficiency, or neoplastic diseases.12,13 However, these studies commonly used extremely high amounts of IL-15 to observe any discernible change. As we have shown previously,22 IL-15Rα+ cells cannot only present IL-15 but also recycle the IL-15/IL-15Rα complex between the cell surface and endosomal compartments without degradation of the complex. This is unlike most other cytokines and defines IL-15 as a unique cytokine with relatively long biologic activity in vivo. We speculate that IL-15Rα+ cells, after binding IL-15, may retain the complex and act as reservoir for IL-15 in vivo, in addition to their capacity to present IL-15 in trans. We actually have some preliminary results suggesting that IL-15Rα+ environmental cells (activated cells or those from the IL-15Rα transgenic mice) help maintain the IL-15 in the circulation in mice much longer (for 5-10 days) than normal environmental cells (for 1-2 days) (N.S. and Y.T., unpublished observation). We anticipate that the controlled induction of IL-15Rα expression by nonlymphoid cells may dramatically improve the efficacy of IL-15 actions in vivo.
Schluns et al36 have recently demonstrated that parenchymal- and bone marrow–derived IL-15Rα+ cells contribute distinctly to IL-15 trans-presentation to CD8 memory T cells. Our results showed that MC38, colon epithelial cells in origin, can present IL-15 to NK cells. It is possible that such cell type-specific requirements play an important role for IL-15 trans-presentation in vivo, but broader types of cells may be capable of presenting IL-15 in trans when circumstances allow, particularly in the context of lymphoid malignancy or immunologic disorders.
Our studies demonstrated the relevance of IL-15 trans-presentation for diverse IL-15 functions in vivo. IL-15 trans-presentation may be the only viable means by which IL-15 under physiologic concentrations activates IL-15Rα- NK cells in vivo. Another potential venue in which the trans-presentation may operate would be the maintenance of antigen-specific memory CD8 T cells. As was proposed by a recent publication,20 memory CD8 T cells may be sustained by periodic exposure to IL-15 presented in trans. The IL-15Rα, in addition to IL-15, can be transduced onto target cells to augment the immune response in the context of gene therapy approaches to cure cancer as well as some immunologic diseases.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2003-12-4187.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Figure 4. Lack of IL-15Rα expression on NK cells. (A) Flow cytometry of IL-15Rα expression on ex vivo NK cells. NK cells were expanded from bone marrow precursors from C57BL/6 mice in the presence of 70 nM IL-15 for 10 days. The cells were stripped of any bound cytokine by treating briefly in acetic acid–phosphate-buffered saline (PBS; pH 3)22 and then stained using anti–IL-15Rα polyclonal antisera (Santa Cruz), followed by a staining with phycoerythrin (PE) anti–goat IgG (Sigma, St Louis, MO). As a positive control, dendritic cells were expanded from bone marrow precursors in the presence of 1 nM GM-CSF (PeproTech) and were stimulated by lipopolysaccharide (LPS; 1μgmL-1) and interferon-γ (10 nM) for 24 hours to augment their expression of IL-15Rα. (B) Lack of proliferative responses of NK cells to low doses of IL-15. NK cells expanded by IL-15 as described in panel A (> 90% were NK1.1+, data not shown) were incubated with the indicated concentrations of IL-15 for 20 hours and pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 4 hours to measure their DNA synthesis. More than 10 nM IL-15 was needed to induce meaningful proliferative responses in these NK cells, although less than 100 pM IL-2 induced an almost maximum proliferative response from these cells. (C) Induction of NK proliferation by the IL-15trans-presentation. PT-18 cells24,25 were transfected with a human IL-15Rα expression construct, and clones expressing this molecule were selected using G418. The IL-15Rα+ PT-18 cells were first irradiated by 50 Gy of x-ray on the previous day of the experiment and then incubated with indicated concentrations of soluble IL-15 for 60 minutes at 37°C. After an extensive wash with PBS, 100 000 of these IL-15–bearing PT-18 cells were added to equal numbers of NK cells and incubated for 20 hours, followed by a pulse with 1μCi (0.037 MBq)3[H] thymidine for 4 hours. A similar experiment was undertaken by incubating IL-15Rα+ MC38 cells with effector NK cells, but those MC38 cells were lysed almost instantaneously, so that we could not observe NK cell proliferation (data not shown). (D) Strong induction of NK-cell mediated killing of IL-15Rα+ MC38 cells. IL-15Rα+ or IL-15Rα- (parental) MC38 cells were incubated with 0.5 nM IL-15 for 2 hours concurrent with the 51Cr labeling prior to the cytotoxicity assay. The cells were then extensively washed in PBS to remove any free IL-15. Twenty-five thousand labeled (with IL-15 and 51Cr) cells were mixed with various numbers of effector NK cells for 6 hours, and the cell-bound and released radioactivity were measured.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2003-12-4187/6/m_zh80020572830004.jpeg?Expires=1765957730&Signature=Mq20j5VsseadVp8tdQYh5eV-qzQlkd~FQfU2CxR~rrPSSoPhP23zByDPzcUiSfXcvHa1n-l2gRg4ul-4QsMbwf4KMnd-7mwYNRoPwKTCdUQqpqd9O5z0OiaE6jltWi55MQPCZefAQw8Q6K6v5rutQ9tlvH5K-PwcxgmO11dc2Hw2VRcGbMEXkGzEVvlulc59b8mY2tAjQOUBGGe3nZaC1GR6NA4feIYDWnHEqKsTmIoJMd8VN~moo06Ikrzamo6e3TEc0~P2lkpBwXA-d3jR53sfUCkBP4dZ730xLkjhADUjaIDYAfepn88VD7YEofIdNKX-7Z-JLtr5d7~niItTyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Survival curves of wt or NK-depleted C57BL/6 mice injected with parental and IL-15Rα-transfected MC38 cells. (A) Expression levels of IL-15Rα on MC38 transfectants. Flow cytometry data showing the expression levels of mouse IL-15Rα on MC38 transfectants. (Left) Parental MC38/isotype control staining of all MC38 clones; (middle) MC38-mouse IL-15Rα (moderate); (right) MC38-mouse IL-15Rα (high). (B) Survival of wt mice challenged by IL-15Rα–transfected MC38 cells. Two million parental or IL-15Rα (moderate or high expression)–transfected MC38cells were injected via the tail vain into C57BL/6 mice (n = 11 for parental MC38 injected mice, and n = 6 for mice injected with IL-15Rα+[moderate] or IL-15Rα+MC38 cells, n = 4 for NK-depleted wt mice injected with IL-15Rα+MC38), and their survival was monitored for 60 days. The survival of mice injected with IL-15Rα+ (high) MC38 cells was significantly longer (P < .001) than that of mice injected with the parental MC38 cells. The left panel shows the levels of IL-15Rα expression on these MC38 transfectants as assessed by flow cytometry. When the IL-15Rα+ MC 38 cells were injected into mice that had been depleted of either NK cells or CD8 T cells, only NK-depleted mice allowed the development of IL-15Rα+ (high) fatal MC38 cell tumors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2003-12-4187/6/m_zh80020572830005.jpeg?Expires=1765957730&Signature=S4MmVFuxoDfx25xqGf1t9LK-7RAgyGRDUzpEwALHtgm4ebizmxj-xBSrDRZ9u71et4-hcBVYno7pT8MSZQ4gj77seHblqt15GsfgPxDhTfuZgL8NQ2~c~pPZxvJT~MbGutFyTSRRLX71bLfAi~auS2w8m-IHu-C98poSL0KG6hqn~jBzjujqQHKn-8rb5B-kFZkr8w95maCkv0zA~8c-ZdX6ZgGRB45lEwd3JKSsUMs13i0v96-Tj79wXcQN5fofxuMeIrBKaJN5XiFtf5l0~Uj2SeEappNwZ5m2WObbMBqbFZZ15DmpGLUZ0QWwY8hcoCjpHXi43E-CUvRTHbP2ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal