Abstract
This international clinical trial evaluated the safety and efficacy of recombinant factor IX (rFIX) in previously untreated patients (PUPs) with severe or moderately severe hemophilia B (FIX activity, ≤ 3 IU/dL). Sixty-three PUPs aged younger than 1 month to 14 years received rFIX (median treatment duration, 37 months; range, 4-64 months). Mean rFIX recovery (0.68 ± 0.27 IU/dL per IU/kg) remained constant over 5 years and was similar in infants (1 month to < 2 years) and children (2 to < 12 years). Fifty-four PUPs used rFIX (median dose, 62.7 IU/kg per infusion; range, 8.2-292 IU/kg) to treat 997 hemorrhages. Bleeding was well controlled, with 75% of hemorrhages requiring only one rFIX infusion. Response to rFIX was “excellent” or “good” in 94% of cases. Effective hemostasis was achieved in 32 PUPs receiving rFIX for routine prophylaxis, with 91% of prophylaxis responses rated “excellent.” rFIX administered for 30 surgical procedures in 23 PUPs achieved hemostasis for all rated procedures. Five patients experienced allergic-type manifestations, including 2 (3%) patients who developed FIX inhibitors (both > 5 BU/dL). rFIX was well tolerated, with no associated thrombotic events or evidence of viral transmission. These data indicate that rFIX is a safe and effective treatment for PUPs with hemophilia B.
Introduction
Hemophilia B is an X-linked recessive bleeding disorder resulting from factor IX (FIX) deficiency. Bleeding is characterized by spontaneous or trauma-induced hemorrhage into joints, muscles, and soft tissues. Hemorrhage into internal organs and the central nervous system may also occur.1-3 The goal of hemophilia treatment is to treat or prevent hemorrhage, reduce disabling joint and tissue damage, and improve quality of life and life expectancy. Historically, the mainstay of hemophilia B therapy has been the replacement of deficient FIX with pooled plasma or plasma derivatives. Although early plasma-derived FIX (pdFIX) products and prothrombin complex concentrates (PCCs) improved the clinical outcome for patients with hemophilia B, their use was associated with several complications, including thrombosis, disseminated intravascular coagulation, altered immune function, and transmission of HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV).4-6
In the past decade, the development of high-purity pdFIX preparations has reduced the thrombogenic risks associated with FIX replacement therapy.7-9 In addition, the introduction of improved viral attenuation methods during manufacture has reduced the risk of viral transmission with pdFIX products.10 Nevertheless, current viral attenuation methods may not eliminate nonenveloped viruses, including hepatitis A (HAV) and parvovirus, or blood-borne prions associated with transmissible spongiform encephalopathies, such as variant Creutzfeldt-Jacob disease.10,11 Concern also remains regarding the transmission of blood-borne pathogens with pdFIX products, despite manufacturing improvements such as the introduction of nanofiltration steps.11
The cloning of human FIX12 led to the expression of human recombinant FIX (rFIX) in Chinese hamster ovary (CHO) cells.13 Commercially available rFIX (BeneFIX; Wyeth Pharmaceuticals, Philadelphia, PA) is structurally and functionally similar to pdFIX, although minor differences in the posttranslational sulfation and phosphorylation of rFIX have been associated with a lower in vivo recovery.14-16 rFIX is not exposed to human- or animal-derived proteins at any stage in manufacture or formulation, and therefore has an enhanced viral safety profile compared with current pdFIX products.17-19
Clinical data demonstrating the efficacy and safety of rFIX for the treatment of hemorrhage and in prophylactic and surgical settings in previously treated patients (PTPs) have recently been published.20,21 We present here the findings of the largest international trial conducted in previously untreated patients (PUPs) with severe or moderately severe hemophilia B performed to date. This study investigated the efficacy and safety of rFIX in PUPs for the treatment or prevention of bleeding episodes.
Patients and methods
Patients
PUPs with severe (FIX activity of < 1 IU/dL) or moderately severe (FIX activity of 1-3 IU/dL) hemophilia B were eligible for participation. Other inclusion criteria included normal platelet count, normal liver function (with the exception of normalizing hyperbilirubinemia of the newborn), and normal renal function (serum creatinine level ≤ 1.25 times the upper limit of the normal range). Patients were excluded if they had been previously exposed to blood or blood products or had a positive FIX inhibitor as measured by the Bethesda inhibitor assay. A family history of FIX inhibitors was not a reason for exclusion. Other exclusion criteria included existence of another coagulation disorder (except for vitamin K deficiency of the newborn); infection with HIV-1, HIV-2, HAV, HBV, or HCV; a serious medical or social condition; or use of an investigational drug within 30 days prior to study entry.
Approval for the study was obtained by the institutional review boards or ethics committees at each of the participating centers listed in the “Appendix.” According to the Declaration of Helsinki, written informed consent was obtained for all participating study subjects.
Design and procedures
This international, multicenter, open-label, single-cohort study was conducted at 41 centers in the United States, Canada, Belgium, Denmark, France, Germany, Italy, The Netherlands, and the United Kingdom. Screening evaluations were performed at the initial visit. All patients who satisfied eligibility criteria at the baseline visit (day 1, within 30 days following the screening visit) were enrolled in the study. On day 1, rFIX was administered in the clinic by intravenous infusion for rFIX recovery and 24-hour survival. For recovery and survival assessments, plasma rFIX activity was measured prior to rFIX infusion, as soon as possible after infusion (preferably within 30 minutes of infusion), and 4 and 24 hours after infusion. rFIX was administered at a dose of 50 IU/kg for recovery and 24-hour survival assessments if the patient was in a nonbleeding state. Thereafter, rFIX was administered for on-demand treatment of any bleeding episode at a dose deemed appropriate by the investigator, or as a prophylactic treatment to prevent either hemorrhage or surgery-related bleeding (continuous infusion or bolus dosing or both allowed). Treatment dosage was based on standard guidelines6 according to the severity and nature of the hemorrhage or surgery and was adjusted for patient body weight and FIX activity measured during treatment.
Patients who received rFIX for on-demand treatment or routine prophylaxis continued on the study for at least 2 years, up to a maximum of 5 years or 100 exposure days (EDs), whichever occurred first. Patients who completed the core phase were permitted to continue treatment with rFIX for an additional year in the extension phase of the protocol. This allowed continued treatment of patients who were unable to obtain commercial rFIX (prior to approval and availability).
Efficacy assessments
Response ratings to assess bleeding control and prevention. Efficacy for each rFIX infusion used for on-demand treatment of bleeding was assessed 24 hours after the dose or just prior to the next dose. Efficacy was rated by the patient/guardian or investigator using a 4-point scale of “excellent,” “good,” “moderate,” or “no response,” consistent with the rating scale used in the rFIX hemophilia clinical study of PTPs.20 Surgical hemostasis was assessed by the investigator and surgeon, who estimated blood loss and assessed response to surgical prophylaxis using an identical 4-point scale.
For routine prophylaxis, the investigator rated response to treatment at 3-month intervals on a 3-point scale: “excellent” (prophylaxis treatment completely prevented spontaneous musculoskeletal bleeding, no change in dosing regimen necessary); “effective” (adequately prevented spontaneous musculoskeletal bleeding episodes as demonstrated by a lower than expected incidence of spontaneous musculoskeletal bleeding episodes); or “inadequate” (inadequate prevention of bleeding requiring a change in dosing regimen).
Pharmacokinetic studies. To evaluate the long-term stability of rFIX pharmacokinetics, assessments for rFIX recovery and 24-hour survival were made at baseline and every 6 months thereafter after a washout period of at least 4 days. Recovery of rFIX was defined as the maximum FIX activity (IU/dL) obtained within 30 minutes following a rFIX infusion, per dose of rFIX (IU/kg) infused. Recovery values were corrected for any preinfusion plasma FIX activity. If possible, rFIX recovery and 24-hour survival were measured when the patient was in a nonbleeding state.
Safety assessments
All patients had routine safety assessments 1 month after the first rFIX infusion. Thereafter, patients receiving on-demand treatment returned 2 months following the first rFIX infusion and then at 3-month intervals. Patients who received routine prophylaxis or who were frequently treated (≥ 2 new bleeding episodes per month) returned at monthly intervals or after every 5 EDs (whichever was sooner) until 20 rFIX EDs had occurred; patients then returned every 3 months. Safety was assessed by routine clinical assessments and clinical laboratory evaluations that included hematology; serum chemistry; serology for HAV, HBV, HCV, HIV-1 and HIV-2; FIX inhibitor by Bethesda assay; and anti-FIX antibody (enzyme-linked immunosorbent assay [ELISA]). Two independent ELISA tests were used to detect anti-FIX antibody responses—one to measure responses to rFIX, the other to measure responses to pdFIX. BeneFIX and pdFIX (Mononine; Aventis Behring, Kankakee, IL) were used to coat the wells of microtiter plates before the addition of the patient's serum at various dilutions, starting with an initial dilution of 1:25. Antibody bound to FIX was detected with a horseradish peroxidase–conjugated protein A reagent, followed by a horseradish peroxidase substrate to generate a color reaction that was detected spectrophotometrically.
Laboratory assessments
FIX activity was measured using a one-stage clotting assay. FIX inhibitor analyses were performed after a 4-day washout period using the classical Bethesda assay at the central laboratory. A positive inhibitor titer was defined as equal to or more than 0.6 BU/dL. Local laboratories also performed FIX inhibitor analyses. Anti-FIX antibodies were determined at the central laboratory by ELISA. FIX genotype analyses were also performed.
Statistics
All patients who enrolled in the study and received at least one dose of rFIX were included in the intent-to-treat analysis. Descriptive statistics including mean, median, range, and SD were used to describe the data.
Results
Patients
A total of 67 patients were enrolled in the study; 63 patients received at least one dose of rFIX and 4 patients remained untreated. Baseline characteristics of the 63 treated patients are shown in Table 1. Forty of the 63 patients completed the study. Of the 23 patients who did not complete the study, 12 patients withdrew consent (10 for reasons related to infrequent bleeding, burden of venipuncture, difficulties with transportation to the study site, or patient relocation away from the study site; 2 provided no reason for withdrawal of consent); 4 patients discontinued treatment due to protocol violation or noncompliance; 4 patients were withdrawn at the sponsor's or investigator's discretion (related to infrequent bleeding and commercialization of BeneFIX); 2 patients discontinued treatment due to FIX inhibitor development; and 1 patient was lost to follow-up.
Baseline patient demographics of 63 patients treated with rFIX
Characteristic | |
| Sex, no. (%) | |
| Male | 62 (98.4) |
| Female | 1 (1.6) |
| Race, no. (%) | |
| White | 54 (85.7) |
| Hispanic | 5 (7.9) |
| Black | 2 (3.2) |
| Black-Asian | 1 (1.6) |
| Mixed race | 1 (1.6) |
| Median age, mo (range) | 9 (0-168) |
| Mean weight, kg (SD) | 11.6 (8.9)† |
| Positive family history of hemophilia, no. (%) | 37 (58.7) |
| Positive family history of inhibitor, no. (%) | 1 (1.6) |
| Severity of hemophilia B*, no. (%) | |
| Severe (less than 1% FIX activity) | 40 (64.5)† |
| Moderately severe (1%-3% FIX activity) | 22 (35.5)† |
Characteristic | |
| Sex, no. (%) | |
| Male | 62 (98.4) |
| Female | 1 (1.6) |
| Race, no. (%) | |
| White | 54 (85.7) |
| Hispanic | 5 (7.9) |
| Black | 2 (3.2) |
| Black-Asian | 1 (1.6) |
| Mixed race | 1 (1.6) |
| Median age, mo (range) | 9 (0-168) |
| Mean weight, kg (SD) | 11.6 (8.9)† |
| Positive family history of hemophilia, no. (%) | 37 (58.7) |
| Positive family history of inhibitor, no. (%) | 1 (1.6) |
| Severity of hemophilia B*, no. (%) | |
| Severe (less than 1% FIX activity) | 40 (64.5)† |
| Moderately severe (1%-3% FIX activity) | 22 (35.5)† |
Patients were categorized according to their FIX activity prior to receiving the first dose of rFIX.
no. = 62.
Patients were followed over a median interval of 37 months (range, 4-64 months; mean, 38.1 ± 16.4 months). A total of 5032 infusions with a cumulative total of 5 741 189 IU rFIX were administered to patients receiving on-demand, prophylactic, and surgery-related treatment (Table 2).
Summary of exposure to rFIX
. | rFIX infusion type*† . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | On-demand treatment . | Prophylaxis‡ . | Surgery related§ . | Total . | |||
| No. of patients∥ | 54 | 42 | 23 | 56 | |||
| Total rFIX administered, IU/patient | |||||||
| Cumulative | 1 742 860 | 3 791 388 | 206 941 | 5 741 189 | |||
| Median (range) | 20 515 (690-240 293) | 83 825 (530-411 780) | 6130 (2000-31 580) | 91 620 (1260-483 140) | |||
| Dose/infusion, IU/kg | |||||||
| Median (range) | 62.70 (8.20-292.00) | 60.60 (9.70-230.40) | 70.00 (29.30-260.00) | 61.40 (8.20-292.00) | |||
| Mean ± SD | 75.58 ± 42.47 | 71.57 ± 36.89 | 89.87 ± 51.95 | 73.49 ± 39.47 | |||
| No. of infusions | |||||||
| Cumulative | 1505 | 3320 | 207 | 5032 | |||
| Median (range) | 19.00 (1-108) | 71.50 (1-212) | 6.00 (1-40) | 100.00 (1-239) | |||
| Mean ± SD | 27.87 ± 27.76 | 79.05 ± 68.74 | 9.00 ± 8.55 | 89.86 ± 74.04 | |||
| Duration of exposure, d | |||||||
| Cumulative | 1443 | 3311 | 117 | 4858 | |||
| Median (range) | 19.00 (1-107) | 71.50 (1-212) | 4.00 (1-20) | 93.50 (1-225) | |||
| Mean ± SD | 26.72 ± 26.41 | 78.83 ± 68.46 | 5.09 ± 3.96 | 86.75 ± 71.73 | |||
. | rFIX infusion type*† . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | On-demand treatment . | Prophylaxis‡ . | Surgery related§ . | Total . | |||
| No. of patients∥ | 54 | 42 | 23 | 56 | |||
| Total rFIX administered, IU/patient | |||||||
| Cumulative | 1 742 860 | 3 791 388 | 206 941 | 5 741 189 | |||
| Median (range) | 20 515 (690-240 293) | 83 825 (530-411 780) | 6130 (2000-31 580) | 91 620 (1260-483 140) | |||
| Dose/infusion, IU/kg | |||||||
| Median (range) | 62.70 (8.20-292.00) | 60.60 (9.70-230.40) | 70.00 (29.30-260.00) | 61.40 (8.20-292.00) | |||
| Mean ± SD | 75.58 ± 42.47 | 71.57 ± 36.89 | 89.87 ± 51.95 | 73.49 ± 39.47 | |||
| No. of infusions | |||||||
| Cumulative | 1505 | 3320 | 207 | 5032 | |||
| Median (range) | 19.00 (1-108) | 71.50 (1-212) | 6.00 (1-40) | 100.00 (1-239) | |||
| Mean ± SD | 27.87 ± 27.76 | 79.05 ± 68.74 | 9.00 ± 8.55 | 89.86 ± 74.04 | |||
| Duration of exposure, d | |||||||
| Cumulative | 1443 | 3311 | 117 | 4858 | |||
| Median (range) | 19.00 (1-107) | 71.50 (1-212) | 4.00 (1-20) | 93.50 (1-225) | |||
| Mean ± SD | 26.72 ± 26.41 | 78.83 ± 68.46 | 5.09 ± 3.96 | 86.75 ± 71.73 | |||
Excludes data for 5 patients due to inadequate source documentation at 3 study sites.
Excludes data for 2 patients from start of inhibitor development.
Includes primary and secondary (routine and intermittent) prophylaxis.
Excludes continuous infusions.
A patient may have received doses more than once a day for different reasons.
rFIX recovery
Of the 63 treated patients, recovery assessments were performed in 59 patients. These 59 patients received a total of 286 rFIX infusions, with a cumulative total of 327 238 IU and a mean dose of 64.2 ± 28.1 IU/kg (range, 14.2-222.2 IU/kg). A total of 202 recovery samples from 58 patients who had at least one recovery assessment within 30 minutes of rFIX infusion in the presence or absence of hemorrhage were analyzed, demonstrating a mean rise in FIX activity of 0.68 ± 0.27 IU/dL per IU/kg. Of these 202 samples, 93 samples from 38 patients were collected after an infusion of the target rFIX recovery dose of 50 IU/kg (± 10%), with a mean recovery of 0.68 ± 0.29 IU/dL per IU/kg. When this analysis was further restricted to patients who were in a nonbleeding state and who received the target rFIX recovery dose of 50 IU/kg (± 10%; 89 recovery samples from 36 patients), a similar rise in FIX activity of 0.67 ± 0.25 IU/dL per IU/kg was measured.
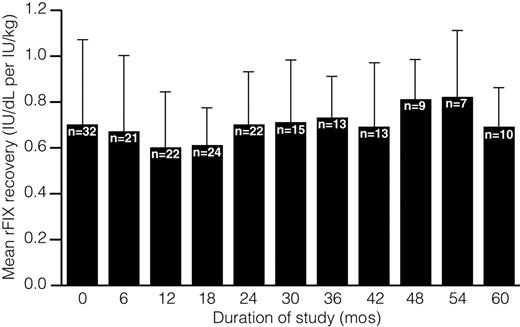
Data from 57 patients who underwent repeat rFIX recovery testing demonstrate that the average incremental rFIX recovery was consistent over time (Figure 1). To evaluate the effect of patient age on rFIX recovery, samples were categorized according to the age of the patient at the time of recovery sample collection. Recovery data for a given patient could be included in more than one age group, as the patient aged over the course of the study. There was no difference in the recovery of rFIX between infants aged 1 month to younger than 2 years (n = 33; mean increase in FIX activity 0.66 ± 0.36 IU/dL per IU/kg) and children aged 2 to less than 12 years; (n = 48; mean increase in FIX activity 0.68 ± 0.21 IU/dL per IU/kg). The increase in FIX activity in the neonate (n = 1; 0 to < 1 month) and adolescent (n = 3; ≥ 12 years) groups was 0.46 IU/dL per IU/kg and 0.93 ± 0.41 IU/dL per IU/kg, respectively. However, the small sample size of these groups meant that a comparison with infant and children groups could not be made. Insufficient numbers of blood samples were collected 24 hours following rFIX infusion, precluding the assessment of 24-hour rFIX survival in the study population as originally planned.
Average incremental rFIX recovery over duration of study. rFIX recovery, defined as the maximum FIX activity obtained 30 minutes or less after rFIX infusion, was assessed for 57 patients who had repeat recovery testing at scheduled follow-up visits.
Average incremental rFIX recovery over duration of study. rFIX recovery, defined as the maximum FIX activity obtained 30 minutes or less after rFIX infusion, was assessed for 57 patients who had repeat recovery testing at scheduled follow-up visits.
On-demand treatment
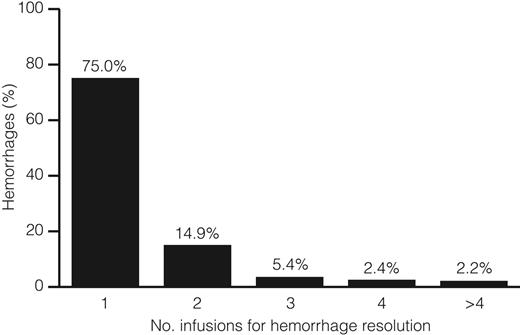
The efficacy evaluation was performed on 58 of the 63 treated patients; 5 patients were excluded from this analysis because of inadequate source documentation at 3 study sites. Of the 58 patients analyzed, 54 (93%) received rFIX for on-demand treatment of any bleeding episode (Table 2). These patients received a total of 1505 infusions for on-demand treatment of 997 hemorrhages. Of these 997 hemorrhages, 327 (32.8%) were hemarthroses, 491 (49.2%) were soft tissue/muscle hemorrhages, and 179 (18%) were multiple site/other hemorrhages. All bleeding episodes were controlled by rFIX treatment. The majority of bleeding episodes (748 of 997; 75%) were resolved by one rFIX infusion (Figure 2), and 938 of 997 (94.1%) had an “excellent” or “good” response to the first rFIX infusion administered for on-demand treatment (Figure 3A). The percentage of responses rated as “excellent” or “good” were similar for the treatment of bleeding in joints (93%), soft tissue/muscle (94%), and multisite hemorrhage locations (98%). Only 10 of 997 (1%) of bleeding episodes had a “no response” rating to the first rFIX infusion. These bleeds subsequently resolved with additional treatment.
Summary of number of on-demand rFIX infusions required for resolution of 997 hemorrhages.
Summary of number of on-demand rFIX infusions required for resolution of 997 hemorrhages.
Response ratings of rFIX. Response ratings of rFIX for (A) 997 infusions for on-demand treatment (n = 54), (B) routine prophylaxis (n = 32), and (C) surgical prophylaxis for 30 surgical procedures (n = 23).
Response ratings of rFIX. Response ratings of rFIX for (A) 997 infusions for on-demand treatment (n = 54), (B) routine prophylaxis (n = 32), and (C) surgical prophylaxis for 30 surgical procedures (n = 23).
Routine prophylaxis
Of the 42 patients who received rFIX for primary or secondary prophylaxis (Table 2), 32 were on a routine prophylactic schedule and the remainder received intermittent rFIX prophylaxis. Twenty-four patients received routine infusions of rFIX 2 or more times per week (mean infusion dose, 72.5 ± 37.1 IU/kg for a mean duration of 13.4 ± 8.2 months; range, 1-25 months). The remaining 8 patients received a prophylactic rFIX infusion once a week (mean rFIX dose, 75.9 ± 17.9 IU/kg; mean duration of 17.6 ± 7.4 months; range, 4-28 months). Overall, 157 of 172 (91.3%) of the prophylaxis regimen responses were rated as “excellent,” indicating that prophylaxis treatment completely prevented spontaneous musculoskeletal bleeding, with no change in dosing regimen necessary during the prior 3 months (the general interval between assessments). In addition, 11 of 172 (6.4%) of the prophylaxis regimen responses were rated as “effective,” with only 3 of 172 (1.7%) rated as “inadequate” (Figure 3B). The percentage of “excellent” ratings was higher in patients who received 2 or more infusions per week (94.1%), compared with patients who received a single infusion per week (84.9%).
Of the 32 patients on a routine prophylaxis schedule, 5 (16%) did not experience any breakthrough bleeding. The remaining 27 (84%) experienced 246 bleeding episodes. The etiology of 231 of 246 bleeding episodes was identified as follows: 175 of 231 (76%) were injury-related and 56 of 231 (24%) were spontaneous hemorrhages. The etiology of 15 bleeds was unknown. The majority (50 of 56; 89%) of spontaneous hemorrhages occurred more than 48 hours after infusion, whereas only 6 of 56 (11%) occurred within 48 hours after infusion. The percentage of patients who had early spontaneous breakthrough bleeding was lower in those who received 2 or more infusions per week (3 of 24; 13%) compared with those who received 1 infusion per week (2 of 8 patients; 25%).
Surgical prophylaxis
Twenty-three of the 58 patients included in the efficacy analysis received rFIX for the prevention of intraoperative and postoperative bleeding in 30 surgical procedures (Table 2), including 12 permanent venous access placements, 7 circumcisions, 1 dental procedure, and 10 “other” procedures. Of these, 4 patients undergoing 5 surgical procedures received rFIX by continuous infusion regimens, and 19 patients received rFIX by bolus dosing only. Of all infusions administered for surgical procedures, 29 of 30 (96.7%) provided “excellent” or “good” hemostasis, and 1 of 30 (3.3%) was “not rated” (Figure 3C). Blood loss was recorded as less than or equal to 5 mL or minimal during the majority (26 of 30; 87%) of surgical procedures. Otherwise bleeding was as anticipated for the procedure in a patient with normal hemostasis.
Safety assessments
Adverse events. Of the 63 patients who received rFIX during the study, 11 (17%) patients had 22 adverse events (AEs) that were deemed related or of unknown relationship to rFIX treatment (Table 3). These included 5 patients who experienced 8 events of potential allergic manifestation within 48 hours of rFIX infusion (3 mild [wheeze, rash, urticaria]; 3 moderate [1 dyspnea, 2 urticaria]; 2 severe [dyspnea and rigors]). Two of these patients had FIX inhibitors (1 with moderate dyspnea and urticaria, 1 with severe rigors), and 1 patient (urticaria and rash) without evidence of a FIX inhibitor had 1 positive FIX ELISA result that was not confirmed by Western blot. The events of potential allergic manifestation resolved in all patients. Patients who experienced these events remained on rFIX for the duration of the study, with the exception of the 2 patients who developed inhibitors. There were no events of potential allergic-type manifestation that occurred more than 48 hours after infusion that were related to rFIX. Most treatment-related AEs were mild or moderate in severity. A total of 4 serious AEs (SAEs) related to rFIX were reported in 3 of 63 (5%) patients and included FIX inhibitor development (2 patients; 3%), dyspnea (1 patient; 2%), and rigors (1 patient; 2%). No life-threatening AEs related to rFIX were reported. Patients who received rFIX for surgical prophylaxis did not experience any clinically significant AEs that were considered to be definitely related to rFIX.
Summary of AEs that are related or of unknown relationship to rFIX
Adverse event . | No. of patients (%) . | No. of events . |
|---|---|---|
| Infection* | 2 (3) | 2 |
| Rigors (chills) | 1 (2) | 1 |
| Injection site reaction | 1 (2) | 1 |
| Photosensitivity reaction | 1 (2) | 1 |
| Diarrhea | 1 (2) | 5 |
| FIX inhibitor | 2 (3) | 2 |
| Elevated alkaline phosphatase | 1 (2) | 1 |
| Elevated AST | 1 (2) | 1 |
| Elevated ALT | 1 (2) | 1 |
| Dyspnea | 2 (3) | 2 |
| Asthma | 1 (2) | 1 |
| Urticaria | 3 (5) | 3 |
| Rash | 1 (2) | 1 |
Adverse event . | No. of patients (%) . | No. of events . |
|---|---|---|
| Infection* | 2 (3) | 2 |
| Rigors (chills) | 1 (2) | 1 |
| Injection site reaction | 1 (2) | 1 |
| Photosensitivity reaction | 1 (2) | 1 |
| Diarrhea | 1 (2) | 5 |
| FIX inhibitor | 2 (3) | 2 |
| Elevated alkaline phosphatase | 1 (2) | 1 |
| Elevated AST | 1 (2) | 1 |
| Elevated ALT | 1 (2) | 1 |
| Dyspnea | 2 (3) | 2 |
| Asthma | 1 (2) | 1 |
| Urticaria | 3 (5) | 3 |
| Rash | 1 (2) | 1 |
Eleven patients had a total of 22 AEs. These patients may appear in more than one AE category and may have had more than one occurrence of a specific AE. Table includes AEs that were of definite, probable, possible, or unknown relationship to rFIX and excludes all AEs that occurred before study drug dosing.
Includes one event of hepatitis A seroconversion and one event of parvovirus B19 seroconversion, each in one patient; the relationship of both events to rFIX was unknown.
There were no thrombotic complications considered to be related to rFIX treatment. One individual experienced a clot in an intravenous access device (port-a-cath) while on a routine prophylaxis regimen. This resolved with urokinase treatment and was judged as unrelated to rFIX. Events of red blood cell (RBC) agglutination (the visual observation of RBC clumping in intravenous tubing/syringe during rFIX administration) were reported for 2 patients. These events resolved without clinical sequelae.
Inhibitors. All 63 treated patients received at least one FIX inhibitor test by Bethesda assay following rFIX exposure. Inhibitor testing was routinely performed 1 month after initiating therapy with rFIX, with repeat testing at least every 3 months thereafter. Only 2 (3%) patients developed high-titer FIX inhibitors (> 5 BU/dL), which in both cases were corroborated with positive anti-FIX antibody results by ELISA and Western blotting. Genotype analyses of the 2 patients who developed FIX inhibitors revealed that the F9 gene contained a point mutation, 6460C > T (mutation R29X); however, one of these patients also had a silent mutation in the coding region for the signal peptide of the protein, indicating that the patients were not related. One patient developed a low-titer inhibitor (3.5 BU/dL) after 15 EDs (approximately 5 months after his first dose of rFIX), which increased to a high-titer inhibitor peaking at 17.9 BU/dL before the patient was treated with activated recombinant factor FVII (rFVIIa). The other patient had an inhibitor detected on ED 7, which was approximately 7 weeks following his first dose of rFIX (5 BU/dL assayed at central laboratory). The inhibitor titer increased to a peak titer of 42 BU/dL; the patient became unresponsive to rFIX and was switched to an activated PCC (aPCC) followed by rFVIIa therapy. Both patients were withdrawn from the study after inhibitor detection, but continued to undergo follow-up assessments. The FIX inhibitor values diminished over time in these patients to 0 and 0.4 BU/dL, respectively, at the time of the last follow-up measurement. Four patients with no evidence of FIX inhibitor had transient positive anti-FIX antibody results by ELISA that were not corroborated by Western blot (1 occasion in 3 patients; 2 occasions in 1 patient).
Clinical chemistry and hematology. The hematologic abnormalities that were reported as AEs included anemia, leukopenia, iron deficiency anemia, thrombocytopenia, leukocytosis, and pancytopenia. None of these AEs were deemed related to rFIX. One patient had increased levels of serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) at month 6, which were reported as AEs of unknown relationship to rFIX. These results returned to within normal limits during the course of the study.
Viral serology. All 63 patients tested negative for HIV-1 and HIV-2. One patient tested positive for HAV at months 33 and 36, but the final study laboratory follow-up assay was negative. Documentation of prior HAV vaccination was not available for this patient. According to the investigator, the patient was never clinically ill and liver function tests were normal. Hence, this transient laboratory abnormality was considered not clinically important and probably due to a false-positive result by the investigator who reported the transient HAV laboratory abnormality as an AE with unknown relationship to rFIX (Table 3). There was no evidence that rFIX was associated with HAV, HBV, or HCV transmission based on serology findings and clinical assessments. An event of parvovirus B19 seroconversion was reported for one patient who was seropositive for parvovirus B19 during routine local laboratory testing on a single occasion. Confirmatory polymerase chain reaction testing was not performed. According to the investigator, the patient was asymptomatic, and the AE was reported as having an unknown relationship to rFIX.
Discussion
This study provides an evaluation of the long-term safety and efficacy of rFIX in 63 PUPs with severe and moderately severe hemophilia B. Overall, these 63 patients received approximately 6 million IU rFIX for on-demand treatment, routine prophylaxis, and surgical prophylaxis. The study population was representative of PUPs from North America and Europe ranging in age from younger than 1 month to 14 years at study entry. Moreover, the types of hemorrhages treated in this study were also characteristic of those occurring in this young patient population with severe or moderately severe hemophilia B, indicating that the safety and efficacy results are representative of the PUP population.
Previous studies with rFIX in PTPs with hemophilia B have shown that rFIX recovery tends to increase with patient age and that there is large interindividual variability in the disposition of FIX activity.20,22 Ideally, rFIX dosing should be based on the knowledge of the pharmacokinetic parameters of FIX activity in each patient.22 However, the number of plasma samples that can be drawn from young children is limited, and formal pharmacokinetic studies and individual dose titrations were impractical in the current study. As a result, we focused our pharmacokinetic assessments on recovery alone. rFIX recovery was similar for the 58 patients who received any dose of rFIX compared with 38 patients who received the 50/IU/kg target dose of rFIX (recovery, 0.68 ± 0.27 IU/dL per IU/kg and 0.68 ± 0.29 IU/dL per IU/kg rFIX, respectively). In addition, the recovery of rFIX in these PUPs was similar to that for PTPs younger than 15 years old (0.66 ± 0.22 IU/dL per IU/kg) treated with rFIX.20 Data for the recovery of rFIX in PUP subgroups were similar for infants and children and the recovery of rFIX in PUPs remained stable over 5 years, demonstrating the long-term consistency of this product.
The results presented in this study demonstrate that rFIX is safe and clinically effective in the treatment and prevention of bleeding of PUPs with hemophilia B. All hemorrhages treated with on-demand rFIX were ultimately well controlled, and in the majority of cases (90%), bleeding responded to 1 or 2 rFIX infusions. In patients receiving rFIX for prophylaxis, the frequency of spontaneous bleeding events increased following increasing time from rFIX prophylactic infusion, as would be expected for an effective prophylactic regimen. The percentage of patients who had spontaneous breakthrough bleeding was also lower in patients who received at least 2 infusions per week compared with patients who received 1 infusion per week, indicating a trend toward reduced breakthrough bleeding with more frequent rFIX dosing. In the surgical setting, rFIX administered for surgical bleeding prophylaxis achieved hemostasis for all rated surgical procedures, with minimal blood loss.
Although this was not a direct comparative study, the hemostatic efficacy of rFIX was rated against the investigators' previous experience with pdFIX using on-demand and surgical prophylactic regimens. The majority of responses to rFIX on-demand treatment were rated “excellent” or “good” (94%), and when responses were stratified by location of bleeding (joints, soft tissue/muscle, and multisite hemorrhage locations), the percentage of “excellent” or “good” responses remained high, ranging between 93% and 98%. Although 7 patients were rated as having “no response,” satisfactory efficacy was achieved with additional rFIX treatment for those without inhibitors. It is possible that the initial “no response” rating may have been attributable to delayed or inadequate rFIX dosing, which may occur in home treatment programs. All rated patients who were treated with prophylactic rFIX in the surgical setting had “excellent” or “good” responses. Most responses to rFIX routine prophylaxis were rated “excellent” (91%), indicating that prophylaxis treatment during the preceding 3 months completely prevented spontaneous musculoskeletal bleeding episodes and that no change in rFIX regimen was necessary. These responses to rFIX for on-demand and prophylactic treatment regimens are similar to those reported in other studies of pdFIX in patients with hemophilia B9,23-26 and for rFIX in PTPs.20
In the current study, the incidence of AEs related to rFIX treatment was low, occurring in 17% of patients, with most related AEs reported as mild or moderate in severity. The incidence of related SAEs, including dyspnea, chills, and the development of FIX inhibitor was low and at a level expected for any FIX replacement product.27 The use of pdFIX for the treatment of hemophilia B in PUPs may be complicated by safety issues such as the development of inhibitors to FIX, allergic reactions, and thrombosis.5,28-30 The development of inhibitors occurs in 1% to 3% of patients with hemophilia B,29 but may be higher in individuals with severe disease.29,31 In the current study, the incidence of FIX inhibitor was 2 of 63 (3%) patients, which is consistent with previous reports of hemophilia B patients treated with pdFIX products9,32 or PTPs treated with rFIX.20 Inhibitory antibodies occur most frequently in hemophilia B patients with major derangements of the F9 gene, due to large deletions, stop codons, and frame shift mutations.33,34 The 2 patients who developed a FIX inhibitor in the current study had the same genetic mutation responsible for severe hemophilia B (6460C > T point mutation, resulting in substitution of a stop codon “TGA” for arginine 29 [R29X] in the GLA domain of the FIX protein sequence). This mutation has been associated with a high risk for inhibitor development33,34 and anaphylactoid reactions following therapy with pdFIX.35 Of interest, genotype analysis also revealed that 2 additional patients with the same mutation did not develop inhibitors. Because the 2 patients who developed inhibitors were unrelated, and 2 additional patients with the same mutation did not develop inhibitors, the existence of other immune response modifiers should be considered in addition to the contribution of the R29X mutation itself. Postmarketing surveillance studies to monitor inhibitor development, combined with genotypic analyses, will enable further investigations into the relationship of genotype and other factors that impact inhibitor development.
There is a potential for allergic-type reactions with FIX infusion, especially in PUPs who develop inhibitors.30 Indeed, data collected in the North American Immune Tolerance Registry showed that allergic events accounted for 79% of AEs among patients with hemophilia B undergoing FIX immune tolerance therapy for inhibitor irradication.36 Complete F9 gene deletions confer a risk of anaphylaxis, in addition to inhibitor development33-35 ; however, the occurrence of potential acute allergic-type manifestations related to rFIX was low in this study. Only 5 patients experienced a total of 8 potential allergic-type manifestations within 48 hours following an infusion that were considered to be related to rFIX, including urticaria, dyspnea, rash, chills, and wheeze. Two of these 5 patients had FIX inhibitors, and 1 patient without an inhibitor had an isolated transiently positive FIX ELISA result that was not corroborated by Western blot. These potential allergic reactions resolved, and patients remained on rFIX for the duration of the study, with the exception of the 2 patients who developed inhibitors.
PCCs were an early mode of FIX replacement therapy used in patients with hemophilia B and were associated with thrombogenic complications due to the accumulation and delayed clearance of activated and nonactivated clotting factor proteins.8 The development of high-purity pdFIX concentrates led to the successful treatment of patients with hemophilia B who previously experienced hypercoagulable events or thromboembolic phenomena (or both) after using intermediate-purity PCCs.7,37 As expected, there was no evidence that rFIX significantly increased thrombin generation in this study; only one patient had a thrombotic event (a blood clot associated with a port site), which was deemed unrelated to rFIX. Events of RBC agglutination in the tubing/syringe have been reported with the administration of rFIX, but no AEs have been associated with these events.38 This phenomenon, attributable to the low ionic strength of the reconstituted rFIX, may be limited or prevented by avoiding significant mixing of blood with the reconstituted rFIX formulation. In the current study, there were 2 events of RBC agglutination; neither case was associated with a reported AE.
Viral transmission is a major safety concern associated with the use of plasma-derived concentrates.19 As anticipated, there was no evidence that rFIX was associated with the transmission of HIV, HAV, HBV, or HCV in this study based on serology findings and clinical assessments, therefore confirming the viral safety of rFIX.20 The report of transient HAV positivity in one patient was attributed to a false-positive result. One patient was noted to be seropositive for parvovirus B19 on a single occasion during routine local laboratory testing, but remained asymptomatic. Even though this event occurred during the patient's study participation and the causal relationship to rFIX was reported as unknown, this event is most likely unrelated to rFIX.
Human parvovirus B19 is ubiquitous and is responsible for erythema infectiosum (fifth disease) in children.39 Because this infection is common,40 seroconversion from a community-acquired infection is most likely. Parvovirus B19 seroprevalence was shown to be 32% in age-matched control pediatric patients with hemophilia who had not previously received transfusions of blood products.41 Furthermore, human parvovirus B19 has resisted cultivation in conventional cell lines, including CHO cells, which are used to manufacture rFIX.42 Also, viral clearance studies used to validate the rFIX purification process demonstrate significant removal of all model viruses tested, including bovine parvovirus (overall log10 removal value of 12.0), which is the model for the virus class that includes human parvovirus B19 (Data on file at Wyeth Pharmaceuticals). Therefore, the prevalence of this disease in the community, coupled with the safeguards built into the manufacturing process of rFIX, make it highly unlikely that parvovirus B19 infection via rFIX administration can be the cause of seroconversion.
Overall, there was no direct evidence to indicate transmission of any viral illness associated with rFIX use. Given the enhanced safety profile of rFIX compared with currently available plasma-derived products, rFIX provides a safe and clinically effective option for the treatment and prevention of bleeding in PUPs with severe or moderately severe hemophilia B for on-demand treatment, routine prophylaxis, and surgical prophylaxis. A study to corroborate these results in children younger than 6 years of age regardless of prior FIX treatment is ongoing.
Appendix
The following institutions and investigators participated in this study as members of the Recombinant Factor IX Study Group: Thomas Abshire (AFLAC Cancer Center and Blood Disorders Service, Emory University, Atlanta, GA); Steven Arkin (Mount Sinai Medical Center, New York, NY); Diana Beardsley (Yale University School of Medicine, New Haven, CT); Jonathan Bernstein (Children's Center for Cancer and Blood Diseases of Las Vegas, NV); Victor S. Blanchette (Hospital for Sick Children, Toronto, ON, Canada); Renate Bluetters-Sawatzki (Justus-Liebig-Universitat Giessen, Giessen, Germany); Doreen Brettler (University of Massachusetts Memorial Health Care, Worcester); Elisabeth Chalmers (Royal Hospital for Sick Children, Glasgow, United Kingdom); Segolene Claeyssens (CHU Purpan Centre Regionale de l'Hemophilie, Toulouse, France); Alice Cohen (Newark Beth Israel Medical Center, NJ); Peter Collins (University Hospital of Wales, Cardiff, United Kingdom); Michele Damay (Centre Hospitalier du Mans Service de Pediatrie, Le Mans, France); Michael Delorme (University of Western Ontario, London, ON, Canada); Annie Derlon (CHU de Caen-Hôpital Côte de Nacre, Caen, France); Franklin Desposito (St Michael's Medical Center, Newark, NJ); Jorge Di Paola (University of Iowa Hospitals and Clinics Pediatrics, Iowa City); Claire Gazengel (Groupe Hopitalier Necker-Enfants Malades Centre de Traitements de l'Hémophilie, Paris, France); Joan Gill (Blood Center of Southeastern Wisconsin, Milwaukee); Alessandro Gringeri (University of Milan, Milano, Italy); Michael Hamon (Derriford Hospital Hematology Department, Plymouth, United Kingdom); Margaret Ann Heisel (Minneapolis Children's Medical Center, MN); W. Keith Hoots (University of Texas-Houston Medical School); Anne Hurlet (St. Michael's Medical Center, Newark, NJ); Lawrence Jardine (University of Western Ontario, London, ON, Canada); C. Thomas Kisker (University of Iowa Hospitals and Clinics Pediatrics, Iowa City, IA); Christine Lee (Royal Free Hospital and School of Medicine, London, United Kingdom); Jeanne M. Lusher (Children's Hospital of Michigan Division of Hematology, Detroit); Marilyn J. Manco-Johnson (University of Colorado Health Sciences Center, Aurora); Catherine S. Manno (The Children's Hospital of Philadelphia, PA); Alison Matsunaga (Children's Hospital Medical Center Hematology/Oncology Department, Oakland, CA); Patricia McCusker (University of Western Ontario, London, ON, Canada); Massimo Morfini (Azienda Ospedaliera Careggi Hematology Department and Hemophilia Center, Firenze, Italy); Gaetano Muleo (Centro Emofilia Ospedale Civile Pugliese, Catanzaro, Italy); Claude Negrier (Hopital Edouard Herriot Centre Régional de Traitement de l'Hémophilie, Lyon, France); Idith Ortiz (University of Puerto Rico School of Medicine, San Juan); K. John Pasi (Royal Free Hospital and School of Medicine, London, United Kingdom); Claire Philipp (UMDNJ Robert Wood Johnson School of Medicine, New Brunswick, NJ); Hartmut Pollmann (Klinik und Poliklinik für Kinderheilkunde, Münster, Germany); Man-Chiu Poon (University of Calgary Foothills Hospital, Calgary, AB, Canada); Georges-Etienne Rivard (Hospital Ste-Justine Division of Hematology, Montreal, QC, Canada); Chantal Rothschild (Groupe Hopitalier Necker-Enfants Malades Centre de Traitements de l'Hémophilie, Paris, France); Pedro J. Santiago Borrero (University of Puerto Rico School of Medicine, San Juan, PR); Elma Scheibel (Rigshospital Juliane Marie Center Hemophilia Centre, Copenhagen, Denmark); Caroline Schoepfer (Centre Hospitalier du Mans Service de Pediatrie, Le Mans, France); Amy D. Shapiro (Indiana Hemophilia and Thrombosis Center, Indianapolis); Rinah Shopnick (Hemophilia and Thrombosis Center of Nevada, Las Vegas); RYJ Tamminga (University Hospital Groningen, Division of Haemostasis and Thrombosis Groningen, The Netherlands); Arthur Thompson (Puget Sound Blood Center Hemophilia Program, Seattle, WA); and Josef Vermylen (University Hospital Gasthuisberg Functiemetingen Bloedingsziekten, Leuven, Belgium).
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-06-2283.
A complete list of the members of the Recombinant Factor IX Study Group appears in the “Appendix.”
Supported by Wyeth Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Steve Sommer, City of Hope National Medical Center, Duarte, CA, for FIX genotype analyses; Dr James Meade, UNC Coagulation Laboratory, University of North Carolina, Chapel Hill, for analyses of FIX activity and inhibitor data; and Elizabeth A. Anderson of Wyeth Research for valuable contributions to the analysis of data. Wyeth Research was the sponsor of this clinical investigation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal