Abstract
Markers of inflammation (eg, interleukin-6 [IL-6]), and endothelial perturbation (von Willebrand factor [VWF], circulating endothelial cells [CECs]) are altered in acute coronary syndromes (ACS). We hypothesized that CECs and IL-6 levels during the first 48 hours of ACS would predict 30-day and 1-year major cardiovascular end points (MACE). A total of 156 patients with ACS were included. Blood was drawn on admission (baseline) and 48 hours later for plasma VWF, IL-6 (both enzyme-linked immunosorbent assay [ELISA]), and CECs (CD146 immunomagnetic separation). CEC phenotyping was performed by indirect immunoperoxidase staining. At 30 days, 48 patients had a MACE, a predicted by baseline and 48-hour CECs and IL-6 levels, 48-hour VWF levels, and by the “admission–48 hour change” (Δ) in CECs, VWF, and IL-6 (all P = .002). On multivariate analysis, 48-hour CECs (P < .001) were the strongest predictor of MACE, followed by ΔIL-6 (P = .01) and ΔVWF (P = .048); 48-hour CECs were the only predictor of death (P = .007). At 1 year, 65 patients had MACE, predicted by 48-hour CECs and ΔIL-6 levels (P < .001); age (P = .046) and 48-hour CECs (P < .001) were the only predictors of death. CECs stained 93% positive for endothelial nitric oxide synthase (eNOS) but were less than 1% positive for CD34, CD36, and CD45 and less than 3% for CD31. Like raised VWF, abnormal CECs and IL-6 levels during the first 48 hours of ACS were strongly associated with 30-day MACE. CECs at 48 hours were the only independent predictor of both death and MACE at 30 days and 1 year, indicating the crucial role of endothelial/vascular damage in ACS pathophysiology.
Introduction
The endothelium plays a critical role in the pathogenesis of vascular disease. Endothelial dysfunction implies functional alterations in physiologic integrity and may be assessed by tests on pharmacologic- or shear stress–induced endothelium-dependent vasodilatation and on plasma markers of endothelial perturbation.1 However, endothelial dysfunction can perhaps be distinguished from endothelial damage, as the latter may be represented by the anatomic disruption, or even detachment, of the endothelium from the vascular internal elastic lamina.1,2 A novel method for assessing endothelial damage, as circulating endothelial cells (CECs) in the peripheral blood, has been developed.3 Increased CECs in the peripheral blood have been reported in various pathologic conditions involving severe endothelial perturbation, including inflammatory disease, acute myocardial infarction, unstable angina, and critical limb ischemia.3-5 The demonstration of an increased number of CECs may be the most direct evidence of endothelial injury or damage in these conditions and perhaps also reflects the degree of severity of the insult(s) toward the endothelium, especially since these cells are rarely found in the blood of healthy persons. Thus, increased numbers of CECs may yet be a prognostic indicator in patients with vascular disease, but (as far as we are aware) this is still unproven.
However, CECs are not the only index of endothelial perturbation. Plasma von Willebrand factor (VWF, marking endothelial damage or dysfunction) may contribute directly to thrombosis, is increased in all major risk factors for atherosclerosis and in clear cardiovascular disease, and increased levels predict poor long-term outcome.1,6,7 Acute changes in VWF levels are also important. For example, in patients with non-ST elevation myocardial infarction (non-STEMI) or unstable angina pectoris (UAP), increasing plasma VWF and other molecules relevant to thrombosis and hemostasis (ie, plasminogen activator inhibitor) during the first 48 hours of presentation to hospital are an independent predictor of adverse cardiovascular outcomes at 14-days' and 30-days' follow-up.8-10
Attention has also focused on the potential role of plasma markers of inflammation as predictors of cardiovascular events.11 Elevated levels of the proinflammatory cytokine interleukin-6 (IL-6) at 48 hours after presentation with acute coronary syndromes (ACS) have been shown to be closely associated with an adverse in-hospital prognosis, even without a rise in serum troponin T.12 Similarly, increasing levels of IL-6 over the first 72 hours of hospitalization were a powerful predictor of 30-day outcomes in patients presenting with non-STEMI or UAP, when compared with the other plasma indices, including VWF.13
We hypothesized that increased baseline plasma IL-6 and the numbers of CECs and/or increasing levels of these markers during the first 24 to 48 hours of presentation (ie, an “acute release” Δ) predict short- as well as long-term major adverse cardiovascular events (MACE) in patients with acute coronary disease. To test this hypothesis, we studied consecutive patients admitted with STEMI, non-STEMI, or UAP and used levels of VWF (previously shown to rise under these conditions8-10 ) as a comparator and positive control. A subhypothesis stated that there was a difference in the research indexes according to STEMI or non-STEMI status. For disease-specific comparative purposes and to provide a perspective, we compared levels in ACS with patients with clinically stable coronary artery disease (CAD) and healthy control subjects and stained CECs for leukocyte, progenitor, and other markers.
Patients, materials, and methods
Patients
Patients consecutively admitted to our coronary care unit with chest pain or discomfort at rest, associated with ST-segment, T-wave changes or both on a standard electrocardiogram (ECG) occurring within 24 hours of their symptom onset were prospectively recruited. The diagnosis of STEMI was defined as the concurrence of prolonged chest pain or discomfort with persistent ST-segment elevation of greater than 1 mm in 2 or more contiguous leads or with presumed new left bundle-branch block with cardiac enzymes (total creatine kinase and creatine kinase MB fraction [CK-MB]) above twice the upper normal limit.14 The diagnosis of non-STEMI included the presence of typical angina at rest associated with acute and transient ST-segment or T-wave changes with cardiac enzymes above twice the upper normal limit, raised troponin I levels to at least “high risk” values (> 0.6 ng/mL), or both.14 Patients with clinical or ECG features of non-STEMI but with normal cardiac enzymes plus normal troponin levels were classified as UAP. All patients received standard therapy that included aspirin, low-molecular-weight heparin, intravenous nitrates, statin, β-blocker, angiotensin converting enzyme (ACE) inhibitor, and so forth where appropriate. The global risk profile of the patients on admission was also assessed with the thrombolysis in myocardial infarction (TIMI) risk score for STEMI15 and for UAP/non-STEMI.16 One modification was made to apply the risk score in the present study: a history of MI, percutaneous coronary intervention (PCI), or coronary bypass grafting (CABG) surgery was substituted for known coronary artery stenosis greater than 50%.
Baseline values of VWF, IL-6, and CECs of acute patients were compared with 36 age- and sex-matched patients with clinically stable CAD and 40 healthy control subjects. Stable CAD patients were recruited from those who had recently completed a 12-week course of cardiac rehabilitation program in our hospital for previous (> 3 months) MI or coronary revascularization. Healthy control subjects, defined by careful history, examination, and basic blood tests, were recruited from members of the hospital staff and from those relatives attending the hospital cardiac rehabilitation with the stable CAD patients. These subjects were asked to abstain from tobacco and alcoholic and caffeine-containing beverages during the evening before blood sampling.
None of the acute or stable patients or healthy control subjects had a history of renal or liver disease, malignancy, connective tissue disease, deep vein thrombosis or pulmonary embolism, recent infections, inflammatory disorders, or were taking regular nonsteroidal anti-inflammatory drugs or on anticoagulants. To avoid possible influences of percutaneous coronary intervention (PCI) or catheter-related acute-phase reactions on the measured parameters, patients requiring urgent PCI or coronary angiographic investigation within the first 48 hours of admission were excluded from this study. Informed consent was obtained from all patients and control subjects. The protocol was approved by the local ethical committee.
Clinical follow-up
All acute patients were followed for MACE, defined as the occurrence, at 30 days and 1 year, of a composite end point of cardiovascular death, non-fatal MI, or refractory angina with ECG ST-T segment changes requiring hospitalization or urgent coronary revascularization. Nonfatal MI was defined by a history of angina with ST-T segment changes on ECG and elevated cardiac enzyme (total CK and CK-MB levels above twice the upper-normal limit or raised troponin levels to > 0.6 ng/mL).14 Patients with events occurring between admission and the 48-hour sampling were excluded from the analysis.
Blood samples and laboratory analyses
Blood was drawn on admission (baseline) and 48 hours thereafter. The first 4 mL of blood was discarded. All samples for plasma were immediately placed on ice and were centrifuged at 3000 rpm/1000g at 4°C for 20 minutes within 1 hour of collection to obtain citrated plasma. Plasma was divided into aliquots and stored at –70°C to allow batch analysis. All laboratory work was performed in blinded fashion with respect to the identity of the samples and clinical events.
VWF and IL-6 levels were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) using commercial reagents (Dako-Patts, Ely, United Kingdom, and R&D Systems, Abingdon, United Kingdom, respectively). The lower limits of detection were 0.5 IU/dL for VWF and 5 pg/mL for IL-6. Intra- and interassay coefficients of variation (CVs) were less than 5% and less than 10%, respectively. Blood for CECs was collected in sodium fluoride and prepared for immunomagnetic separation within 1 hour and counted by a single observer under epifluorescence microscopy (Zeiss, Welwyn Garden City, Herts, United Kingdom). The detailed methodology for capturing CECs (using anti-CD146–coated immunomagnetic Dynabeads; Dynal, Warrington, Cheshire, United Kingdom) and criteria for counting CECs have been described in detail elsewhere.4,5 Intra(n = 40) and interassay (n = 20) CVs were less than 5% and less than 10%, respectively; the inter- and intraobserver CVs of the method in our laboratory were less than 5%.5
For immunophenotyping, CECs were stained by indirect immunoperoxidase by standard techniques at room temperature. Briefly, CECs were air dried to glass slides and rehydrated in phosphate-buffered saline (PBS) plus 10% normal swine serum (Dako, Ely, United Kingdom) for 10 minutes. Following a PBS wash, a 1/50 dilution of monoclonal antibodies (all Becton Dickinson, Oxford, United Kingdom) to CD31 (platelet endothelial cell adhesion molecule-1 [PECAM-1]), CD34 (marking bone marrow–derived progenitor stem cells), CD36 (the thrombospondin receptor, marking microvascular endothelial cells, platelets, and monocytes or macrophages), or CD45 (leukocyte common antigen) was applied to different slides for an hour, followed by a PBS wash. Color was developed by the Dako (catalog no.K3468; Ely, United Kingdom) LSAB-2 System, using first a 10-minute biotin step, washes, a 10-minute streptavidin step, washes, and 5 minutes of diaminobenzidine (DAB) substrate and chromogen. Slides were washed and taken through alcohol to xylene and mounted under a coverslip. Autofluorescence was retained: positive cells stained black. Positive controls were normal Ficoll-prepared peripheral blood mononuclear cells.
Using endothelial nitric oxide synthase (eNOS) staining, additional CEC phenotyping experiments were performed to confirm their endothelial nature. Blood (8 mL) was obtained from each patient, and CECs were separated as discussed in paragraph 2 of this section. The final 2 washes were performed in Earle balanced salt solution (EBSS; Sigma, Poole, United Kingdom) and resuspended in 100 μL EBSS. Twenty microliters was added onto X-tra Adhesive Slides (Surgipath, Newtown, United Kingdom), and cells were allowed to adhere electrostatically onto each reaction field (RF) for 10 minutes in a humidification chamber to form a monolayer. The slides were fixed with ice-cold 50 μL/RF 2% formaldehyde (Sigma) in EBSS for 20 minutes. Following 3 washes in EBSS, each RF was blocked with 2% fetal bovine serum (Gibco, Paisley, United Kingdom) in EBSS for 10 minutes. Slides were then washed in 0.1% Saponin (wash buffer saponin [WBS]; Sigma) in EBSS. Endogenous peroxidase activity was blocked by incubating with 1% hydrogen peroxide (Sigma) in WBS followed by the avidin-biotin blocking kit (Vector Laboratories, Peterborough, United Kingdom) in the dark. After 2 washes, 20 μL (in WBS) biotinylated antihuman endothelial nitric oxide synthase antibody (R&D Systems, Abingdon, United Kingdom) was incubated at a concentration of 50 μg/mL for 30 minutes, in the dark. After 3 washes in WBS, each RF was incubated with Vectastain Elite ABC-peroxidase (Vector Laboratories) in the dark. After 3 washes in distilled water color substrate (Vector Laboratories, Peterborough, United Kingdom) was added to each RF for 8 minutes. The slides were counterstained with hematoxylin, dehydrated in ethanol, and cleared, and cover slips were mounted with 1,3-diethyl-8-phenylxanthine (DPX). Quality control for eNOS was with human umbilical vein endothelial cells (HUVECs; harvested from fresh human umbilical cord by standard techniques) as “positive controls” and with circulating neutrophils isolated from venous blood as “negative controls.”
Power calculations
Previous data from 68 patients with ACS indicated that the change of VWF levels during the first 48 hours was about 2-fold higher in patients who experienced a 30-day end point (n = 27, 40% of patents) than in those without an end point (P = .02). At 1-year follow-up, 38 patients (56%) had at least 1 end point.8,9 However, we more conservatively predicted that only 30% of our cohort would experience at least 1 end point at 1-year follow-up. Because we intended to measure and assess several indexes, we decided to recruit over twice as many patients (ie, n = 156). As this number of patients far exceeds our previous data showing raised CECs in ACS4 and critical limb ischemia,5 we are, therefore, confident of minimizing the risk of types 1 and 2 error. Of the 156 patients, 68 had STEMI and 44 non-STEMI, providing the 1-β power of 0.8 to detect a P value less than .05 difference of 0.4 of a standard deviation in a continuously distributed variable index such as VWF. We also recruited 36 patients with stable CAD and 40 healthy control subjects to provide perspective. As these subjects do not test a hypothesis, a power calculation is not needed, although the numbers provide as much power as our previous CEC data.4,5
Statistical analysis
The Anderson-Darling test was applied to continuously variable data to define distribution. Results are expressed as mean and standard deviation (SD) or as medians with interquartile ranges (IQRs), as appropriate. Between group or intergroup comparisons were made using the chi-squared test for categorical variables and using the unpaired t test and 1-way analysis of variance (ANOVA) or the Mann-Whitney U test and Kruskal-Wallis test for continuous data, as appropriate. Correlations were performed by the Spearman correlation methods. Potential associations between clinical or biologic parameters and the composite end point were first tested by univariate procedures using the unpaired t test or chi-squared tests. The “acute release” of VWF, IL-6, and CECs was simply defined as the difference (Δ) between their plasma concentrations at 48 hours and baseline (48 hours – baseline). A multivariate logistic regression with forward stepwise conditional method was used for the analysis of 30-day end point using plasma marker parameters, including the basal values, and clinical variables that have a P value less than .20 in the univariate procedure. The odds ratios (ORs) with their 95% confidence intervals (CIs) of each significant predictor were computed. The comparisons for cumulative probability of event-free survival between patients in the median and less than median values (ie, high versus low levels) of each measured indexes were performed using Kaplan-Meier survival analyses with log-rank test. Cox stepwise regression was used to test predictors of adverse outcome at 1 year, adjusted for known cardiovascular risk factors (age, sex, active smoking, body mass index, past history of stroke or transient ischemic attack, congestive heart failure, hypertension, diabetes mellitus, family history of coronary heart disease [CHD]) and the basal values of the biologic indexes, including cardiac enzyme rise, etc. All tests were 2-tailed, and probability values were considered significant at the .05 level. All statistical analyses were performed using SPSS software, version 11 (SPSS, Chicago, IL).
Results
Cross-sectional data
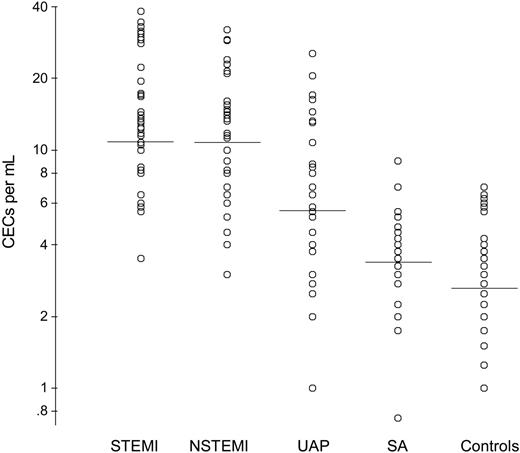
The demographic, clinical, and baseline research data from the 156 patients with ACS, the 36 patients with stable CAD, and the 40 healthy control subjects are shown in Table 1. As expected,4,5,8-13 the baseline CECs (Figure 1), VWF, and IL-6 levels in the patients with ACS were higher than the patients with stable CAD and the healthy control subjects. In the 156 patients with ACS, VWF correlated significantly with CECs (r = 0.44, P < .001), VWF correlated with IL-6 (r = 0.25, P = .002), and CECs correlated with IL-6 (r = 0.55, P < .001).
Baseline demographic and clinical characteristics, circulating endothelial cells, plasma levels of VWF and IL-6 in acute coronary syndrome, stable coronary artery disease, and healthy control subjects
. | Acute coronary syndrome, n = 156 . | Stable CAD, n = 36 . | Control subjects, n = 40 . |
|---|---|---|---|
| Age, y, mean ± SD | 65 ± 12 | 60 ± 12 | 60 ± 8 |
| Male, n (%) | 110 (71) | 26 (72) | 27 (68) |
| BMI, kg/m2, mean ± SD | 27 ± 4 | 27 ± 4 | 28 ± 5 |
| Active smoker, n (%) | 54 (35) | 5 (13) | 4 (10) |
| Systolic BP, mm Hg, mean ± SD | 131 ± 18 | 136 ± 23 | 135 ± 16 |
| Cholesterol, mmol/L, mean ± SD | 5.7 ± 0.9 | 3.6 ± 0.7 | 5.6 ± 0.7 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 |
| Creatinine, μmol/L, mean ± SD | 89 ± 23 | 88 ± 17 | 79 ± 17 |
| Glucose, mmol/L, mean ± SD | 7.4 ± 2.9 | 6.2 ± 2.6 | 5.0 ± 0.7 |
| Research indexes | |||
| VWF, IU/dL, mean ± SD | 215 ± 59 | 147 ± 32 | 121 ± 20 |
| IL-6, pg/mL, median (IQR) | 27.2 (12.8-61.7) | 13.4 (8.2-27.7) | 10.4 (6.9-14.7) |
| CECs, cells/mL, median (IQR) | 10.63 (5.75-14.5) | 3.13 (2.25-4.0) | 2.75 (1.56-3.94) |
. | Acute coronary syndrome, n = 156 . | Stable CAD, n = 36 . | Control subjects, n = 40 . |
|---|---|---|---|
| Age, y, mean ± SD | 65 ± 12 | 60 ± 12 | 60 ± 8 |
| Male, n (%) | 110 (71) | 26 (72) | 27 (68) |
| BMI, kg/m2, mean ± SD | 27 ± 4 | 27 ± 4 | 28 ± 5 |
| Active smoker, n (%) | 54 (35) | 5 (13) | 4 (10) |
| Systolic BP, mm Hg, mean ± SD | 131 ± 18 | 136 ± 23 | 135 ± 16 |
| Cholesterol, mmol/L, mean ± SD | 5.7 ± 0.9 | 3.6 ± 0.7 | 5.6 ± 0.7 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 |
| Creatinine, μmol/L, mean ± SD | 89 ± 23 | 88 ± 17 | 79 ± 17 |
| Glucose, mmol/L, mean ± SD | 7.4 ± 2.9 | 6.2 ± 2.6 | 5.0 ± 0.7 |
| Research indexes | |||
| VWF, IU/dL, mean ± SD | 215 ± 59 | 147 ± 32 | 121 ± 20 |
| IL-6, pg/mL, median (IQR) | 27.2 (12.8-61.7) | 13.4 (8.2-27.7) | 10.4 (6.9-14.7) |
| CECs, cells/mL, median (IQR) | 10.63 (5.75-14.5) | 3.13 (2.25-4.0) | 2.75 (1.56-3.94) |
BMI indicates body mass index; HDL-C, high density lipoprotein cholesterol; CAD, coronary artery disease; VWF, von Willebrand factor; IL-6, interleukin-6; CEC, circulating endothelial cells.
Numbers of circulating endothelial cells (CECs) on admission according to diagnosis and compared with patients with stable angina and healthy control subjects. STEMI indicates ST-segment elevation myocardial infarction; NSTEMI, non-STEMI; UAP, unstable angina pectoris; SA, stable angina.
Numbers of circulating endothelial cells (CECs) on admission according to diagnosis and compared with patients with stable angina and healthy control subjects. STEMI indicates ST-segment elevation myocardial infarction; NSTEMI, non-STEMI; UAP, unstable angina pectoris; SA, stable angina.
Acute (48 hours) changes in research indices in ACS
Comparing baseline with the 48-hour samples, VWF increased by 6.5% to 229 ± 61 IU/dL (P = .001), median IL-6 by 10.3% to 30 pg/mL (IQR, 12.6-81.8; P = .002), and CECs by 6.6% to 11.3 cells/mL (6.2-20.8; P < .001). At 48 hours, VWF again correlated with CECs (r = 0.64, P < .001), VWF correlated with IL-6 (r = 0.44, P < .001), and CECs correlated with IL-6 (r = 0.63, P < .001).
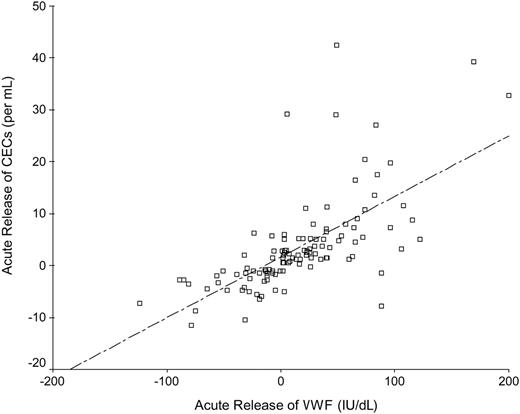
The increase (Δ) of VWF was significantly higher in patients with non-STEMI (median [IQR], 27.6 [0.13-59.2] IU/dL) compared with patients with STEMI (4.0 [–17.8 to 46.3] IU/dL) and UAP (2.6 [–13.3 to 23.8] IU/dL) (P = .04). However, ΔCECs and ΔIL-6 levels were not significantly different among the 3 patients groups (data not shown), although mean/median numbers of CECs, VWF, and IL-6 levels at baseline and at 48 hours were significantly higher in both patients with STEMI and non-STEMI than in patients with UAP (all P < .01). ΔVWF correlated with ΔCECs (r = 0.76, P < .001; Figure 2), ΔVWF correlated with ΔIL-6 (r = 0.44, P < .001), and ΔCECs correlated with ΔIL-6 (r = 0.24, P = .01).
Spearman correlation between the acute release of CECs and the corresponding acute change in plasma levels of VWF.
Spearman correlation between the acute release of CECs and the corresponding acute change in plasma levels of VWF.
30-Day follow-up data
MACE occurred in 48 (31%) patients at 30 days, being 20 with STEMI, 19 with non-STEMI, and 9 with UAP. Death occurred in 10 patients (all cardiovascular) while 33 patients had refractory angina and 2 had non-STEMI. The characteristics of patients are shown in Table 2. There were no statistically significant differences in demographic and clinical variables, the use of standard in-patient therapy, and secondary preventive medications at discharge. Patients who experienced a MACE had significantly higher TIMI risk scores on admission for both STEMI (P = .023) and non-STEMI/UAP (P = .02).
Baseline demographic and clinical characteristics of patients with or without MACE at 30 days
. | With 30-day MACE, n = 48 . | Without 30-day MACE, n = 108 . | P . |
|---|---|---|---|
| Age, y, mean ± SD | 64 ± 12 | 65 ± 12 | .62 |
| Aged 80 y, n (%) | 4 (8.3) | 12 (11) | .6 |
| Male, n (%) | 37 (77) | 73 (68) | .23 |
| Ethnicity, n (%) | |||
| White | 29 (60) | 64 (59) | .51 |
| Indo-Asians | 19 (40) | 39 (36) | |
| BMI, kg/m2, mean ± SD | 27 ± 3.8 | 27 ± 4.7 | .97 |
| Active smoker, n (%) | 16 (33) | 38 (35) | .82 |
| Family history of CHD, n (%) | 5 (10) | 13 (12) | .77 |
| Prior history | |||
| AMI, n (%) | 9 (19) | 23 (21) | .72 |
| PCI, n (%) | 1 (2) | 4 (4) | .69 |
| CABG, n (%) | 2 (4) | 13 (12) | .15 |
| Diabetes, n (%) | 11 (23) | 32 (30) | .39 |
| Hypertension, n (%) | 22 (49) | 48 (44) | .87 |
| Dyslipidemia, n (%) | 13 (27) | 32 (30) | .75 |
| Heart failure, n (%) | 4 (8) | 5 (5) | .36 |
| Angina, n (%) | 9 (19) | 25 (23) | .54 |
| Stroke, n (%) | 2 (4.2) | 5 (4.6) | .89 |
| Clinical parameters | |||
| Heart rate, bpm, mean ± SD | 81 ± 14 | 77 ± 14 | .14 |
| Systolic BP, mm Hg, mean ± SD | 128 ± 19 | 133 ± 17 | .051 |
| Killip class 2, n (%) | 15 (31) | 23 (21) | .18 |
| Baseline blood tests | |||
| Cholesterol, mmol/L, mean ± SD | 5.78 ± 0.8 | 5.7 ± 1.0 | .64 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.14 | 1.0 ± 0.13 | .47 |
| Creatinine, μmol/L, mean ± SD | 92 ± 31 | 88 ± 18 | .36 |
| Glucose, mmol/L, mean ± SD | 7.3 ± 2.6 | 7.4 ± 3.0 | .84 |
| Main ischemic area | |||
| Anterior, n (%) | 34 (71) | 65 (60) | .28 |
| Inferior, n (%) | 9 (21) | 36 (33) | |
| Lateral, n (%) | 4 (8.3) | 7 (6.5) | |
| TIMI risk score for | |||
| STEMI, mean ± SD | 4.4 ± 1.5 | 3.2 ± 2.1 | .023 |
| Non-STEMI/UAP, mean ± SD | 4.3 ± 0.9 | 3.7 ± 1.5 | .02 |
. | With 30-day MACE, n = 48 . | Without 30-day MACE, n = 108 . | P . |
|---|---|---|---|
| Age, y, mean ± SD | 64 ± 12 | 65 ± 12 | .62 |
| Aged 80 y, n (%) | 4 (8.3) | 12 (11) | .6 |
| Male, n (%) | 37 (77) | 73 (68) | .23 |
| Ethnicity, n (%) | |||
| White | 29 (60) | 64 (59) | .51 |
| Indo-Asians | 19 (40) | 39 (36) | |
| BMI, kg/m2, mean ± SD | 27 ± 3.8 | 27 ± 4.7 | .97 |
| Active smoker, n (%) | 16 (33) | 38 (35) | .82 |
| Family history of CHD, n (%) | 5 (10) | 13 (12) | .77 |
| Prior history | |||
| AMI, n (%) | 9 (19) | 23 (21) | .72 |
| PCI, n (%) | 1 (2) | 4 (4) | .69 |
| CABG, n (%) | 2 (4) | 13 (12) | .15 |
| Diabetes, n (%) | 11 (23) | 32 (30) | .39 |
| Hypertension, n (%) | 22 (49) | 48 (44) | .87 |
| Dyslipidemia, n (%) | 13 (27) | 32 (30) | .75 |
| Heart failure, n (%) | 4 (8) | 5 (5) | .36 |
| Angina, n (%) | 9 (19) | 25 (23) | .54 |
| Stroke, n (%) | 2 (4.2) | 5 (4.6) | .89 |
| Clinical parameters | |||
| Heart rate, bpm, mean ± SD | 81 ± 14 | 77 ± 14 | .14 |
| Systolic BP, mm Hg, mean ± SD | 128 ± 19 | 133 ± 17 | .051 |
| Killip class 2, n (%) | 15 (31) | 23 (21) | .18 |
| Baseline blood tests | |||
| Cholesterol, mmol/L, mean ± SD | 5.78 ± 0.8 | 5.7 ± 1.0 | .64 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.14 | 1.0 ± 0.13 | .47 |
| Creatinine, μmol/L, mean ± SD | 92 ± 31 | 88 ± 18 | .36 |
| Glucose, mmol/L, mean ± SD | 7.3 ± 2.6 | 7.4 ± 3.0 | .84 |
| Main ischemic area | |||
| Anterior, n (%) | 34 (71) | 65 (60) | .28 |
| Inferior, n (%) | 9 (21) | 36 (33) | |
| Lateral, n (%) | 4 (8.3) | 7 (6.5) | |
| TIMI risk score for | |||
| STEMI, mean ± SD | 4.4 ± 1.5 | 3.2 ± 2.1 | .023 |
| Non-STEMI/UAP, mean ± SD | 4.3 ± 0.9 | 3.7 ± 1.5 | .02 |
MACE indicates major adverse cardiovascular events; BMI, body mass index; HDL-C, high density lipoprotein cholesterol; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris; CHD, coronary heart disease; TIMI, thrombolysis in myocardial infarction; CABG, coronary artery bypass grating; PCI, percutaneous coronary intervention; BP, blood pressure; AMI, acute myocardial infarction.
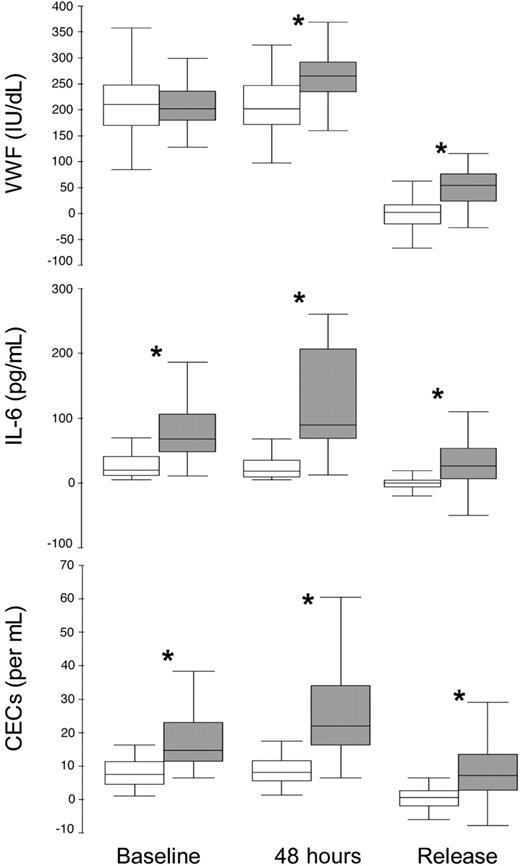
The baseline, 48-hour, and Δ in VWF, IL-6, and CECs according to 30-day MACE are shown in Figure 3. Baseline, 48-hour, and ΔCECs and IL-6, and 48-hour and ΔVWF were higher in those with a MACE at 30 days. In multivariate logistic regression controlling for heart rate, systolic blood pressure, previous history of CABG, use of intravenous nitrates, Killip class, and the basal values of all 3 indexes, 48-hour CECs was the strongest independent predictor for 30-day MACE (OR [95% CI], 1.04 [1.02-1.06]; P < .001), followed by ΔIL-6 (1.03 [1.01-1.04]; P = .01) and ΔVWF (1.02 [1.0-1.03]; P = .04). When 48-hour CEC data were dichotomized into higher or lower than the median value, the independent (adjusted) OR for a MACE with higher (> median) CECs was 17.5 (95% CI, 3.2-95.4; P < .001). 48-Hour CECs was the only single independent predictor of death at 30 days (OR, 1.02 [95% CI, 1.01-1.03]; P = .001).
Baseline and 48-hour and the acute release of the median plasma levels of VWF, IL-6, and numbers of CECs according to MACE at 30 days. Intergroup comparisons *P < .001.
Baseline and 48-hour and the acute release of the median plasma levels of VWF, IL-6, and numbers of CECs according to MACE at 30 days. Intergroup comparisons *P < .001.
In the STEMI subgroup, adjusting for the corresponding TIMI risk score in addition to clinical variables, baseline CECs, ΔIL-6, and ΔVWF were significant predictors of MACE at 30 days (all P = .03). Conversely, when adjusting for the corresponding TIMI risk score in the non-STEMI/UAP subgroup, 48-hour CECs was the only predictor of 30-day MACE (P < .001).
1-Year follow-up data
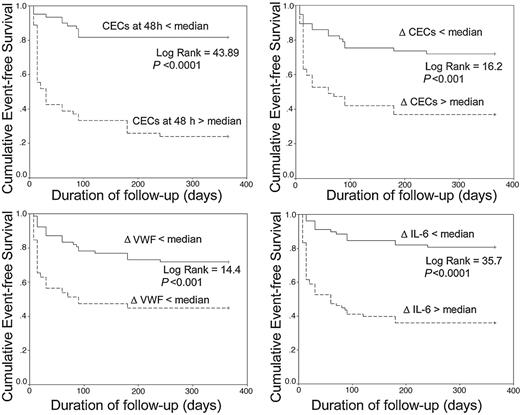
After 1 year, 65 (42%) patients had experienced at least 1 MACE, with 17 deaths (11%). The characteristics of these patients are shown in Table 3. Significant differences between these groups are TIMI risk scores and Killip class on admission. Figure 4 shows the Kaplan-Meier cumulative 1-year event-free survival curves comparing high (= median) versus low (< median) median values of the ΔCECs, ΔVWF, and ΔIL-6, and the high versus low median values of 48-hour CECs. After adjustment for clinical indexes and the basal values of the biologic indices in a Cox regression analysis, 48-hour CECs and ΔIL-6 were significant predictors of MACE (hazard ratio [HR], 1.04 [95% CI 1.02-1.05] and 1.01 [1.005-1.014], respectively; both P < .001). Age (HR, 1.05 [1.0-1.1], P = .046) and 48-hour CECs (HR, 1.06 [1.03-1.1]; P < .001) were the only independent predictors of death. 48-Hour CECs did not correlate with age (r =–0.1, P = .68).
Baseline demographic and clinical characteristics of patients with or without MACE at 1 year
. | With 1-year MACE n = 65 . | Without 1-year MACE n = 91 . | P . |
|---|---|---|---|
| Age, y, mean ± SD | 66 ± 12 | 63 ± 12 | .16 |
| Age of 80 y, n (%) | 7 (10.8) | 9 (10) | .86 |
| Male, n (%) | 50 (77) | 60 (66) | .14 |
| Ethnicity, n (%) | |||
| White | 39 (60) | 54 (59) | .51 |
| Indo-Asians | 25 (39) | 33 (36) | |
| BMI, kg/m2, mean ± SD | 27 ± 4 | 27 ± 5 | .58 |
| Active smoker, n (%) | 21 (32) | 33 (36) | .61 |
| Family history of CHD, n (%) | 7 (11) | 11 (12) | .80 |
| Medical history since index event | |||
| AMI, n (%) | 58 (89) | 72 (79) | .10 |
| Diabetes, n (%) | 15 (23) | 28 (31) | .29 |
| Hypertension, n (%) | 31 (48) | 39 (43) | .55 |
| Stroke, n (%) | 3 (4.6) | 4 (4.4) | .95 |
| Baseline clinical parameters | |||
| Heart rate, bpm, mean ± SD | 78 ± 15 | 76 ± 14 | .76 |
| Systolic BP, mm Hg, mean ± SD | 130 ± 20 | 133 ± 18 | .35 |
| Killip class 2, n (%) | 22 (34) | 16 (18) | .02 |
| Baseline blood tests | |||
| Total cholesterol, mmol/L, mean ± SD | 5.71 ± 0.9 | 5.73 ± 1.0 | .91 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.14 | 1.0 ± 0.13 | .62 |
| Creatinine, μmol/L, mean ± SD | 90 ± 29 | 89 ± 18 | .79 |
| Glucose, mmol/L, mean ± SD | 7.1 ± 2.6 | 7.6 ± 3.1 | .29 |
| Main ischemic area, n (%) | |||
| Anterior | 39 (60) | 60 (66) | .10 |
| Inferior | 18 (28) | 28 (31) | |
| Lateral | 8 (12) | 3 (3.3) | |
| Baseline TIMI risk score for | |||
| STEMI, mean ± SD | 4.7 ± 1.7 | 3.0 ± 1.9 | .001 |
| Non-STEMI/UAP, mean ± SD | 4.6 ± 0.9 | 3.4 ± 1.5 | .001 |
. | With 1-year MACE n = 65 . | Without 1-year MACE n = 91 . | P . |
|---|---|---|---|
| Age, y, mean ± SD | 66 ± 12 | 63 ± 12 | .16 |
| Age of 80 y, n (%) | 7 (10.8) | 9 (10) | .86 |
| Male, n (%) | 50 (77) | 60 (66) | .14 |
| Ethnicity, n (%) | |||
| White | 39 (60) | 54 (59) | .51 |
| Indo-Asians | 25 (39) | 33 (36) | |
| BMI, kg/m2, mean ± SD | 27 ± 4 | 27 ± 5 | .58 |
| Active smoker, n (%) | 21 (32) | 33 (36) | .61 |
| Family history of CHD, n (%) | 7 (11) | 11 (12) | .80 |
| Medical history since index event | |||
| AMI, n (%) | 58 (89) | 72 (79) | .10 |
| Diabetes, n (%) | 15 (23) | 28 (31) | .29 |
| Hypertension, n (%) | 31 (48) | 39 (43) | .55 |
| Stroke, n (%) | 3 (4.6) | 4 (4.4) | .95 |
| Baseline clinical parameters | |||
| Heart rate, bpm, mean ± SD | 78 ± 15 | 76 ± 14 | .76 |
| Systolic BP, mm Hg, mean ± SD | 130 ± 20 | 133 ± 18 | .35 |
| Killip class 2, n (%) | 22 (34) | 16 (18) | .02 |
| Baseline blood tests | |||
| Total cholesterol, mmol/L, mean ± SD | 5.71 ± 0.9 | 5.73 ± 1.0 | .91 |
| HDL-C, mmol/L, mean ± SD | 1.0 ± 0.14 | 1.0 ± 0.13 | .62 |
| Creatinine, μmol/L, mean ± SD | 90 ± 29 | 89 ± 18 | .79 |
| Glucose, mmol/L, mean ± SD | 7.1 ± 2.6 | 7.6 ± 3.1 | .29 |
| Main ischemic area, n (%) | |||
| Anterior | 39 (60) | 60 (66) | .10 |
| Inferior | 18 (28) | 28 (31) | |
| Lateral | 8 (12) | 3 (3.3) | |
| Baseline TIMI risk score for | |||
| STEMI, mean ± SD | 4.7 ± 1.7 | 3.0 ± 1.9 | .001 |
| Non-STEMI/UAP, mean ± SD | 4.6 ± 0.9 | 3.4 ± 1.5 | .001 |
MACE indicates major adverse cardiovascular events; BMI, body mass index; HDL-C, high density lipoprotein cholesterol; TIMI, thrombolysis in myocardial infarction; BP, blood pressure; AMI, acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris.
Kaplan-Meier cumulative 1-year event-free survival curves. Kaplan-Meier cumulative 1-year event-free survival curves between more than median versus less than median values of (A) CECs at 48 hours, (B) ΔCECs, (C) ΔVWF, and (D) ΔIL-6. Δ Indicates acute release of the following: VWF, von Willebrand factor; IL-6, interleukin-6; CEC, circulating endothelial cells.
Kaplan-Meier cumulative 1-year event-free survival curves. Kaplan-Meier cumulative 1-year event-free survival curves between more than median versus less than median values of (A) CECs at 48 hours, (B) ΔCECs, (C) ΔVWF, and (D) ΔIL-6. Δ Indicates acute release of the following: VWF, von Willebrand factor; IL-6, interleukin-6; CEC, circulating endothelial cells.
CEC immunophenotyping
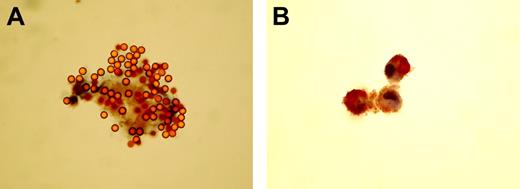
We analyzed CECs from 10 patients with ACS and peripheral blood mononuclear cells (PBMCs) from 1 healthy volunteer, the latter acting as positive control. Of 185 CECs identified by rosetted immunobeads, less than 1% stained positive for CD34 or CD45. In most cases, this amounted to 1 positive event per slide. No CECs stained for CD36, but some PBMCs stained positive. Positive staining for CD31 was present on 2.6% of CECs. We also identified 14 cases of positive-staining cells binding 1 to 3 beads that were noticeably smaller than CECs that we take to be contaminating leukocytes, captured by nonspecific binding. In a separate phenotyping experiment, 93% of 40 CECs identified by immunobeads stained positive for eNOS; in comparison, 100% of HUVECs (positive controls) and 0% of neutrophils (negative controls) stained (Figure 5). Data are mean of 2 observers. eNOS staining was considerably stronger in HUVECs than in CECs.
CECs and HUVECs express eNOS. (A) High-power (650×) light photomicrograph of an endothelial nitric oxide synthase–positive circulating endothelial cell from a patient with a recent acute myocardial infarction. Confirmation that the cell is endothelial is depicted by strong binding to CD146 coated magnetic beads (4μm in diameter). (B) “Positive control” photomicrograph (160×) of a cluster of 3 endothelial nitric oxide synthase–stained human umbilical vein endothelial cells. Note that staining is limited to the cytoplasm where most nitric oxide synthase is located. Images were visualized using an Axiostar Plus microscope (Zeiss, Welwyn Garden City, UK); objective lens, 40× and 10×; eyepiece, 10×. Images were captured using a Camedia C3030 camera, and transfered to JPG using standard smart card technology.
CECs and HUVECs express eNOS. (A) High-power (650×) light photomicrograph of an endothelial nitric oxide synthase–positive circulating endothelial cell from a patient with a recent acute myocardial infarction. Confirmation that the cell is endothelial is depicted by strong binding to CD146 coated magnetic beads (4μm in diameter). (B) “Positive control” photomicrograph (160×) of a cluster of 3 endothelial nitric oxide synthase–stained human umbilical vein endothelial cells. Note that staining is limited to the cytoplasm where most nitric oxide synthase is located. Images were visualized using an Axiostar Plus microscope (Zeiss, Welwyn Garden City, UK); objective lens, 40× and 10×; eyepiece, 10×. Images were captured using a Camedia C3030 camera, and transfered to JPG using standard smart card technology.
Discussion
Montalescot et al8,9 and Collet et al10 have amply and elegantly demonstrated that raised VWF in the peri-ACS period predicts MACEs. We confirm their data but also show that IL-6 and another vascular index, CECs, are also valuable in predicting those at risk of a MACE. In patients with acute coronary disease, particularly those with myocardial damage, elevated numbers of CECs can be readily demonstrated.4,5 The present study confirms these observations and in addition, also shows for the first time that, among the measured indexes, elevated numbers of CECs are the only independent predictor of death at 30 days and at 1 year. Taken together, these results suggest that serious damage and increasing shedding of the vascular endothelium into the circulation during the initial period of the coronary ischemic insult may in part play a role in the short- and long-term pathogenesis of recurrent cardiovascular events.
The pathophysiologic basis of ACS includes thrombosis, hemostasis, and disturbed vascular function, with rupture of a vulnerable plaque, leading to exposure of the blood constituents to the subendothelial surface and plaque core.17,18 The resultant thrombosis leads to a variable degree of flow occlusion, the extent of which (possibly) differentiates the 3 acute coronary syndromes, STEMI, non-STEMI, and UAP. These, in turn, may lead to a variable degree of disruption and shedding of the endothelium, presumably from the infarct-related coronary artery and its territorial endomyocardium. Indeed, if the risk factor theory of cardiovascular disease is correct, then many mural and/or intracardiac endothelial cells will be damaged before these acute events and, thus, easier to remove and so transform into CECs by the process of infarction and/or spasmosis. Increased VWF in subjects with chronic disease and/or risk factors (reviewed in Blann1 ), and raised CECs in “atherosclerosis-free” pulmonary hypertension19 support this hypothesis. Hence, we speculate that increased CECs in patients with evidence of acute myocardial damage (STEMI and non-STEMI) compared with those without such evidence (UAP) probably reflect a greater degree of endothelial injury or damage associated with the more severe ischemic insult in the former group. Given the importance and diversity of physiologic functions of the endothelium, it seems likely that a greater degree of endothelial dysfunction, injury, or damage can lead to a greater degree of thrombosis and disturbed blood flow1,2 and thereby constitute the higher recurrent MACE rate observed in patients with STEMI and non-STEMI than in patients with UAP.
The potential for CECs to participate in additional disease such as thrombosis and microinfarction has been previously suggested in studies of patients in acute crises in sickle cell anemia20-22 and in patients with systemic lupus erythematosus (SLE).23 Once shed, CECs, if viable, may be capable of further potentiating vascular injury by the production of prothrombotic mediators (eg, VWF, tissue factor) and engaging in heterotypic aggregation with neutrophils or platelets, with resultant vascular thrombosis.20-23 Indeed, acute inflammation (IL-6), endothelial cell activation, damage, and dysfunction (VWF) are closely related determinants of the onset and course of acute coronary disease.24 Thus, our results are consistent with previous studies indicating that the acute release of IL-612,13 and VWF8-10 during the first 48 hours of presentation of ACS were significant independent predictors of adverse events at 30 days. In addition, we also show that the acute release of IL-6, a proinflammatory cytokine, is an independent predictor of adverse events at 1-year follow-up. Further support for the role of VWF and IL-6 in the course of acute coronary disease are the strong correlations between the numbers of CECs and the levels of VWF and IL-6. However, the precise mechanisms underlying interactions between VWF, IL-6, and CECs in the pathogenesis of recurrent cardiovascular event are unclear, probably complex, and certainly require further investigation.25
Several studies and reviews have addressed the origin and cellular nature of CECs3-5,20-23,25-27 and their relation to endothelial progenitor cells (EPCs).25,28-32 In this study, and our previous studies, CECs were defined using an endothelial-specific antigen CD146, which is almost exclusively expressed on mature endothelial cells.3,26 Furthermore, we found CECs to be almost entirely eNOS-staining positive, but effectively CD31 (PECAM-1), CD34 (EPC), CD36 (microvascular cells), and CD45 (leukocyte common antigen) negative. Thus, we assume that our cells are mural-endothelial in origin (eNOS being endothelial specific) and simply not just part of a repair process because EPC derived from the bone marrow are CD146– and CD34+.28-30 However, we acknowledge the possibility that as CD34– circulating cells may differentiate into endothelial cells ex vivo, they may have a role in the repair process in vivo.29,30 Further studies of CEC phenotypes are required to elucidate the pathogenesis of endothelial cell damage.
Finally, although it is clear that CECs are a novel tool in the assessment of endothelial cell damage, there are other such tools. Other investigations of endothelial dysfunction and injury in patients with CAD have used plasma increased VWF, thrombomodulin, and E-selectin alongside impaired brachial artery flow-mediated (endothelium-dependent) dilatation (FMD).1,2 Indeed, Solovey et al22 used cell surface thrombomodulin to help define their CD146+ CECs. Our present and previous studies have found strong relationships between numbers of CECs and VWF levels5 and inversely between FMD and VWF.33 Recently, increased CD146+ and annexin V+ (ie, apoptotic) CECs have been inversely correlated with brachial artery FMD and elevated tissue factor in young women with SLE.34 Our recent case-control study of congestive heart failure reports also an inverse correlation between FMD and CECs (r =–0.423, P = .002), alongside positive relationships between FMD and VWF (r =–0.461, P = .001) and between CECs and VWF (r = 0.29, P = .032).35 These data provide additional support for the concept of CECs as an additional marker of endothelial perturbation.
In conclusion, the present study suggests increased and increasing numbers of CECs in patients with acute coronary syndromes could be indicative of the severity of the ischemic episode and progressive of active disease, respectively. Despite the overwhelming mass of data on the vascular origin of CD146+ CECs, a very minor doubt remains as some spleen cells may coexpress several relevant markers.36 Nevertheless, our data suggest that numbers of CECs, which are closely associated with high VWF and IL-6 levels, may be a novel prognostic indicator of both short- and long-term adverse events in acute vascular disease.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-03-1106.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank James Devey for the immunoperoxidase work, and acknowledge the support of the Peel Medical Research Trust and the Sandwell and West Birmingham Hospitals NHS Trust Research and Development program for the Haemostasis Thrombosis and Vascular Biology Unit.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal