Abstract
Donor T-cell recognition of host alloantigens presented by host antigen-presenting cells (APCs) is necessary for the induction of graft-versus-host disease (GVHD), but whether direct alloreactivity is sufficient for the propagation of GVHD is unknown. In this study, we demonstrate that GVHD cannot be effectively propagated through the direct pathway of allorecognition. Rather, donor T-cell recognition of antigens through the indirect pathway is necessary for the perpetuation of GVHD. Furthermore, GVHD results in the breaking of self tolerance, resulting in the emergence of donor T cells that can cause autoimmune disease in syngeneic recipients. Notably, GVHD-induced autoreactivity is donor APC dependent, transferable into secondary hosts, and involves cells of the innate immune system. These results indicate that donor T-cell--mediated pathologic damage during GVHD becomes donor APC dependent and provide a mechanistic explanation for the long-standing observation that GVHD is associated with autoimmune clinical manifestations. (Blood. 2005;105:4885-4891)
Introduction
Graft-versus-host disease (GVHD) is a complex pathophysiologic process that has been divided into acute and chronic phases that have distinctive clinical features and occur with different temporal kinetics after transplantation.1-3 Acute GVHD presents as an inflammatory syndrome that generally targets a restricted set of organs consisting of the skin, liver, and intestinal tract. Chronic GVHD, on the other hand, has more protean manifestations and is characterized by the emergence of clinical features that bear close resemblance to what is observed in autoimmune disorders, such as systemic lupus erythematosus, Sjögren syndrome, scleroderma, and rheumatoid arthritis.4-6 In these patients, autoantibody formation is a common feature, providing evidence for the premise that chronic GVHD may be attributable, in part, to autoreactive donor T cells.7,8 Since, in most patients, chronic GVHD develops in the setting of preexisting acute GVHD, a long-standing, unresolved issue has been how acute GVHD, which is initiated by alloreactive donor T cells, evolves into chronic GVHD whereby autoreactive donor T cells have been speculated to play a role in the pathophysiology of this syndrome.8
Alloreactive donor T cells can be activated by recognition of peptides presented within the context of either self-major histocompatibility complex (MHC) molecules (indirect allorecognition) or allogeneic MHC molecules present in the recipient (direct allorecognition). Experimental studies in murine models have convincingly shown that GVHD is initiated by donor T-cell recognition of host alloantigens presented by host antigen-presenting cells (APCs),9,10 through the direct alloreactive pathway.11 As host APCs are eventually eliminated, donor T cells recognize host antigens presented on donor APCs via the indirect pathway of allorecognition.12,13 Recent studies suggest that donor APCs are required for maximizing GVHD severity once it is initiated within the context of host APCs,14 possibly by cross-presentation of host antigens to donor T cells. The extent to which the repopulation of the APC compartment by donor APCs affects the ability of donor T cells to recognize self antigens, however, is unknown. In this study, we examined whether GVHD alters donor T-cell reactivity to self antigens and the degree to which this is dependent on the presence of donor APCs. These results demonstrate that, during the course of GVHD, breaking of tolerance against self occurs, which results in donor T cells acquiring the ability to recognize both self and recipient antigens in a donor APC-dependent manner.
Material and methods
Mice
C57BL/6 (B6) (H-2b, CD45.2), B6.SJL (H-2b, CD45.1), Balb/cJ (H-2d), B10.BR (H-2k), B6.129S7-Rag-1-deficient (H-2b), and C.129S7-Rag-1-deficient (H-2d) mice were obtained from The Jackson Laboratories (Bar Harbor, ME). All animals were housed in microisolator cages at the Animal Resource Center of the Medical College of Wisconsin, which has been accredited by the American Association for Laboratory Animal Care (AALAC). Mice received regular mouse chow and acidified tap water ad libitum.
Induction of GVHD and adoptive transfer experiments
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco-modified media and passed through sterile mesh filters to obtain single-cell suspensions. BM was T cell-depleted (TCD) in vitro with anti-Thy 1.2 monoclonal antibody (clone 30-H12, rat immunoglobulin G2b [IgG2b]; American Tissue Culture Collection, Rockville MD) plus low-toxicity rabbit complement (C-6 Diagnostics, Mequon, WI). Lethally irradiated Balb mice (900 cGy) received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. In other experiments, lethally irradiated B10.BR (900 cGy) mice received transplants of TCD B6 BM plus 2 × 106 B6 spleen cells. Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 62 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Spleen cells from B6→Balb or B6→B10.BR chimeras undergoing GVHD were analyzed by flow cytometry to confirm complete donor cell engraftment prior to transfer into unirradiated B6 or Balb Rag mice. To obtain highly enriched populations of CD4+ and CD8+ T cells, splenic T-cell subpopulations were positively selected using the MACS magnetic cell separation system (Miltenyi Biotech, Auburn, CA), as previously described.15
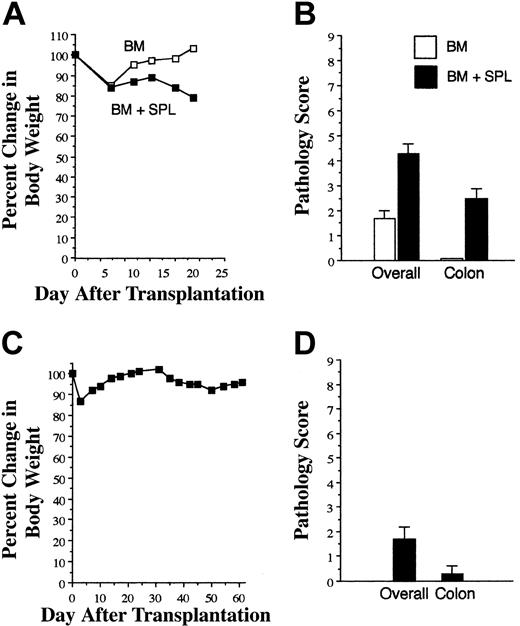
GVHD is not effectively propagated through direct allorecognition. (A) Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM alone (□, n = 3) or together with 3 × 105 B6 spleen cells (∼ 1 × 105 T cells; ▪, n = 8). Mice were killed 18 to 24 days after bone marrow transplantation (BMT). (A) Serial weight curves are shown in panel A. Mean overall GVHD score and pathology score in the colon are shown in panel B. Results are representative of 5 independent experiments. Pooled spleen cell suspensions from mice that had received transplants as in panel A (containing either 0.4 or 1 × 106 T cells in 2 independent experiments) were then transplanted along with B6 Rag-1 BM (10 × 106 cells) into lethally irradiated Balb mice (n = 7). Data indicate percentage of body weight change (C) and mean overall and colon GVHD scores (D). Error bars depict SEM.
GVHD is not effectively propagated through direct allorecognition. (A) Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM alone (□, n = 3) or together with 3 × 105 B6 spleen cells (∼ 1 × 105 T cells; ▪, n = 8). Mice were killed 18 to 24 days after bone marrow transplantation (BMT). (A) Serial weight curves are shown in panel A. Mean overall GVHD score and pathology score in the colon are shown in panel B. Results are representative of 5 independent experiments. Pooled spleen cell suspensions from mice that had received transplants as in panel A (containing either 0.4 or 1 × 106 T cells in 2 independent experiments) were then transplanted along with B6 Rag-1 BM (10 × 106 cells) into lethally irradiated Balb mice (n = 7). Data indicate percentage of body weight change (C) and mean overall and colon GVHD scores (D). Error bars depict SEM.
Generation of BM chimeras
B6 Rag mice were lethally irradiated (1000 cGy) and received transplants of 10 × 106 Balb Rag BM cells on day 0. In reciprocal studies, lethally irradiated Balb Rag mice (900 cGy) received transplants of 10 × 106 B6 Rag BM cells. Mice were bled 2 months after transplantation to confirm complete donor cell engraftment.
Flow cytometric analysis and assessment of chimerism
Monoclonal antibodies conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to assess chimerism in marrow transplant recipients. PE-anti-CD8 (clone CT-CD8a, rat IgG2a) and tricolor (TC)-anti-CD4 (clone CT-CD4, rat IgG2a) were obtained from Caltag (San Francisco, CA). PE-anti-Mac1 (clone M1/70 rat IgG2b), PE-anti-CD4 (clone GK1.5, rat IgG2b), FITC-anti-Thy1.2 (clone 30-H12, rat IgG2b), FITC-CD45.1 (clone A20, mouse IgG2a), FITC-anti-Gr-1 (clone RB6-8C5, rat IgG2b), FITC-anti-CD11c (clone HL3, hamster IgG), and FITC-anti-H-2Kb (clone AF6-88.5, mouse IgG2a) were all purchased from Pharmingen (San Diego, CA). Spleen cells were obtained from chimeras at defined intervals after transplantation, processed into single-cell suspensions, and stained for 2- or 3-color analysis. Red cells were removed when necessary by hypotonic lysis. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). At least 10 thousand cells were analyzed for each determination whenever possible.
Histologic analysis
Representative samples of lung, liver, pancreas, small intestines, kidney, and colon were obtained from recipients that received a transplant and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin. A semiquantitative scoring system was used to account for histologic changes consistent with GVHD in primary transplant recipients. Changes in representative organs deemed to be compatible with GVHD were as follows: lung (peribronchial or perivascular mononuclear infiltration and interstitial inflammation), colon (crypt cell apoptosis, crypt destruction, goblet cell depletion, and lamina propria lymphocytic infiltration), and liver (periportal infiltration and bile duct necrosis). The scoring system that was used categorized 0 as normal, 1 for mild, 2 for moderate, and 3 for severe for each of these organs (maximum score of 9/mouse). The severity of colitis in Rag mice that received GVHD T cells was classified as mild if there was depletion of goblet cells and minimal inflammation in the lamina propria; moderate if there were crypt cell abscesses, loss of crypts, and mononuclear infiltration extending into the submucosa; and severe if there was transmural inflammation and ulceration of the surface lining with purulent exudate in the colonic lumen (maximum score of 3/mouse). All slides were coded and read in a blinded fashion. Images were visualized using a Nikon Eclipse E400 microscope and a Nikon Plan APO 10 ×/0.45 objective lens (Nikon, Tokyo, Japan). Image acquisition was performed with a Zeiss Axiom camera and Axiovision 3.0.6 SP2 software (Zeiss, Berlin, Germany).
Immunohistochemistry
Tissues for immunoperoxidase studies were cut into 4-μm sections and fixed to charged slides. Antibody retrieval was performed using a pH 6, citrate, heat-induced epitope retrieval method. CD3 antibody (Polyclonal; Cell Marque, Hot Springs, AR) was used at a 1:500 dilution, and sections were stained on a DAKO Autostainer (Carpinteria, CA) at 25°C. Positive and negative controls were performed with each run of samples.
Statistics
Survival curves were constructed using the Kaplan-Meier product limit estimator and compared using the log-rank test. Group comparisons of total numbers of T cells, white blood cell subpopulations, spleen cells, and pathology scores were performed using the Mann Whitney U test or the Student t test. A P value no greater than .05 was deemed to be significant in all experiments.
Results
Reexposure of donor T cells from GVHD mice to host APCs and host alloantigens attenuates GVHD severity
We first examined whether donor T cells from mice undergoing GVHD could propagate the disease when reexposed to host alloantigens in the context of host APCs. B6→Balb chimeras with GVHD were killed 18 to 24 days after transplantation, and spleen cells from fully donor T cell-engrafted animals (hence-forth referred to as GVHD T cells) were transplanted, along with B6 Rag BM, into lethally irradiated Balb mice. At the time of transfer, GVHD animals had lost 15% to 20% of their body weight (Figure 1A) and had moderate GVHD with the colon as the primary GVHD target organ (Figure 1B). Lethally irradiated secondary Balb recipients, which had received a 4- to 10-fold higher dose of GVHD T cells, all survived for 60 days after transplantation without appreciable weight loss (Figure 1C) or histologic evidence of significant GVHD (Figure 1D). Thus, GVHD T cells had a significantly reduced ability to propagate GVHD after reencounter with host APCs and host alloantigens in lethally irradiated recipients.
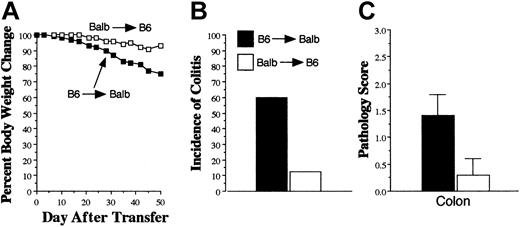
Donor APCs are required for the propagation of GVHD. Lethally irradiated (900 cGy) Balb Rag mice received transplants of B6 Rag BM to create chimeric animals in which APCs were of donor origin. In reciprocal studies, lethally irradiated (1000 cGy) B6 Rag animals received transplants of Balb Rag BM. Chimeric mice were bled 60 days after transplantation, and peripheral blood cells were determined to be more than 95% donor origin. Spleen cells (containing 0.6 × 106 T cells) from B6→Balb mice undergoing GVHD were then adoptively transferred into either B6 Rag BM→Balb Rag (▪, n = 10) or Balb Rag BM→B6 Rag (□, n = 8) chimeras 2 months after their initial transplantation. B6 Rag animals (n = 19) that received spleen cells containing 1 × 106 T cells from normal B6 mice served as pathologic controls. Weight loss (A), incidence (B), and severity of colitis (C) are depicted. Results are cumulative data from 2 experiments. Error bars depict SEM.
Donor APCs are required for the propagation of GVHD. Lethally irradiated (900 cGy) Balb Rag mice received transplants of B6 Rag BM to create chimeric animals in which APCs were of donor origin. In reciprocal studies, lethally irradiated (1000 cGy) B6 Rag animals received transplants of Balb Rag BM. Chimeric mice were bled 60 days after transplantation, and peripheral blood cells were determined to be more than 95% donor origin. Spleen cells (containing 0.6 × 106 T cells) from B6→Balb mice undergoing GVHD were then adoptively transferred into either B6 Rag BM→Balb Rag (▪, n = 10) or Balb Rag BM→B6 Rag (□, n = 8) chimeras 2 months after their initial transplantation. B6 Rag animals (n = 19) that received spleen cells containing 1 × 106 T cells from normal B6 mice served as pathologic controls. Weight loss (A), incidence (B), and severity of colitis (C) are depicted. Results are cumulative data from 2 experiments. Error bars depict SEM.
Donor APCs are required for the propagation of GVHD
During GVHD, there is elimination of host APCs and repopulation by donor APCs,16,17 which are able to present host alloantigens to donor T cells through the indirect pathway of allorecognition. To determine whether this pathway was required for the progression of GVHD, lethally irradiated Balb Rag mice received transplants of B6 Rag BM to create chimeric animals in which APCs were of donor (B6) origin. Spleen cells (containing 0.6 × 106 T cells) from B6→Balb mice with GVHD 3 to 4 weeks after BMT were then adoptively transferred into these chimeric animals. At the time of transfer, all dendritic (CD11c+) cells in the spleens of primary GVHD mice were of donor origin (data not shown). B6 Rag BM→Balb Rag chimeras that received transferred GVHD T cells had pronounced weight loss (Figure 2A), necessitating that mice be killed (31-50 days after transfer) in order that histologic analysis could be performed prior to demise. When compared with B6 Rag mice that received T cells from normal B6 animals and served as pathologic controls, there were no abnormalities observed in the small intestines, kidney, liver, lung, or pancreas of chimeric mice. Examination of the colon, however, which was the major target organ in primary GVHD animals, revealed the presence of colitis in 60% (6 of 10) of Balb recipients reconstituted with B6 APCs (Figure 2B). This was in contrast to reciprocally transplanted Balb Rag BM→B6 Rag mice in which weight loss was negligible, and only 1 (12%) of 8 mice developed colitis after transfer of GVHD T cells. The mean pathology score for colonic damage that accounted for both the incidence and severity of colitis in the 2 groups was significantly greater in B6 Rag BM→Balb Rag chimeras (mean, 1.4 ± 0.4 versus 0.3 ± 0.3; P = .036) (Figure 2C). These data indicated that GVHD T cells were capable of transferring disease into secondary recipients that had first been repopulated with donor APCs.
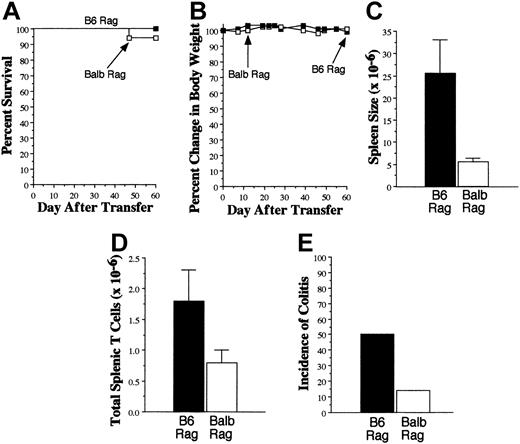
GVHD T cells cause colitis when transferred into syngeneic Rag mice. Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. Mice were killed 20 to 27 days after transplantation. Spleen cell suspensions were then transferred into either B6 (▪, n = 18) or Balb (□, n = 17) Rag mice. In most experiments, spleen cells were pooled from multiple GVHD animals before transfer into Rag recipients. Mice in both cohorts received an equivalent number of T cells in the spleen cell suspension, ranging between 0.3 and 1 × 106 in replicate experiments. Survival (A), serial weight curves (B), spleen cellularity (C), and total number of splenic T cells (D) are shown. Data in panels C and D are presented as the mean ± SEM. (E) Incidence of colitis in B6 and Balb Rag recipients of GVHD T cells. Data are derived from 6 independent experiments.
GVHD T cells cause colitis when transferred into syngeneic Rag mice. Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. Mice were killed 20 to 27 days after transplantation. Spleen cell suspensions were then transferred into either B6 (▪, n = 18) or Balb (□, n = 17) Rag mice. In most experiments, spleen cells were pooled from multiple GVHD animals before transfer into Rag recipients. Mice in both cohorts received an equivalent number of T cells in the spleen cell suspension, ranging between 0.3 and 1 × 106 in replicate experiments. Survival (A), serial weight curves (B), spleen cellularity (C), and total number of splenic T cells (D) are shown. Data in panels C and D are presented as the mean ± SEM. (E) Incidence of colitis in B6 and Balb Rag recipients of GVHD T cells. Data are derived from 6 independent experiments.
Donor T cells from GVHD mice induce autoimmune colitis
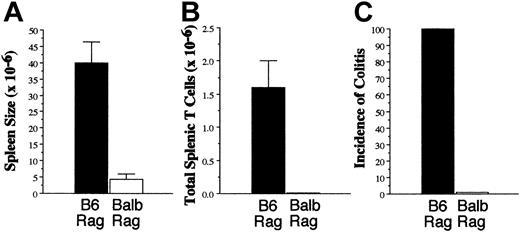
The long-standing clinical association between GVHD and autoimmune manifestations,4-7 coupled with our data indicating a requirement for donor APCs in the propagation of GVHD, led us to consider whether GVHD T cells might be capable of responding to self antigens presented by self APCs. To examine this question, spleen cells from lethally irradiated B6→Balb GVHD mice, transplanted as in Figure 1A, were adoptively transferred into nonirradiated B6 or Balb Rag animals. Adoptive transfer of GVHD T cells into either group resulted in no significant mortality (Figure 3A) or weight loss (Figure 3B), although spleen sizes of B6 Rag mice were significantly greater than in Balb Rag animals (25.5 × 106 versus 5.6 × 106, P < .001) (Figure 3C). There was also a statistically significant increase in the absolute number of splenic T cells in B6 Rag mice (1.8 × 106 versus 0.8 × 106, P < .05) (Figure 3D). Since the mean number of T cells transferred into recipients was 0.78 × 106 for B6 Rag and 0.72 × 106 for Balb Rag mice, this was indicative of a 2-fold expansion in the spleens of syngeneic recipients. Examination of the relative percentage of splenic CD4+ versus CD8+ T cells in both groups also demonstrated a statistically significant increase in the CD4/CD8 ratio in B6 versus Balb Rag recipients (4.3 ± 0.6 versus 1.6 ± 0.2, P = .001), indicating that a preferential expansion of CD4+ T cells had occurred. To determine whether expansion of GVHD T cells differed from that of normal T cells in syngeneic Rag animals, spleen cells from normal B6 mice containing 1 × 106 T cells were transferred into B6 Rag animals (n = 19). The mean absolute number of donor T cells in these animals was 2.5 × 106 cells after 60 to 70 days, indicating that the fold expansion of GVHD T cells was similar to that of normal T cells.
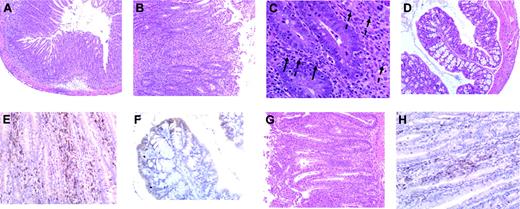
To determine whether T-cell expansion in B6 Rag mice was associated with any pathology, histologic examination was performed on representative tissue samples from the colon, small intestines, pancreas, liver, lung, and kidney of both B6 and Balb Rag animals. The only pathologic abnormalities observed were in the colon where colitis occurred in 50% of B6 Rag recipients (n = 18) but only 14% of Balb Rag mice (n = 17) (P = .038 by Fisher exact test) (Figure 3E). Microscopic examination of colitic tissue in B6 Rag recipients revealed marked infiltration of mononuclear cells into the lamina propria accompanied by depletion of goblet cells and sloughing of the mucosa into the colonic lumen (Figure 4A). There was also transmural inflammation extending out into the mesentery (Figure 4B). Neutrophils (dashed arrows) and eosinophils (solid arrows) also colocalized with T cells to histologically damaged tissue (Figure 4C), indicative of an associated innate immune response. Quantification of pathologic damage demonstrated that affected animals had moderate-severe colitis (mean pathology score, 2.4 ± 0.2). In contrast, the vast majority of Balb Rag recipients had no evidence of colitis with normal-appearing colonic mucosa, preservation of goblet cells, and absence of inflammation in the lamina propria and muscularis mucosa (Figure 4D). Immunohistochemical staining of paraffin-fixed sections of the colon in B6 Rag recipients demonstrated extensive CD3+ T-cell infiltration throughout the lamina propria extending across the wall of the colon into the surrounding mesentery (Figure 4E) but no B-cell involvement (data not shown). Areas of T-cell infiltration corresponded to sites of tissue damage (ie, crypt abscesses, crypt cell apoptosis). In contrast, there was no detectable T-cell infiltrate when histologically normal colonic tissue from Balb Rag mice that received GVHD T cells was examined (Figure 4F), indicating that these cells did not nonspecifically migrate to the colon. The relative lack of pathologic damage in nonirradiated Balb Rag mice was further evidence (see also Figure 1) that GVHD could not be effectively propagated through the direct alloreactive pathway.
Colitis is characterized by extensive infiltration of T cells and granulocytes. (A) Histologic analysis of colitis in B6 Rag recipients that received B6→Balb GVHD T cells, revealing marked infiltration of mononuclear cells into the lamina propria accompanied by depletion of goblet cells and sloughing of the mucosa into the colonic lumen along with (B) transmural inflammation extending out into the mesentery indicative of serosal inflammation (original magnification, × 200). (C) Neutrophils (dashed arrows) and eosinophils (solid arrows) in regions of histologically damaged colonic tissue. (D) Colonic mucosa in Balb-Rag recipients showing no evidence of colitis with preservation of goblet cells and the absence of inflammation in the lamina propria and muscularis mucosa. (E) Immunohistochemical staining showing extensive CD3+ T-cell infiltration (brown staining cells) throughout the lamina propria extending across the wall of the colon into the surrounding mesentery corresponding to sites of tissue damage in B6 Rag animals (ie, crypt abscesses, crypt cell apoptosis). (F) Absence of GVHD T-cell infiltration into the mucosa or lamina propria of Balb Rag mice. (G) Colonic tissue from B6 Rag recipients of B6→B10.BR spleen cells showing similar pathologic damage and (H) CD3+ T-cell infiltration as in panels A and E, respectively.
Colitis is characterized by extensive infiltration of T cells and granulocytes. (A) Histologic analysis of colitis in B6 Rag recipients that received B6→Balb GVHD T cells, revealing marked infiltration of mononuclear cells into the lamina propria accompanied by depletion of goblet cells and sloughing of the mucosa into the colonic lumen along with (B) transmural inflammation extending out into the mesentery indicative of serosal inflammation (original magnification, × 200). (C) Neutrophils (dashed arrows) and eosinophils (solid arrows) in regions of histologically damaged colonic tissue. (D) Colonic mucosa in Balb-Rag recipients showing no evidence of colitis with preservation of goblet cells and the absence of inflammation in the lamina propria and muscularis mucosa. (E) Immunohistochemical staining showing extensive CD3+ T-cell infiltration (brown staining cells) throughout the lamina propria extending across the wall of the colon into the surrounding mesentery corresponding to sites of tissue damage in B6 Rag animals (ie, crypt abscesses, crypt cell apoptosis). (F) Absence of GVHD T-cell infiltration into the mucosa or lamina propria of Balb Rag mice. (G) Colonic tissue from B6 Rag recipients of B6→B10.BR spleen cells showing similar pathologic damage and (H) CD3+ T-cell infiltration as in panels A and E, respectively.
Development of autoimmune colitis is not murine model dependent
To exclude the possibility that the observed pathologic lesions were model dependent, we performed similar transplantation studies using a B6[H-2b]→B10.BR[H-2k] murine model. Spleen cells from lethally irradiated B6→B10.BR mice undergoing GVHD were adoptively transferred into nonirradiated B6 Rag animals. A total of 7 independent experiments comprising a total of 42 recipient mice were conducted. The absolute number of donor T cells recovered from the spleens of recipient B6 Rag mice averaged 1.5 ± 0.3 × 106, which was an approximate 1.7-fold expansion, similar to what was observed in the B6→Balb murine model. Transfer of GVHD T cells into B6 Rag recipients resulted in colitis in 43% of recipients with pathologic abnormalities (Figure 4G) and extensive T-cell infiltration (Figure 4H) that were identical to those observed in B6 Rag recipients of B6→Balb GVHD T cells.
Autoimmunity is propagated by CD4+ T cells
To confirm that T cells were responsible for the induction of autoimmunity in syngeneic recipients, we purified CD4+ and CD8+ T cells from the spleens of primary (B6→Balb) GVHD mice and cotransferred them into B6 Rag animals (0.5 × 106 cells per mouse). The absolute number of CD4+ and CD8+ T cells transferred into these mice was 0.4 × 106 and 0.1 × 106, respectively. All B6 Rag mice (n = 4) developed profound (> 20%) weight loss within 35 days of transfer, necessitating that animals be killed. Histologic analysis revealed the presence of severe colitis in all recipients (data not shown). Since we had previously observed a preferential expansion of CD4+ T cells in the spleens of B6 Rag recipients of GVHD T cells, we considered that CD4+ T cells might be capable of causing autoimmune colitis in the absence of CD8+ T cells. To test this hypothesis, purified CD4+ T cells from primary GVHD animals (0.5 × 106) were transferred into B6 Rag mice. All recipients (n = 5) had significant weight loss (mean, 30% pretransplantation weight loss) and histologic evidence of colitis (mean pathology score, 2.2 ± 0.4) within 30 days of transfer. Thus, CD4+ T cells were sufficient for the induction of autoimmunity.
Autoimmunity can be serially passaged through B6 Rag mice
Whereas GVHD could not be easily transferred in either irradiated (Figure 1) or nonirradiated (Figure 3) Balb secondary recipients, we examined whether autoimmunity could be serially passaged in B6 syngeneic mice. Spleen cells from B6→Balb chimeras undergoing GVHD were adoptively transferred into B6 Rag mice for 67 days after which time these mice were killed, and spleen cells were transferred into new secondary B6 or Balb Rag recipients for an additional 2 months (n = 4/group). Both the spleen size (mean, 39.9 × 106 versus 4.3 × 106, P = .029) (Figure 5A) and the absolute number of donor splenic T cells (mean, 1.6 × 106 versus 0.01 × 106, P = .03) (Figure 5B) were significantly increased in B6 versus Balb Rag mice, similar to what was observed in primary transfer recipients (Figure 2). Histologic analysis revealed moderate-severe colitis in all B6 Rag animals, while no pathology was observed in the colons of Balb Rag mice (Figure 5C). These results indicated that autoreactive T cells maintained their capability to transfer disease even after parking these cells in syngeneic recipients for 2 months.
Autoimmunity can be transferred in syngeneic recipients. Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. Spleen cells were obtained from GVHD animals 22 days after BMT and transferred into B6 Rag recipients for 67 days. Mice were then killed, and pooled spleen cells were transferred into either B6 or Balb Rag animals (n = 4/group). Mice were killed 61 days after transfer and analyzed for overall spleen cellularity (A), absolute number of splenic T cells (B), and incidence of colitis (C). Data are presented as the mean ± SEM.
Autoimmunity can be transferred in syngeneic recipients. Lethally irradiated (900 cGy) Balb mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. Spleen cells were obtained from GVHD animals 22 days after BMT and transferred into B6 Rag recipients for 67 days. Mice were then killed, and pooled spleen cells were transferred into either B6 or Balb Rag animals (n = 4/group). Mice were killed 61 days after transfer and analyzed for overall spleen cellularity (A), absolute number of splenic T cells (B), and incidence of colitis (C). Data are presented as the mean ± SEM.
Expansion of the innate immune system in mice with autoimmunity
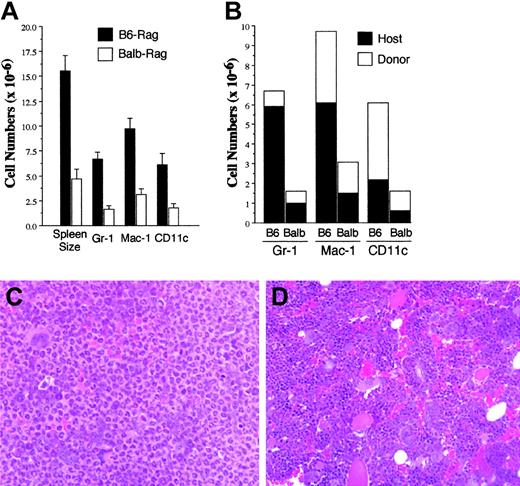
Phenotypic analysis of spleen cells in B6 Rag recipients of GVHD T cells revealed that the majority of cells were not T cells (Figure 3C versus 3D). Since spleen cells were used for adoptive transfer and host Rag mice have endogenous hematopoietic cells, we reasoned that these non-T cells might be comprised of both donor and host cell populations. To determine both the phenotype and origin of these cells, we used a congenic primary GVHD model (B6.SJL→Balb) to distinguish donor (CD45.1) from host (CD45.2) cells in B6 Rag mice. Histologic analysis of mice 60 to 70 days after transfer revealed colitis in 67% of B6 Rag mice but only 16% of Balb Rag animals (n = 6/group). As previously observed (Figure 3), there was an increase in overall spleen cellularity in B6 versus Balb Rag mice (15.5 × 106 versus 4.7 × 106, P = .002) (Figure 6A). This was accompanied by statistically significant increases in the absolute numbers of Gr-1+, Mac-1+, and CD11c+ cells relative to what was observed in Balb Rag recipients (P ≤ .002 for all group comparisons). Most Gr-1+ and Mac-1+ cells were of host origin, while donor-derived CD11c+ cells constituted most dendritic-cell population (Figure 6B). Histologic examination of the bone marrow revealed marked neutrophilic hyperplasia in B6 Rag (Figure 6C) in comparison to Balb Rag mice where normal representation of all hematopoietic elements was present, and there was no evidence of preferential granulocytic expansion (Figure 6D). Thus, the development of autoimmunity was associated with granulocytic infiltration in histologically damaged target organs (Figure 4C) as well as significant increases in innate immune cells in both bone marrow and spleen.
Expansion of the innate immune system in mice with autoimmunity. Lethally irradiated Balb mice received transplants of TCD B6.SJL (CD45.1) BM plus 3 × 105 B6.SJL spleen cells. Spleen cells were obtained from GVHD mice 3 to 4 weeks after BMT and transferred into either B6 (n = 6) or Balb (n = 6) Rag mice. Mice in each cohort were killed approximately 2 months after transfer and analyzed for spleen cellularity and absolute numbers of Gr-1+, Mac-1+, and CD11c+ cells (A) as well as the absolute numbers of Gr-1+, Mac-1+, and CD11c+ cells that were of donor (CD45.1) or host (CD45.2) origin (B). Data are cumulative results from 2 independent experiments. Histology of representative bone marrows from B6 (C) or Balb (D) Rag mice that received spleen cells from lethally irradiated Balb mice that had received transplants of TCD B6 BM plus B6 spleen cells to induce GVHD (original magnification, × 200). Marrow from B6 Rag mouse shows an almost homogenous population of mature neutrophils, while marrow from Balb Rag mouse demonstrates a heterogenous population of erythroid, myeloid, and megakaryocytic cells. In panels A and B, error bars indicate standard error measurement (SEM).
Expansion of the innate immune system in mice with autoimmunity. Lethally irradiated Balb mice received transplants of TCD B6.SJL (CD45.1) BM plus 3 × 105 B6.SJL spleen cells. Spleen cells were obtained from GVHD mice 3 to 4 weeks after BMT and transferred into either B6 (n = 6) or Balb (n = 6) Rag mice. Mice in each cohort were killed approximately 2 months after transfer and analyzed for spleen cellularity and absolute numbers of Gr-1+, Mac-1+, and CD11c+ cells (A) as well as the absolute numbers of Gr-1+, Mac-1+, and CD11c+ cells that were of donor (CD45.1) or host (CD45.2) origin (B). Data are cumulative results from 2 independent experiments. Histology of representative bone marrows from B6 (C) or Balb (D) Rag mice that received spleen cells from lethally irradiated Balb mice that had received transplants of TCD B6 BM plus B6 spleen cells to induce GVHD (original magnification, × 200). Marrow from B6 Rag mouse shows an almost homogenous population of mature neutrophils, while marrow from Balb Rag mouse demonstrates a heterogenous population of erythroid, myeloid, and megakaryocytic cells. In panels A and B, error bars indicate standard error measurement (SEM).
Discussion
Presentation of host alloantigens on host APCs is a prerequisite for the initiation of GVHD.9 Elimination of host APCs, however, occurs early after transplantation after which time recipients with ongoing GVHD become repopulated with donor APCs.16,17 A central question in GVHD pathophysiology is, therefore, the relative role of host versus donor APCs in the propagation of the disease. Our results indicate that T cells from GVHD animals that have reconstituted with donor APCs have a markedly reduced capacity to transfer GVHD when reexposed to host APCs and host antigens, even when secondary recipients are given 4- to 10-fold higher doses of GVHD T cells. While the ability to serially transfer GVHD was not completely abrogated, it was substantially attenuated when compared with the severity of GVHD induced by a lower dose of T cells in primary recipients. This was observed regardless of whether transfer of these cells was performed in an inflammatory (Figure 1) or noninflammatory (Figure 3) environment. These data indicate that T cells from mice with established GVHD have a reduced ability to propagate the disease through the direct alloreactive pathway. These results are consistent with prior studies conducted many years ago in nonirradiated parent→F1 (first filial generation) models of GVHD in which serial transfer of GVHD occurred only when massive numbers of cells were administered to secondary recipients.18 Since tissue damage is a progressive and ongoing event in GVHD, this suggests that propagation of GVHD must be dependent upon an alternative pathway of allorecognition. Our data demonstrating that equivalent numbers of GVHD T cells, which are largely incapable of recognizing Balb tissues in the presence of Balb APCs, can cause pathologic damage when donor type APC repopulation has occurred in these same animals (Figure 2) are evidence that perpetuation of GVHD is dependent upon indirect allorecognition. This presumably occurs through cross-presentation of host antigens to donor APCs and is consistent with data from Matte et al14 who showed that the presence of functional donor APCs intensified GVHD severity, once it had been initiated by donor T cell-host APC interactions.
A long-standing question in the field of allogeneic stem cell transplantation has been why some patients with GVHD develop autoimmune manifestations. Several explanations have been advanced in an effort to resolve this paradox. One is that increased apoptosis after allogeneic BMT19-21 predisposes patients to the development of autoimmunity by causing surface exposure of autoantigens22 which then can be recognized by T cells and induce autoantibody formation and class I-restricted responses.23-26 To date, however, there has been no formal proof that increased apoptosis results in the breaking of T-cell tolerance to self antigens. An alternative explanation is that acute GVHD results in thymic damage which impairs normal thymic selection.27,28 Prior studies have shown that clonally dysregulated T cells emerge from the thymus with antihost specificity29,30 ; however, whether these T cells are truly autoreactive has not been definitively shown. Our studies now demonstrate that T-cell tolerance to self antigens is broken during GVHD, resulting in the emergence of autoreactive T cells that are capable of causing pathologic damage. Recognition of self antigens was dependent upon donor APCs, as reconstitution of B6 Rag mice with Balb APCs almost completely abrogated the ability of GVHD T cells to cause autoimmune colitis (Figure 2). At the pathologic level, GVHD in allogeneic recipients reconstituted with donor APCs was indistinguishable from autoimmunity in syngeneic recipients, indicating that donor T cell-donor APC interactions, and not the MHC background of host tissue antigens, were the determinant factor in the elicitation of pathology. This resulting transition in GVHD pathobiology, whereby pathologic damage mediated by donor T cells can occur in syngeneic or allogeneic recipients as long as mice have been repopulated with donor APCs, would potentially allow for T-cell reactivity to a broader repertoire of antigens in primary GVHD animals. This scenario also provides a mechanistic explanation for why autoimmunity is able to develop in the primary GVHD setting and why chronic GVHD is characterized by more widespread organ involvement.
An important question is whether the T cells that induce colitis under these 2 different conditions are the same or different. One possible explanation is that the same T cells are responsible for both autoreactivity and indirect alloreactivity. In this scenario, donor T cells acquire the capability of responding to either host or donor antigens within the context of donor APCs. An alternative explanation is that autoreactive donor T cells are distinct from those that cause colitis through the indirect pathway. This explanation would support a model of GVHD whereby there is recruitment of bystander T cells that are not alloreactive but lose self tolerance. The ability of alloreactive T cells to influence survival of bystander nonalloreactive T cells has been previously demonstrated,19 but whether these T cells are able to affect bystander T-cell tolerance to self antigens is unknown. Addressing this question in future studies may provide new insights into how autoimmunity develops within the context of GVHD.
Autoimmune clinical manifestations typically occur later during the course of GVHD when the disease is classified as chronic.3-5 What is not known, however, is whether autoreactive T cells are present early after BMT or whether these cells emerge later to coincide with these clinical features. In that regard, Parkman's group demonstrated that both alloreactive cytotoxic CD8+ and autoreactive noncytotoxic CD4+ T cells could be cloned from the peripheral blood of mice 10 to 14 days after BMT.31,32 In contrast, by 50 days after transplantation, only noncytotoxic CD4+ T cells were present. These data suggested that CD4+ T cells were responsible for autoreactivity and emerged in the setting of alloreactivity early after allogeneic BMT. Our studies are consistent with these data as autoreactive T cells were present as early as 3 to 4 weeks after BMT at a time when primary mice had pathologic evidence of GVHD in the colon. Furthermore, our data also provide definitive proof that CD4+ T cells from GVHD mice are able to induce autoimmunity as purified CD4+ T cells from these animals were able to cause colitis when transferred into syngeneic recipients.
The development of colitis as the primary histologic abnormality in B6 Rag mice is similar to what has been observed in nontransplantation murine models of inflammatory bowel disease whereby an inadequate regulatory response has been implicated as one of the primary mechanisms responsible for pathology.33-37 The preferential targeting of the colon may be attributable to the fact that mucosal microflora function, in essence, as self antigens.36 This observation raises the question as to whether autoimmunity is the result of impaired T-cell regulation arising in the setting of GVHD. While the relationship between GVHD and regulatory T cells has not been defined, a recent study38 has shown an inverse correlation between GVHD severity and forkhead box p3 (foxp3) expression, suggesting that absence of effective T-cell regulation may play a role in the pathophysiology of GVHD. The extent to which this might predispose GVHD recipients to develop autoimmune manifestations is unknown but has precedence in other experimental and clinical settings in which defective or absent T-cell regulation induces autoimmunity.39-41 This premise is also supported by the granulocytic hyperplasia in the spleen and bone marrow and the marked neutrophilic infiltration in the colon that we observed in these animals. Similar findings have been made in mice with interleukin 2 receptor β (IL-2Rβ) deficiency, whereby dysregulation of T cells is associated with hypergranulopoiesis in spleen, bone marrow, and other tissues sites,42 and in Janus kinase 3 (JAK 3)-deficient mice, whereby constitutive T-cell activation occurs.43
An alternative, but not mutually exclusive, explanation for the development of autoimmunity is that acute GVHD results in thymic damage that impairs normal thymic selection. Direct injury to the thymus during GVHD has been well documented, and this appears to be a major mechanism to explain the impaired T-cell reconstitution that occurs after BMT.44,45 This has been shown to result in the escape of T cells with reactivity against host antigens,30,46 although whether these cells can respond to self antigens has not been previously examined. Impaired negative selection in the thymus of syngeneic BM chimeras has also been shown to result in clinical features resembling what is observed in acute GVHD.47 This was attributable to the emergence of an autoreactive T-cell repertoire and the concurrent absence of a regulatory T-cell population. These studies, however, did not assess whether T cells from GVHD mice were capable of causing autoimmunity. To address these questions, experiments are currently under way to define whether defective or absent T-cell regulation, impaired thymic selection, or a combination of the 2 are responsible for the development of autoimmunity during GVHD.
In summary, our studies suggest a model whereby primary GVHD is initiated by donor T-cell recognition of recipient alloantigens presented in the context of host APCs, or what has been termed direct alloreactivity. Eventually host APCs are eliminated and replaced by donor APCs that become the predominant APC population and present host antigens to donor T cells via the indirect pathway of allorecognition. At some time point during this transition, there is a breaking of tolerance against self, which results in donor T cells acquiring the ability to recognize both host and self antigens, but only in the context of donor APCs. These data are indicative of the critical role that donor APCs play in the propagation of both alloreactivity and autoreactivity and, by extension, GVHD. These results also provide an explanation for why autoimmunity is a component of GVHD in allogeneic marrow transplant recipients.
Prepublished online as Blood First Edition Paper, March 3, 2005; DOI 10.1182/blood-2004-12-4980.
Supported by grants from the National Institutes of Health (HL64603) and the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI).
E.T. performed the research and analyzed data, R.K. analyzed the pathologic data, and W.R.D. designed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal