Abstract
Interleukin-4 (IL-4), a major T-helper type 2 (Th2) cytokine, primes dendritic cells (DCs) for IL-12 production, suggesting a negative feedback loop to prevent dysregulated Th2 inflammation, such as allergy. We previously showed that human thymic stromal lymphopoietin (TSLP), highly expressed by keratinocytes of atopic dermatitis, activates CD11c+ DCs to induce the differentiation of naive CD4+ and CD8+ T cells into proallergic effectors. Here we show that TSLP primes DCs to produce large amounts of IL-12 after CD40 ligand stimulation, similar to IL-4 priming of DCs. In contrast to IL-4 priming, DCs activated with TSLP and CD40 ligand induce the differentiation of naive CD4+ T cells into effectors producing both Th1 and Th2 cytokines, a unique profile that is reminiscent of the late phase of allergy. Thus, TSLP is a major regulatory cytokine for IL-12 production by DCs, and TSLP-activated DCs could promote the persistence of Th2 inflammation even in the presence of IL-12-inducing signals. (Blood. 2005;105:4749-4751)
Introduction
In T-helper type 2 (Th2) responses, excessive production of interleukin-4 (IL-4), IL-13, and IL-5 should be regulated by the appropriate negative feedback loops to prevent allergic diseases.1,2 One of the mechanisms that negatively regulate Th2 responses is IL-12 production by IL-4- or IL-13-primed dendritic cells (DCs), which subsequently switches the immune response toward a Th1 profile.3,4 Quantitative5-7 or qualitative8 defectiveness of IL-12 production was suggested to be involved in dysregulated Th2 responses in allergic subjects or experimental models of allergy. However, extrinsic factors present in the cell microenvironment may lead to the maintenance of the Th2 phenotype even in the presence of physiologic levels of functional IL-12.
Human thymic stromal lymphopoietin (TSLP), an IL-7-like cytokine,9,10 strongly activates immature CD11c+ DCs. TSLP-activated DCs (TSLP DCs) induce a robust expansion of allogeneic and autologous T cells11-13 and induce the differentiation of allogeneic naive CD4+ and CD8+ T cells into proallergic effectors.11,12 TSLP is highly expressed by keratinocytes of atopic dermatitis,11 making it a potential key player in the physiopathology of allergy.
Because TSLP is an important cytokine for the induction of inflammatory Th2 responses, we have analyzed the ability of TSLP to prime DCs for the production of IL-12 and the consequences of such priming on the polarization of naive CD4+ T cells.
Study design
DC purification, culture
CD11c+ DCs were purified from adult blood buffy coats to reach greater than 99% purity, as described.11,13 DCs (0.5 × 106/mL in flat-bottom 96-well plates) were cultured in RPMI containing 10% fetal calf serum (FCS), 1% pyruvate, 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and penicillin/streptomycin with TSLP (15 ng/mL), IL-4 (50 U/mL), IL-7 (50 ng/mL), lipopolysaccharide (LPS, 1 μg/mL), poly(I:C) (100 μg/mL), CD40 ligand (CD40L)-transfected L-fibroblasts (2.5 × 104/well), culture medium alone, or a combination of these conditions.
DC IL-12 production
IL-12 production was measured in DC culture supernatants by high-sensitivity protein enzyme-linked immunosorbent assay (ELISA) for bioactive IL-12p70 or the IL-12p40 sub-unit (R&D Systems, Minneapolis, MN). A first set of supernatants was obtained after 24-hour culture of DCs. For a secondary stimulation, 24-hour cultured DCs were washed thoroughly to remove any remaining culture supernatant. Viable DCs were counted using trypan blue exclusion of dead cells and were reseeded in complete culture medium at equal cell numbers and concentrations with various DC activators. A second set of supernatants was obtained after 24 hours of restimulation.
DC T-cell coculture and T-cell cytokine production
After 24 hours of culture, 104 viable DCs were cocultured with 5 × 104 freshly purified allogeneic naive CD4+CD45RA+ T cells (purity greater than 95%) in round-bottom 96-well plates, as described.11,13 After 6 days of coculture, primed T cells were washed to remove all cytokines and were restimulated with anti-CD3 and anti-CD28 for protein ELISA or with phorbol 12-myristate 13-acetate (PMA) and ionomycin for intracellular cytokine staining, as described.11,13
Results and discussion
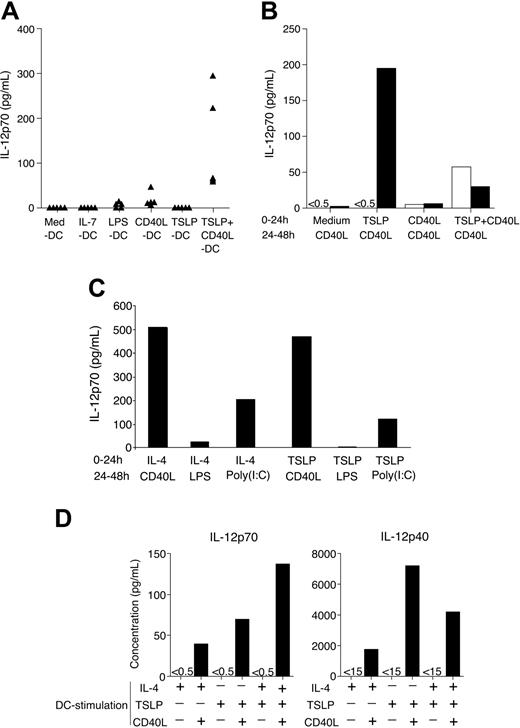
TSLP DCs did not produce detectable amounts of bioactive IL-12, whereas CD40L consistently induced low amounts of IL-12, as previously described11 (Figure 1A). Importantly, TSLP strongly enhanced bioactive IL-12 production by CD40L-activated DCs (CD40L DCs) (Figure 1A-D). In parallel, surface expression of the costimulatory molecules CD40, CD80, and CD86 were increased when DCs were activated with CD40L and TSLP compared with each stimulus alone (Gilliet et al12 and data not shown), indicating a strong synergy between TSLP and CD40L.
TSLP enhances CD40L-induced bioactive IL-12p70 production by CD11c+ DCs. (A) Blood CD11c+ DCs were activated for 24 hours in different conditions, and IL-12p70 was measured in the culture supernatant by high-sensitivity protein ELISA. Each triangle represents IL-12p70 production by DCs in a different donor. (B-C) CD11c+ DCs were first primed for 24 hours in different conditions, and IL-12p70 was measured by protein ELISA in this first set of supernatants (0-24 hours; □). After extensive washing of the DCs, equal numbers of cells from each initial condition were restimulated for 24 hours, and IL-12p70 was measured in the culture supernatant (24-48 hours; ▪). (B) Priming for 24 hours with medium alone, TSLP, CD40L, or TSLP+CD40L and subsequent 24-hour restimulation with CD40L for all culture conditions. (C) Priming for 24 hours with IL-4 or TSLP (0-24 hours; no IL-12 production) followed by 24-hour restimulation with CD40L, lipopolysaccharide (LPS), or poly(I:C) (24-48 hours). (D) CD11c+ DCs were primed for 24 hours with IL-4, TSLP, CD40L, or a combination of these conditions. IL-12p70 and IL-12p40 were measured in the culture supernatant by protein ELISA. Data shown are from 1 of 3 representative independent experiments.
TSLP enhances CD40L-induced bioactive IL-12p70 production by CD11c+ DCs. (A) Blood CD11c+ DCs were activated for 24 hours in different conditions, and IL-12p70 was measured in the culture supernatant by high-sensitivity protein ELISA. Each triangle represents IL-12p70 production by DCs in a different donor. (B-C) CD11c+ DCs were first primed for 24 hours in different conditions, and IL-12p70 was measured by protein ELISA in this first set of supernatants (0-24 hours; □). After extensive washing of the DCs, equal numbers of cells from each initial condition were restimulated for 24 hours, and IL-12p70 was measured in the culture supernatant (24-48 hours; ▪). (B) Priming for 24 hours with medium alone, TSLP, CD40L, or TSLP+CD40L and subsequent 24-hour restimulation with CD40L for all culture conditions. (C) Priming for 24 hours with IL-4 or TSLP (0-24 hours; no IL-12 production) followed by 24-hour restimulation with CD40L, lipopolysaccharide (LPS), or poly(I:C) (24-48 hours). (D) CD11c+ DCs were primed for 24 hours with IL-4, TSLP, CD40L, or a combination of these conditions. IL-12p70 and IL-12p40 were measured in the culture supernatant by protein ELISA. Data shown are from 1 of 3 representative independent experiments.
To check whether concomitant TSLP and CD40L stimulation was required to induce high levels of IL-12, we primed DCs for 24 hours with TSLP, CD40L, or both. Cells from all conditions were then thoroughly washed and subsequently were restimulated for 24 hours with CD40L. After 24-hour restimulation with CD40L, TSLP-primed DCs produced the highest levels of bioactive IL-12 (Figure 1B). TSLP + CD40L-primed DCs (TSLP+CD40L DCs), but not CD40L DCs, still produced significant amounts of IL-12 after 24-hour CD40L restimulation, indicating that the DCs were not exhausted.
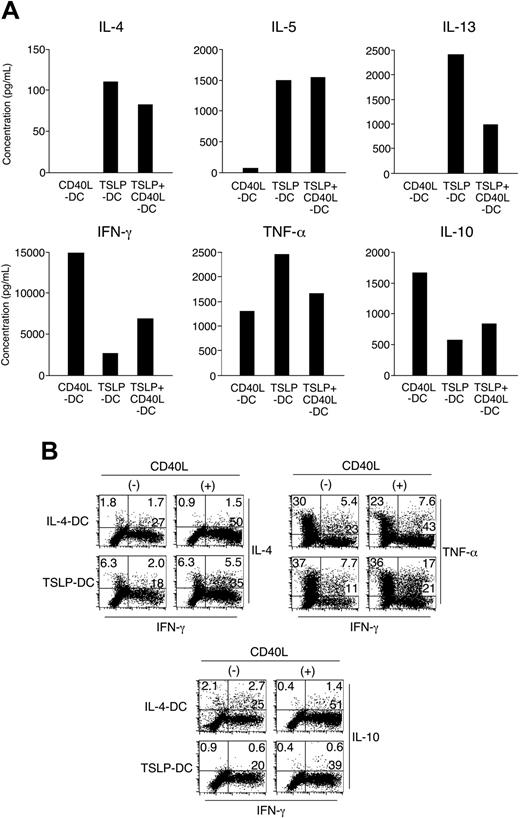
CD11c+ DCs activated by TSLP together with CD40L prime naive CD4+ T cells to produce Th1 and Th2 cytokines. (A) DCs activated with TSLP, CD40L, or both stimuli were used to prime naive CD4+ T cells. After 6 days of coculture, T cells were restimulated for 24 hours with anti-CD3 and anti-CD28, and T cell-derived cytokines were measured in the culture supernatant using ELISA. (B) DCs activated by TSLP or IL-4 with or without CD40L were used to prime naive CD4+ T cells. After 6 days of coculture, T cells were restimulated for 5 hours with PMA and ionomycin, and T cell-derived cytokine production was determined by intracellular cytokine staining. Numbers indicate the percent of cells in each quadrant. Data shown are from 1 of 3 representative independent experiments.
CD11c+ DCs activated by TSLP together with CD40L prime naive CD4+ T cells to produce Th1 and Th2 cytokines. (A) DCs activated with TSLP, CD40L, or both stimuli were used to prime naive CD4+ T cells. After 6 days of coculture, T cells were restimulated for 24 hours with anti-CD3 and anti-CD28, and T cell-derived cytokines were measured in the culture supernatant using ELISA. (B) DCs activated by TSLP or IL-4 with or without CD40L were used to prime naive CD4+ T cells. After 6 days of coculture, T cells were restimulated for 5 hours with PMA and ionomycin, and T cell-derived cytokine production was determined by intracellular cytokine staining. Numbers indicate the percent of cells in each quadrant. Data shown are from 1 of 3 representative independent experiments.
Because IL-4 is described as a key regulatory cytokine for IL-12 production by DCs,14 we compared IL-4 and TSLP for their ability to prime DCs for IL-12 production. Neither cytokine induced IL-12 by itself (Figure 1C-D), but each strongly primed DCs to produce comparably high levels of bioactive IL-12 in response to CD40L or poly(I:C) stimulation (Figure 1C).
We next compared the differential effect of TSLP and IL-4 activation of DCs on IL-12p70 and IL-12p40 production. TSLP priming had much greater efficiency than IL-4 for CD40L-induced IL-12p40 compared with IL-12p70 production (Figure 1D). When TSLP was used with IL-4, we observed an additive effect on CD40L-induced IL-12p70 production that was not present for IL-12p40 production by DCs. One report3 demonstrates that IL-4 priming inhibited IL-12p40 production at early time points during treatment (less than 24 hours). TSLP priming enhances IL-12p40 production, but this effect may be suppressed by additional IL-4 priming. In contrast, CD40L-induced production of IL-12p35, which is a subunit of the IL-12p70 heterodimer, may be enhanced by IL-4 and TSLP priming, resulting in an additive effect and the highest production of IL-12p70.
Next, we examined the cytokine-producing capacity of CD4+ T cells primed by activated DCs. CD4+ T cells, primed for 6 days by TSLP DCs and restimulated for 24 hours with anti-CD3 and anti-CD28, produced high levels of the Th2 cytokines IL-4, IL-5, and IL-13 and of tumor necrosis factor-α (TNF-α) but low levels of IL-10 and interferon-γ (IFN-γ), as previously described11 (Figure 2A). Importantly, T cells primed by TSLP+CD40L DCs produced increased levels of IFN-γ, as predicted by the IL-12 production of DCs, but conserved their ability to produce high levels of IL-4, IL-5, IL-13, and TNF-α. Intracellular cytokine staining of primed T cells restimulated with PMA plus ionomycin confirmed these findings (Figure 2B). IFN-γ-producing cells increased from approximately 20% after priming with TSLP DCs to 40% after priming with TSLP+CD40L DCs. Parallel to this increase in IFN-γ-producing T cells, the percentage of IL-4-producing cells increased from 8.3% to 11.8% and included up to 5.5% IL-4 and IFN-γ double producers. TNF-α-producing cells also increased, with 17% of TNF-α and IFN-γ double producers. Similar to TSLP DCs, the proportion of IFN-γ-producing T cells increased from 28% after priming with IL-4 DCs to 52% with IL-4+CD40L DCs. However, the percentage of IL-4- or IL-10-producing T cells decreased, consistent with a down-regulation of the Th2 phenotype.
In this study, we show that human TSLP is a novel regulatory cytokine for IL-12p70 production with specific characteristics promoting the development of dysregulated Th2 responses. TSLP shares 3 important functional properties with IL-4: (1) It promotes the in vitro generation of Th2 cytokine-producing T cells, (2) it does not directly induce IL-12p70 production by DCs, and (3) it primes DCs to produce high levels of IL-12p70 in response to CD40L stimulation. However, DCs primed with TSLP and CD40L, though producing high levels of IL-12, induce naive CD4+ T cells to differentiate into effectors producing both Th1 and Th2 cytokines. These results, together with the finding that TSLP+CD40L DCs prime CD8+ T cells to produce Th1 and Th2 cytokines,12 suggest that IL-12-mediated negative regulation of Th2 responses is not effective in TSLP-induced Th2 inflammation and leads to a mixed Th1 and Th2 profile, which is reminiscent of the late phase of allergy.15,16
The present study and the finding that TSLP is produced by keratinocytes in acute and chronic lesions of atopic dermatitis11 suggest that TSLP is important for the initiation and the maintenance of the allergic reaction and should encourage therapeutic interventions to suppress TSLP in allergic diseases.
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-09-3622.
Supported by an INSERM Avenir grant (V.S.). DNAX is supported by the Schering-Plough Corporation.
At the time of the study, several of the authors (N.W., S.H., S.A., Y.-J.L., V.S.) were employed by a company (DNAX Research Institute) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jim Cupp for cell sorting and Drs Giorgio Trinchieri and Xiao-Feng Qin for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal