Abstract
The BCR-ABL1 fusion kinase is frequently associated with chronic myeloid leukemia and B-cell acute lymphoblastic leukemia but is rare in T-cell acute lymphoblastic leukemia (T-ALL). We recently identified NUP214-ABL1 as a variant ABL1 fusion gene in 6% of T-ALL patients. Here we describe the identification of another ABL1 fusion, EML1-ABL1, in a T-ALL patient with a cryptic t(9;14)(q34;q32) associated with deletion of CDKN2A (p16) and expression of TLX1 (HOX11). Echinoderm microtubule-associated protein-like 1-Abelson 1 (EML1-ABL1) is a constitutively phosphorylated tyrosine kinase that transforms Ba/F3 cells to growth factor-independent growth through activation of survival and proliferation pathways, including extracellular signal-related kinase 1/2 (Erk1/2), signal transducers and activators of transcription 5 (Stat5), and Lyn kinase. Deletion of the coiled-coil domain of EML1 abrogated the transforming properties of the fusion kinase. EML1-ABL1 and breakpoint cluster region (BCR)-ABL1 were equally sensitive to the tyrosine kinase inhibitor imatinib. These data further demonstrate the involvement of ABL1 fusions in the pathogenesis of T-ALL and identify EML1-ABL1 as a novel therapeutic target of imatinib. (Blood. 2005;105:4849-4852)
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is frequently characterized by chromosomal rearrangements leading to ectopic expression of transcription factors (including TLX1, TLX3, LMO1, LYL1) or the generation of chimeric transcription factors (including SIL-TAL1 or MLL fusions).1,2 In addition, mutations in protein tyrosine kinases (LCK and FLT3) have also been identified in a small subset of T-ALL cases.3,4 In contrast to B-cell acute lymphoblastic leukemia (B-ALL), the BCR-ABL1 oncogene is only rarely implicated in the pathogenesis of T-ALL1,5-7 but we recently identified a variant ABL1 fusion gene, NUP214-ABL1, in approximately 6% of T-ALL patients.8 NUP214-ABL1 was highly associated with ectopic expression of TLX1 or TLX3 and deletion of CDKN2A.8 Here we report the identification and characterization of EML1-ABL1, another variant ABL1 fusion gene that is generated by the t(9;14)(q34;q32), which is not detectable by standard cytogenetics.
Study design
Patients
A total of 116 T-ALL patients were screened for ABL1 rearrangements. The 16-year-old female patient with a cryptic t(9;14) presented with very high leukocytosis (455 × 109/L), with 99% blasts with the phenotype of cortical thymocytes, and normal karyotype. She is in first complete remission 15 months after diagnosis. This study was approved by the Ethical Committee of the Medical Faculty of the University of Leuven. Informed consent was obtained from all subjects.
FISH
Fluorescence in situ hybridization (FISH) was performed using standard protocols. Metaphases were hybridized up to 3 times9 using the LSI BCR-ABL ES (Vysis, Downers Grove, IL) translocation probe or bacterial artificial chromosome (BAC) probes RP11-57C19 and RP11-83J21 (BACPAC Resources, Oakland, CA).
RACE and PCR
The 5′-rapid amplification of cDNA ends (5′-RACE) polymerase chain reaction (PCR) was performed as described previously.10 Synthesis of cDNA was performed with the ABL1-R1 primer (5′-gcgtgatgtagttgcttg), followed by PCR with the RACE primers and the nested ABL1 primers ABL1-R2 (5′-acaccattccccattgtgattat) and ABL1-R3 (5′-ccggagcttttcacctttagtta). The presence of the EML1-ABL1 fusion transcript was confirmed by reverse transcriptase-PCR (RT-PCR) using the primers EML1-F (5′-cactcactgggaggtggttt) and ABL1-R2. EML1 expression was detected using primers EML1-F (5′-tagaatagatctcgcgatggcactgtgttaccaaag) and EML1-R (5′-caatgtcacagaatcccagatg). ZNF384 was amplified as described previously.8 Detection of TLX1, TLX2, TLX3, and NKX2-5 expression was performed as described.11
Constructs
The open reading frame of exon 1 to 17 of EML1 was amplified from an IMAGE clone (accession no. BC033043) with primers EML1-F1 (5′-ggaaagatctcagcatggaggacggcttct) and EML1-R (5′-tagaatgcggccgctctggtgagtatcgcattacag). EML1 in which nucleotides 1 to 363 of the open reading frame were deleted (del EML1) was obtained by replacement of EML1-F1 by the EML1-F2 primer (5′-ggaaagatctcagcatggctgtgccagcaaccaaaag). The ABL1 part was amplified from a BCR-ABL construct, using primers ABL1-F (5′-tagaatgcggccgctagcatctga) and ABL1-R (5′-tagaatgaattctacctctgcactatgtcact). The generated EML1/del EML1 parts were ligated together with the ABL1 fragment in the retroviral vector murine stem cell virus-puromycin (MSCV-puro; Clontech, Palo Alto, CA).
Cell culture and retroviral transduction
HEK 293T and Ba/F3 cells were cultured, transfected, and transduced as described previously.12 Transduced Ba/F3 cells were selected with puromycin (2.5 μg/mL) or neomycin (600 μg/mL medium). For Western blotting, Ba/F3 cells were incubated with imatinib for 90 minutes. For growth curves, 105 Ba/F3 cells were seeded in 1 mL medium and viable cells were counted on 3 consecutive days. For dose-response curves, 2 × 105 Ba/F3 cells were seeded in 1 mL medium and incubated in the presence of imatinib for 24 hours. Viable cell numbers were determined with the AQueousOne Solution (Promega, Madison, WI).
Western blotting
Total cell lysates were analyzed by standard procedures using the following antibodies: anti-phospho-ABL1 (Tyr412), anti-ABL1, anti-phospho-extracellular signal-related kinase 1/2 (anti-phospho-ERK1/2; Thr202/Tyr204), and anti-phospho-severe combined immunodeficiency repopulating cell (anti-phospho-SRC) family (Tyr 416; Cell Signaling, Beverly, MA); anti-ERK2, anti-phospho-signal transducers and activators of transcription 5 (anti-phospho-STAT5), anti-STAT5a, and anti-LYN (Santa Cruz Biotechnology, Santa Cruz, CA); antiphosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY); and antimouse/antirabbit peroxidase-labeled antibodies (AP Biotech, Uppsala, Sweden).
Results and discussion
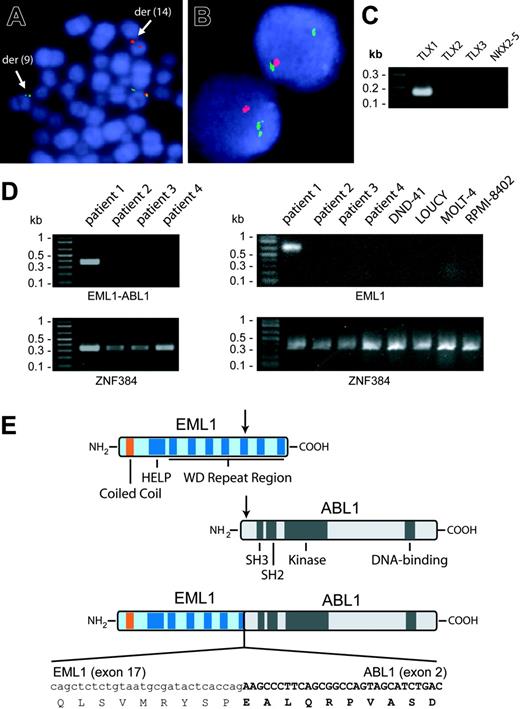
In the process of screening 116 T-ALL patients for ABL1 gene rearrangements by FISH, we detected 6 cases with ABL1 amplification (5 were recently reported),8 1 case with inv(9), and 1 case with a cryptic translocation t(9;14)(q34;q32). Further investigation of the t(9;14) case confirmed that the breakpoint was in intron 1 of ABL1 (Figure 1A). RACE experiments revealed that the t(9;14) generated an in-frame fusion between exon 17 of EML1 (echinoderm microtubule-associated protein-like 1 gene) and exon 2 of ABL1 (Figure 1E).
EML1 was mapped within the Usher syndrome type 1a locus on 14q32 and encodes a protein with high similarity to the echinoderm microtubule-associated protein.13 Unlike other fusion partners of ABL1, EML1 seems to have a more restricted expression pattern,13 and we could not detect its expression in T-ALL cases or cell lines without the t(9;14) (Figure 1D). This suggests that activity of the EML1 promoter in the leukemic cells with the t(9;14) could be a consequence of the translocation.
The EML1-ABL1 fusion gene results in the formation of a 190-kDa EML1-ABL1 protein containing the coiled-coil domain of EML1 and the kinase domain of ABL1 (Figure 1E). RT-PCR confirmed the EML1-ABL1 fusion transcript in the patient with t(9;14) (Figure 1D). Further molecular analysis of this case also revealed hemizygous deletion of the tumor suppressor gene CDKN2A (Figure 1B) as well as ectopic expression of the homeobox gene TLX1 (Figure 1C), 2 known oncogenic events in T-ALL.1,2
Identification of t(9;14)(q34;q32) and detection of EML1-ABL1 and TLX1 expression. (A) FISH with 5′ ABL1 (green signal) and 3′ ABL1 (red signal) probes on metaphase cells of the T-ALL patient with the cryptic t(9;14)(q34;q32). The translocation causes separation of the 2 probes with the 5′ ABL1 probe hybridizing to der(9) and the 3′ ABL1 probe hybridizing to der(14). (B) FISH with CDKN2A probe (red signal) and chromosome 9 centromere probe (green signal) on interphase cells of the patient with t(9;14)(q34;q32). The absence of a second red signal in each cell is caused by hemizygous CDKN2A deletion. FISH data were collected on a Leica DMRB (Wetzlar, Germany) fluorescence microscope equipped with a triple band-pass filter and a cooled black and white charged couple device camera (Photometrics, Tuscon, AZ) run by Quips SmartCapture FISH Imaging Software (Vysis, Downers Grove, IL). (C) Detection of TLX1 transcripts in the patient with t(9;14) by RT-PCR. TLX2, TLX3, or NKX2-5 transcripts were not detected in this patient. (D; Left) RT-PCR detection of the EML1-ABL1 fusion transcript in the patient with t(9;14) (patient 1) and absence of these fusion transcripts in 3 other T-ALL patients (patients 2-4). (Right) RT-PCR analysis of EML1 expression in the 4 patient samples analyzed in the left part of the figure and in 4 T-ALL cell lines. ZNF384 was amplified as a control of RNA quality. (E) Schematic representation of the EML1, ABL1, and EML1-ABL1 fusion proteins. The sequence of the in-frame fusion between exon 17 of EML1 and exon 2 of ABL1 is indicated at the bottom. SH3 indicates Src homology 3.
Identification of t(9;14)(q34;q32) and detection of EML1-ABL1 and TLX1 expression. (A) FISH with 5′ ABL1 (green signal) and 3′ ABL1 (red signal) probes on metaphase cells of the T-ALL patient with the cryptic t(9;14)(q34;q32). The translocation causes separation of the 2 probes with the 5′ ABL1 probe hybridizing to der(9) and the 3′ ABL1 probe hybridizing to der(14). (B) FISH with CDKN2A probe (red signal) and chromosome 9 centromere probe (green signal) on interphase cells of the patient with t(9;14)(q34;q32). The absence of a second red signal in each cell is caused by hemizygous CDKN2A deletion. FISH data were collected on a Leica DMRB (Wetzlar, Germany) fluorescence microscope equipped with a triple band-pass filter and a cooled black and white charged couple device camera (Photometrics, Tuscon, AZ) run by Quips SmartCapture FISH Imaging Software (Vysis, Downers Grove, IL). (C) Detection of TLX1 transcripts in the patient with t(9;14) by RT-PCR. TLX2, TLX3, or NKX2-5 transcripts were not detected in this patient. (D; Left) RT-PCR detection of the EML1-ABL1 fusion transcript in the patient with t(9;14) (patient 1) and absence of these fusion transcripts in 3 other T-ALL patients (patients 2-4). (Right) RT-PCR analysis of EML1 expression in the 4 patient samples analyzed in the left part of the figure and in 4 T-ALL cell lines. ZNF384 was amplified as a control of RNA quality. (E) Schematic representation of the EML1, ABL1, and EML1-ABL1 fusion proteins. The sequence of the in-frame fusion between exon 17 of EML1 and exon 2 of ABL1 is indicated at the bottom. SH3 indicates Src homology 3.
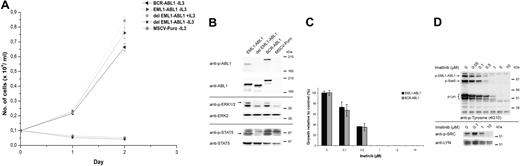
To investigate the oncogenic potential of EML1-ABL1, the fusion gene (as it was identified in the patient) and a variant in which the coiled-coil domain of EML1 was deleted (del EML1-ABL1) were expressed in the interleukin 3 (IL3)-dependent Ba/F3 cell line. EML1-ABL1 expression transformed the Ba/F3 cells to IL3-independent growth, but cells expressing del EML1-ABL1 were not transformed and died upon IL3 withdrawal (Figure 2A). Western blot analysis confirmed that both EML1-ABL1 and del EML1-ABL1 were expressed but only EML1-ABL1 was tyrosine phosphorylated. (Figure 2B). In addition, only Ba/F3 cells expressing EML1-ABL1 showed phosphorylation of Erk1/2 and Stat5 (Figure 2B). These observations demonstrate that the coiled-coil domain of EML1 is required for activation of EML1-ABL1, similar to what was shown for the dimerization domain of ETV6 in the context of ETV6-ABL1.14 The importance of the coiled-coil domain in the context of BCR-ABL1 isless clear for transformation in vitro but is well demonstrated for its in vivo oncogenic properties.15,16
Analysis of transforming properties and imatinib sensitivity of EML1-ABL1. (A) Ba/F3 cells retrovirally transduced with indicated constructs were grown in the absence or presence of IL3. Their mean growth ± SD was recorded over a period of 3 days. (B) Western blot analysis of retroviral-transduced Ba/F3 cells. Constitutive activation of EML1-ABL1 and BCR-ABL1 kinases is shown by immunoblotting with anti-phospho-ABL1 (anti-p-ABL1). Expression of the 3 ABL1 fusion proteins is demonstrated using an anti-ABL1 antibody. Activation of Erk1/2 and Stat5 is demonstrated using anti-phospho-ERK1/2 and anti-phospho-STAT5 antibodies. (C) EML1-ABL1- and BCR-ABL1-transduced Ba/F3 cells were treated with the indicated concentrations of imatinib and cell survival was quantified after 24 hours. Cell survival in the absence of imatinib (= control) was set at 100%; the results represent the average ± SEM of 3 determinations. (D, top panel) Western blot showing the effect of imatinib treatment on EML1-ABL1-expressing Ba/F3 cells. Total cell lysates were analyzed using antiphosphotyrosine (4G10) antibody, indicating a dose-dependent decrease in phosphorylation of EML1-ABL1, Stat5, and Lyn upon imatinib treatment. (D, bottom panel) Decrease of Lyn activity upon imatinib treatment was confirmed by immunoprecipitation of Lyn followed by detection of its phosphorylation on Tyr396 with anti-phospho-SRC. The blot was stripped and reprobed with anti-LYN.
Analysis of transforming properties and imatinib sensitivity of EML1-ABL1. (A) Ba/F3 cells retrovirally transduced with indicated constructs were grown in the absence or presence of IL3. Their mean growth ± SD was recorded over a period of 3 days. (B) Western blot analysis of retroviral-transduced Ba/F3 cells. Constitutive activation of EML1-ABL1 and BCR-ABL1 kinases is shown by immunoblotting with anti-phospho-ABL1 (anti-p-ABL1). Expression of the 3 ABL1 fusion proteins is demonstrated using an anti-ABL1 antibody. Activation of Erk1/2 and Stat5 is demonstrated using anti-phospho-ERK1/2 and anti-phospho-STAT5 antibodies. (C) EML1-ABL1- and BCR-ABL1-transduced Ba/F3 cells were treated with the indicated concentrations of imatinib and cell survival was quantified after 24 hours. Cell survival in the absence of imatinib (= control) was set at 100%; the results represent the average ± SEM of 3 determinations. (D, top panel) Western blot showing the effect of imatinib treatment on EML1-ABL1-expressing Ba/F3 cells. Total cell lysates were analyzed using antiphosphotyrosine (4G10) antibody, indicating a dose-dependent decrease in phosphorylation of EML1-ABL1, Stat5, and Lyn upon imatinib treatment. (D, bottom panel) Decrease of Lyn activity upon imatinib treatment was confirmed by immunoprecipitation of Lyn followed by detection of its phosphorylation on Tyr396 with anti-phospho-SRC. The blot was stripped and reprobed with anti-LYN.
We next tested the sensitivity of EML1-ABL1 to imatinib, a selective inhibitor of ABL1 kinase activity.17 Imatinib concentrations required to inhibit proliferation of the EML1-ABL1- and BCR-ABL1-transformed Ba/F3 cells were comparable (50% inhibitory concentration [IC50] ∼0.2 μM; Figure 2C). The effect of imatinib on EML1-ABL1-expressing Ba/F3 cells was assessed using an antiphosphotyrosine antibody. This confirmed that the major phosphorylated proteins were EML1-ABL1, Stat5, and Lyn and that phosphorylation of these proteins decreased with increasing dose of imatinib (Figure 2D). The phosphorylation status of Lyn, a recently identified critical downstream effector of BCR-ABL1 in B-ALL,18,19 was also determined by immunoprecipitation followed by detection of its phosphorylation on Tyr396 (Figure 2D). This confirmed decreased activity of Lyn upon imatinib treatment of the EML1-ABL1-expressing Ba/F3 cells.
Taken together, our data identify EML1-ABL1 as a constitutively activated tyrosine kinase most likely implicated in the pathogenesis of T-ALL that is similar to BCR-ABL1 in its mode of activation, its activated signaling pathways, and its sensitivity to imatinib.20,21 It remains to be investigated whether the cryptic t(9;14)(q34;q32) accounts for a number of atypical chronic myeloid leukemia cases or BCR-ABL1-negative imatinib responsive myeloproliferative diseases.12,22 The association of EML1-ABL1 with ectopic expression of TLX1 and deletion of CDKN2A is consistent with a multistep pathogenesis of T-ALL. This study further demonstrates the involvement of variant ABL1 fusion genes in the pathogenesis of T-ALL and provides another example of an imatinib-sensitive fusion kinase.
Prepublished online as Blood First Edition Paper, February 15, 2005; DOI 10.1182/blood-2004-12-4897.
Supported by grants from the Belgian Federation against Cancer (J.C.), the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (P.M.), the Belgian Hematological Society (C.G.), and the Franqui-De Roover Foundation (Salus Sanguinis) (C.G.). K.D.K. is an “Aspirant,” J.C. is a postdoctoral researcher, and P.V. is a clinical investigator of the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.”
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This text presents research results of the Belgian program of Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming. The scientific responsibility is assumed by the authors.
K.D.K. performed and designed research and wrote the paper; C.G. and M.D.O. performed the FISH analysis; N.M. and R.S. performed genetic analysis; J.M., I.W., P.V., and A.H. collected samples and designed the FISH study; and P.M. and J.C. designed the genetic and functional studies and wrote the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal