Abstract
Rapamycin is an immunosuppressive compound that is currently used to prevent acute graft rejection in humans. In addition, rapamycin has been shown to allow operational tolerance in murine models. However, a direct effect of rapamycin on T regulatory (Tr) cells, which play a key role in induction and maintenance of peripheral tolerance, has not been demonstrated so far. Here, we provide new evidence that rapamycin selectively expands the murine naturally occurring CD4+CD25+FoxP3+ Tr cells in vitro. These expanded Tr cells suppress proliferation of syngeneic T cells in vitro and prevent allograft rejection in vivo. Interestingly, rapamycin does not block activation-induced cell death and proliferation of CD4+ T cells in vitro. Based on this new mode of action, rapamycin can be used to expand CD4+CD25+FoxP3+ Tr cells for ex vivo cellular therapy in T-cell-mediated diseases. (Blood. 2005;105:4743-4748)
Introduction
Rapamycin, a macrolide antibiotic produced by Streptomyces hygroscopicus, is a new effective drug used to prevent allograft rejection.1 Similarly to the immunosuppressants FK506 and cyclosporin A (CsA), rapamycin exerts its effect by binding to the intracellular immunophilin FK506-binding protein (FKBP12). However, unlike FK506 and CsA, rapamycin does not inhibit T-cell receptor (TCR)-induced calcineurin activity. Rather, the rapamycin-FKBP12 complex inhibits the serine/threonine protein kinase called mammalian target of rapamycin (mTOR), the activation of which is required for protein synthesis and cell-cycle progression. Therefore, rapamycin blocks signaling in response to cytokines/growth factors, whereas FK506 and CsA exert their inhibitory effects by blocking TCR-induced activation (for a review, see Abraham and Wiederrecht2 ). Consistent with this mechanism of action, it has been shown that rapamycin (1) blocks T-cell-cycle progression from G1 to S phase after activation,3 (2) promotes TCR-induced T-cell anergy even in the presence of costimulation,4 and (3) allows induction of operational tolerance.5 However, a direct effect of rapamycin on T regulatory (Tr) cells has not been demonstrated so far.
Tr cells are well-characterized T-cell subsets that play a key role in inducing and maintaining immunologic tolerance. Among the CD4+ Tr cells, the Tr-cell subset that expresses the interleukin 2 receptor α (IL-2Rα) chain (CD4+CD25+) is one of the most extensively characterized so far (for a review, see Fehervari and Sakaguchi6 ). CD4+CD25+ Tr cells are generated in the thymus and are part of the normal peripheral T-cell repertoire. Suppressive CD4+CD25+ Tr cells can be distinguished from activated T cells based on the high constitutive expression of CD25, cytotoxic T-lymphocyte antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and the transcription factor forkhead box P3 (FoxP3). Once generated, thymic CD4+CD25+ Tr cells migrate to peripheral tissues, where they potently suppress proliferation and cytokine production by both CD4+ and CD8+ T cells via a mechanism that requires cell-cell contact.6 CD4+CD25+ Tr cells contribute to tolerance induction after solid organ transplantation and protect from graft-versus-host disease lethality in bone marrow transplantation models.7
Here, we provide new evidence that in vitro long-term exposure of murine CD4+ T cells to rapamycin induces expansion of the naturally occurring CD4+CD25+FoxP3+ Tr cells, which retain their suppressive functions in vitro and in vivo.
Materials and methods
Mice
Balb/c, C57BL/6, and DO11.10 (TCR transgenic [tg] specific for ovalbumin [OVA]) female mice were purchased from Charles River Laboratories (Calco, Italy). All mice were kept under specific pathogen-free conditions.
Flow cytometry and cell sorting
Cells were stained with the indicated antibodies (Abs; all from BD Biosciences, Mountain View, CA), and were analyzed by fluorescence-activated cell sorting (FACS) with a FACScan flow cytometer equipped with CellQuest software (BD Biosciences). To obtain highly purified CD4+CD25+/- T cells, CD4+ T cells were first purified from splenocytes isolated from Balb/c mice by positive selection with αCD4 monoclonal antibody (mAb)-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Thereafter, CD4+ T cells were stained with CyChrome-coupled αCD4 and phycoerythrin (PE)-coupled αCD25 mAbs (BD Biosciences) and CD4+CD25+ T cells were sorted by FACS on a FAC-Star (BD Biosciences).
T-cell cultures
CD4+ T cells were obtained by incubation of splenocytes with αCD4 mAb-coated microbeads and applied onto MiniMacs columns (Miltenyi Biotec). The average purity was 95%.
CD4+ T cells (1 × 106) isolated from spleens of DO11.10 tg mice were stimulated with 4 × 106 antigen-presenting cells (APCs) from Balb/c mice (ie, total splenocytes irradiated 3000 rad) and 0.6 μM OVA323-339 peptide (Primm, Milano, Italy). Three rounds of stimulation of 7 days each were performed. IL-2 (BD Biosciences) was added starting from the second round of stimulation at 50 U/mL. Alternatively, 1 × 106 CD4+ T cells isolated from spleens of Balb/c mice were stimulated with coated 10 μg/mL αCD3 and soluble 1 μg/mL αCD28 mAbs (BD Biosciences). Cells were cultured in the presence of medium alone or 100 nM rapamycin (Sigma, St Louis, MO). Three rounds of stimulation of 7 days each were performed. IL-2 (BD Biosciences) was added starting from the second round of stimulation at 50 U/mL. In separate experiments, sorted CD4+CD25+ T cells isolated from spleens of Balb/c mice were stimulated with coated 10 μg/mL αCD3, soluble 1 μg/mL αCD28 mAbs (BD Biosciences), and 1000 U/mL IL-2. Cells were cultured in the presence of medium alone or 100 nM rapamycin (Sigma). At the beginning of each new stimulation, 1000 U/mL IL-2 was added.
FoxP3 quantitative PCR
Total RNA was extracted with Eurozol (Euroclone, Lugano, Switzerland), and cDNA was synthesized with high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Levels of FoxP3 mRNA were quantified using Assay on Demand real-time polymerase chain reaction (PCR) kits (Applied Biosystems) with TaqMan Universal PCR Master Mix (Applied Biosystems). Levels of 18s rRNA were quantified as internal control by using TaqMan PDAR Eukaryotic 18s Endogenous Controls (Applied Biosystems, assay ID: Mm00475156_m1). Samples were run in duplicate, and relative expression of FoxP3 was determined by normalizing to 18s expression in each set of samples to calculate a fold-change in value.
Suppression experiments
CD4+ T cells isolated from naive Balb/c mice or KJ1-26+ (αOVA-specific TCR) T cells isolated from DO11.10 tg mice were stained with carboxyfluorescein diacetate succinimidylester (CFSE) (Molecular Probes, Eugene, OR) as described elsewhere8 and cultured in 96-well plates (2 × 105/well) coated with 10 μg/mL αCD3 mAb (BD Biosciences) or irradiated splenocytes and OVA. T cells cultured for 3 weeks in medium or rapamycin were added in 1:1 ratio (ie, 105:105) to the culture, and 5 days later the cells were collected and analyzed by FACS. The percentage of CFSE+ cells divided in the presence of cultured cells was compared to percentage of CFSE+ divided cells in the absence of any added cells.
In the transwell experiments, CD4+ T cells isolated from naive Balb/c mice were stained with CFSE (Molecular Probes) and cultured at the bottom of 48 transwell plates (5 × 105/well) coated with 10 μg/mL αCD3 mAb (BD Biosciences). On top of the transwell were seeded T cells cultured for 3 weeks in medium or rapamycin in 1:1 ratio, preactivated with αCD3 mAb for 6 hours. After 5 days of culture, cells from the bottom compartment were collected and analyzed by FACS.
Cell proliferation by CFSE analysis
The proportion of CFSE+ cells proliferating in vitro was calculated as described elsewhere.8 Briefly, the number of cells (events) in a given cycle (division: n) was divided by 2 raised to power n, to calculate the percentage of original precursor cells from which they arose. The sum of original precursors from division 1 to 6 represents the number of precursors cells that proliferated. The percent of CFSE+ divided cells was calculated by (no. of precursors that proliferated1-6/no. of total precursors0-6) × 100.
Islet transplantation
Diabetes was induced in Balb/c mice by intravenous injection of streptozotocin (Sigma) at 170 mg/kg. A diagnosis of diabetes was made after 2 sequential glucose measurements higher than 350 mg/dL. Hand-picked pancreatic islets isolated from C57BL/6 were transplanted under the kidney capsule of recipient Balb/c diabetic mice, as previously described.9
Statistical analysis
All statistical analyses were performed using the Student t test. Kaplan-Meier survival curves were compared by the log-rank test.
Results
Rapamycin does not block activation-induced cell death and proliferation of murine CD4+ T cells
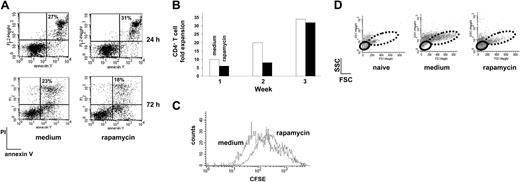
To define the effect of rapamycin on T cells, naive CD4+ T cells from spleens of DO11.10 TCR tg mice were activated with APCs plus OVA in the presence or absence of rapamycin and activation-induced cell death (AICD) was monitored by binding of annexin V. In cells exposed to rapamycin, neither increase nor reduction of apoptosis was observed upon in vitro activation, compared to control cells (Figure 1A). These results confirm that rapamycin does not prevent AICD in CD4+ T cells as already demonstrated in murine splenic mononuclear leukocytes activated with αCD3 mAb,10 and in human peripheral blood mononuclear cells activated in primary mixed lymphocyte cultures.11
To define the effects of a long-term exposure of T cells to rapamycin, naive CD4+ T cells from DO11.10 TCR tg mice were activated in vitro with APCs plus OVA for 3 consecutive weeks in the presence or absence of rapamycin. Fold expansion of T cells was determined after each round of stimulation. T cells activated in the presence of rapamycin had a delayed kinetic of proliferation compared with control cells. However, at the end of the third week of culture the same number of T cells was recovered in control and rapamycin cultures (Figure 1B). Rapamycin binds to FKBP12 and the formed complex inhibits the function of mTOR, which is involved in a broad range of physiologic processes linked to the control of cell cycle.3 Indeed, rapamycin is widely considered as an inhibitor of T-cell cycle by arresting T cells in G1 phase.12-16 Based on this mode of action, rapamycin is used as an immunosuppressive agent for the treatment of transplant rejection. However, patients who received rapamycin did not reveal higher susceptibility to infections, as expected by general immunosuppression.17 Furthermore, it has been demonstrated that rapamycin has some effects in blocking CD4+ T-cell-cycle entry, but the majority of the cells, once they enter the cell cycle, are perfectly capable of dividing.18-20 In line with these observations, our results demonstrate that rapamycin does not block CD4+ T-cell expansion.
After 1, 2 (data not shown), or 3 rounds of stimulation (Figure 1C), CD4+ T cells activated in the presence of rapamycin and restimulated with APCs plus OVA in the absence of rapamycin retained their ability to proliferate, although the overall number of cell divisions was slightly reduced. These data indicate that exposure to rapamycin does not induce anergy in CD4+ T cells. These results are in line with those reported in vitro by Koenen et al11 and in vivo by Ghobrial et al,21 but are in contrast with the findings of Powell and colleagues, who showed that a Th1 cell clone responding to APCs plus antigen becomes anergic when treated with rapamycin.4 One possible explanation for the observed differences might be that, in the aforementioned study, a murine CD4+ T-cell clone stimulated with APC plus antigen and rapamycin overnight was tested, whereas in our experimental model we used a polyclonal T-cell population activated in an antigen-specific way 3 times in the presence of rapamycin.
Although T cells repetitively activated in vitro for 3 weeks in the presence of rapamycin proliferated similarly to control cells, rapamycin-exposed CD4+ T cells were smaller and displayed a more round shape than control cells (Figure 1D). Studies performed in model genetic organisms suggest that cell division and cell growth are normally coordinated yet separable processes and that cells progress through the cell cycle only when sufficient mass, size, and biosynthesis have been reached (for a review, see Schmelzle and Hall22 ). On the contrary, Fingar and colleagues demonstrated that cell growth and cell-cycle progression are separable processes in mammalian cells and that growth to appropriate cell size requires mTOR-dependent signals. In this study it was demonstrated that inhibition of mTOR is the mechanism by which rapamycin reduces cell size in rat fibroblasts and human osteosarcoma cell lines.23 In line with these findings, our data provide evidence that rapamycin blocks CD4+ T-cell growth while allowing their proliferation.
Rapamycin does not block AICD and proliferation of murine CD4+ T cells. (A) DO11.10 tg CD4+ T cells were cultured with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin) and AICD was monitored by FACS after 24 and 72 hours of culture. Percentage of propidium iodide-positive (PI+)-annexin V+ cells is indicated in each dot plot. One representative experiment of 2 is presented. (B) Fold expansion of DO11.10 tg CD4+ T cells 1, 2, and 3 weeks after culture in the presence of APCs plus OVA (medium, □) or APCs plus OVA plus rapamycin (rapamycin, ▪) was evaluated by direct cell counts. One representative experiment of 6 is presented. (C) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were stained with CFSE and restimulated with APCs plus OVA in the absence of the compound and of exogenous IL-2. CFSE dilution was monitored 5 days after activation. One representative experiment of 3 is presented. (D) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were left resting for one additional week with no further stimulation in the presence of IL-2 (50 U/mL). At the end of the 7 days cell size was analyzed by FACS by plotting forward scatter (FSC) versus side scatter (SSC) parameters. Small (solid line) and big (dotted line) cells are circled. Naive CD4+ T cells from a DO11.10 tg mouse were used as control. One representative experiment of 6 is presented.
Rapamycin does not block AICD and proliferation of murine CD4+ T cells. (A) DO11.10 tg CD4+ T cells were cultured with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin) and AICD was monitored by FACS after 24 and 72 hours of culture. Percentage of propidium iodide-positive (PI+)-annexin V+ cells is indicated in each dot plot. One representative experiment of 2 is presented. (B) Fold expansion of DO11.10 tg CD4+ T cells 1, 2, and 3 weeks after culture in the presence of APCs plus OVA (medium, □) or APCs plus OVA plus rapamycin (rapamycin, ▪) was evaluated by direct cell counts. One representative experiment of 6 is presented. (C) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were stained with CFSE and restimulated with APCs plus OVA in the absence of the compound and of exogenous IL-2. CFSE dilution was monitored 5 days after activation. One representative experiment of 3 is presented. (D) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were left resting for one additional week with no further stimulation in the presence of IL-2 (50 U/mL). At the end of the 7 days cell size was analyzed by FACS by plotting forward scatter (FSC) versus side scatter (SSC) parameters. Small (solid line) and big (dotted line) cells are circled. Naive CD4+ T cells from a DO11.10 tg mouse were used as control. One representative experiment of 6 is presented.
Rapamycin expands CD4+CD25+FoxP3+ Tr cells with suppressive ability in vitro
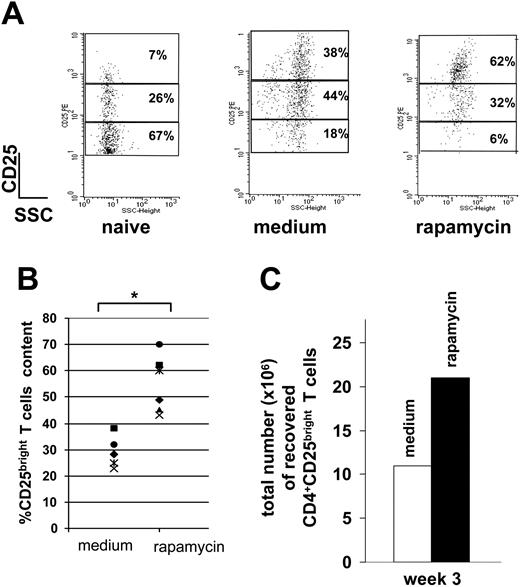
T cells activated in the presence of rapamycin were highly enriched in CD4+CD25bright T cells, which represent the Tr subset among the CD4+CD25+ T-cell population (Figure 2A-B).24,25 Accordingly, the total number of CD4+CD25bright T cells recovered after 3 weeks of culture and repetitive antigen stimulation in the presence of rapamycin was markedly superior to that of control cultures (Figure 2C).
Rapamycin expands CD4+CD25+FoxP3+ Tr cells. (A) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were left resting for 1 week with no further stimulation in the presence of IL-2 (50 U/mL). After 7 days cells were analyzed by FACS. Cells were gated on CD4+CD25+ cells and numbers represent percentages of the 3 different CD25+ subsets (ie, bright, dim, and low). Naive CD4+ T cells from a DO11.10 tg mouse were used as control. One representative experiment of 6 is presented. (B) Content of CD25bright T cells in medium and rapamycin cultures in each of the 6 experiments is presented. *.001 < P ≤ .05. (C) A total of 1 × 106 DO11.10 tg CD4+ T cells (containing 70 000 CD4+CD25bright T cells) were cultured with APCs plus OVA (medium; □) or APCs plus OVA plus rapamycin (rapamycin; ▪). After 3 rounds of stimulation, the total number of CD4+CD25bright T cells was determined by FACS. One representative experiment of 6 is presented.
Rapamycin expands CD4+CD25+FoxP3+ Tr cells. (A) After 3 rounds of stimulation with APCs plus OVA (medium) or APCs plus OVA plus rapamycin (rapamycin), DO11.10 tg CD4+ T cells were left resting for 1 week with no further stimulation in the presence of IL-2 (50 U/mL). After 7 days cells were analyzed by FACS. Cells were gated on CD4+CD25+ cells and numbers represent percentages of the 3 different CD25+ subsets (ie, bright, dim, and low). Naive CD4+ T cells from a DO11.10 tg mouse were used as control. One representative experiment of 6 is presented. (B) Content of CD25bright T cells in medium and rapamycin cultures in each of the 6 experiments is presented. *.001 < P ≤ .05. (C) A total of 1 × 106 DO11.10 tg CD4+ T cells (containing 70 000 CD4+CD25bright T cells) were cultured with APCs plus OVA (medium; □) or APCs plus OVA plus rapamycin (rapamycin; ▪). After 3 rounds of stimulation, the total number of CD4+CD25bright T cells was determined by FACS. One representative experiment of 6 is presented.
Interestingly, rapamycin-exposed CD4+ T cells were able to suppress proliferation of syngeneic naive CD4+ T cells activated in vitro with APCs plus OVA (Figure 3A, left panel). These data clearly indicate that, although cells exposed to rapamycin are not a homogeneous population and contain approximately 60% of CD4+CD25bright Tr cells (Figure 2A), they display a strong suppressive capacity in vitro.
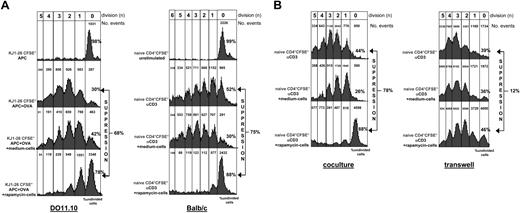
It has been demonstrated that rapamycin profoundly affects the phenotype and function of dendritic cells by reducing their Ag uptake capacity, thereby favoring the differentiation of tolerogenic APCs.26 Thus, the presence of Tr cells in rapamycin-exposed T-cell cultures could be due to an indirect effect of rapamycin on APCs, which become tolerogenic and induce a Tr-cell population, rather than a direct effect on the T cells. To test this hypothesis, we investigated the effects of long-term exposure of T cells to rapamycin in an “APC-free” system. Naive CD4+ T cells from spleens of Balb/c mice were repetitively activated in vitro with αCD3 and αCD28 mAbs for 3 weeks in the presence or absence of rapamycin. As demonstrated for T cells activated with APCs plus OVA, T cells activated with αCD3 and αCD28 mAbs in the absence of APCs, proliferated, did not become anergic, were smaller than control cells (data not shown), and suppressed proliferation of syngeneic naive CD4+ T cells in vitro (Figure 3A, right panel). These data demonstrate that rapamycin induces Tr cells by directly acting on CD4+ T cells.
Rapamycin-exposed T cells suppress proliferation of syngeneic naive CD4+ T cells. (A) Naive KJ1-26+ CD4+ tg T cells isolated from spleens of DO11.10 tg mice were stained with CFSE and were activated with APCs alone or APCs plus OVA. DO11.10 CD4+ T cells activated for 3 weeks with APCs plus OVA (medium cells) or APCs plus OVA plus rapamycin (rapamycin cells) were added in equal number to naive CFSE+ cells (105:105) as shown in the left panel. Alternatively, naive CD4+ cells isolated from spleens of Balb/c mice were labeled with CFSE and cultured alone (unstimulated) or with αCD3 mAb. Balb/c CD4+ T cells activated for 3 weeks with αCD3 plus αCD28 mAbs (medium cells) or αCD3+αCD28 mAbs plus rapamycin (rapamycin cells) were added in equal number to naive CFSE+ cells (105:105) as shown in the right panel. After 5 days of culture, cell division was monitored by levels of CFSE dilution. Histograms show the FACS profile of CD4+CFSE+ T cells. Number of events in each cell division (n) are indicated on top of each peak. The amount of CD4+CFSE+ cells proliferating in the absence or presence of cultured T cells was calculated as described in “Materials and methods” and percentages of undivided cells in each culture condition is indicated. Percentages of suppression in comparison to proliferation of naive control cells is indicated. One representative experiment of 9 (for DO11.10) and 1 of 7 (for Balb/c) is presented. (B) Using the same cells described in panel A (right), the experiment was performed in a transwell system in which responder naive CD4+ T cells were activated with αCD3 mAb at the bottom of the transwell while medium or rapamycin cells were preactivated with αCD3 mAb for 6 hours and then added on top of the transwell (right). Data obtained in the transwell system were compared to data obtained in the coculture system (left). One representative experiment of 3 is presented.
Rapamycin-exposed T cells suppress proliferation of syngeneic naive CD4+ T cells. (A) Naive KJ1-26+ CD4+ tg T cells isolated from spleens of DO11.10 tg mice were stained with CFSE and were activated with APCs alone or APCs plus OVA. DO11.10 CD4+ T cells activated for 3 weeks with APCs plus OVA (medium cells) or APCs plus OVA plus rapamycin (rapamycin cells) were added in equal number to naive CFSE+ cells (105:105) as shown in the left panel. Alternatively, naive CD4+ cells isolated from spleens of Balb/c mice were labeled with CFSE and cultured alone (unstimulated) or with αCD3 mAb. Balb/c CD4+ T cells activated for 3 weeks with αCD3 plus αCD28 mAbs (medium cells) or αCD3+αCD28 mAbs plus rapamycin (rapamycin cells) were added in equal number to naive CFSE+ cells (105:105) as shown in the right panel. After 5 days of culture, cell division was monitored by levels of CFSE dilution. Histograms show the FACS profile of CD4+CFSE+ T cells. Number of events in each cell division (n) are indicated on top of each peak. The amount of CD4+CFSE+ cells proliferating in the absence or presence of cultured T cells was calculated as described in “Materials and methods” and percentages of undivided cells in each culture condition is indicated. Percentages of suppression in comparison to proliferation of naive control cells is indicated. One representative experiment of 9 (for DO11.10) and 1 of 7 (for Balb/c) is presented. (B) Using the same cells described in panel A (right), the experiment was performed in a transwell system in which responder naive CD4+ T cells were activated with αCD3 mAb at the bottom of the transwell while medium or rapamycin cells were preactivated with αCD3 mAb for 6 hours and then added on top of the transwell (right). Data obtained in the transwell system were compared to data obtained in the coculture system (left). One representative experiment of 3 is presented.
The suppressive ability of rapamycin-exposed T cells was also tested in a transwell system in which responder and suppressor cells were kept separate. Rapamycin-exposed T cells were able to suppress proliferation of syngeneic naive CD4+ T cells only in a coculture system (Figure 3B) indicating that their suppressive capacity was strictly dependent on cell-cell contact.
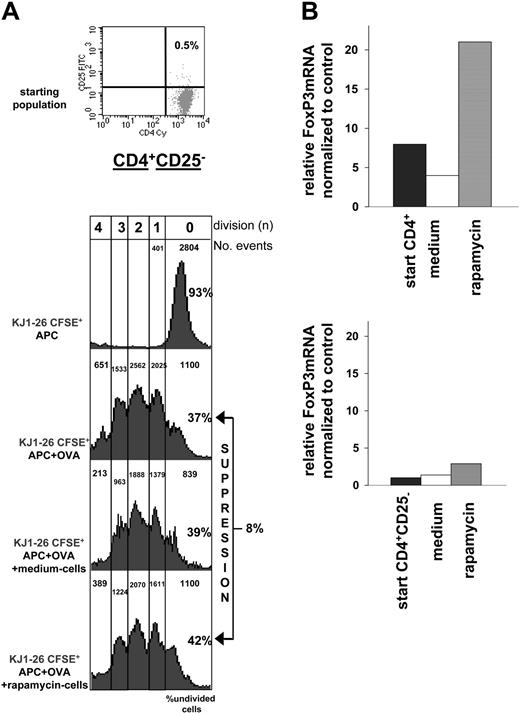
The presence of CD4+CD25+ Tr cells with suppressive activity in rapamycin-exposed T-cell cultures may be due to either a de novo induction of CD25+ Tr cells from CD25- T cells or to a selective expansion of the naturally occurring CD4+CD25+FoxP3+ Tr-cell subset already present in limited amounts at the beginning of the culture (ie, the ∼10% of CD4+CD25bright T cells usually found in a naive spleen). To address this question, CD4+ T cells depleted of the CD25+ Tr cells were cultured for 3 weeks in the presence or absence of rapamycin. In contrast to CD4+ T cells (Figure 3A), CD4+CD25- T cells activated in the presence of rapamycin gave rise to a population of T cells that failed to suppress cell proliferation in vitro (Figure 4A). Accordingly, FoxP3 expression was enhanced in CD4+ T cells exposed to rapamycin but not in CD4+CD25- rapamycin-treated T cells (Figure 4B).
These data indicate that depletion of CD4+CD25+ T cells from the starting cell population does not allow the rapamycin-mediated expansion of Tr cells. However, one cannot exclude the possibility that CD4+CD25+ Tr cells are indispensable in the culture for the generation of rapamycin-mediated induction of Tr cells from a CD4+CD25- T-cell population. To address this point, highly purified sorted CD4+CD25+ T cells (Figure 5A) were activated with αCD3 and αCD28 mAbs and cultured for 3 weeks in the presence or absence of rapamycin. High doses of IL-2 (ie, 1000 U/mL) in the cultures were necessary to expand sorted CD4+CD25+ T cells, which otherwise were anergic (data not shown). T cells activated in the presence of rapamycin had a delayed kinetic of proliferation compared with control cells. However, starting from the third week of culture, CD4+CD25+ T cells activated in the presence of rapamycin greatly expanded, whereas control T cells showed reduced proliferation likely due to exhaustion after repeated TCR stimulation in the presence of high doses of IL-2 (Figure 5B). CD4+CD25+ T cells activated for 3 weeks in the presence of rapamycin contained a higher percentage of CD4+CD25bright T cells compared with control cells (Figure 5C). Accordingly, only rapamycin-exposed CD4+CD25+ T cells suppressed proliferation of syngeneic CD4+ T cells in vitro (Figure 6A) and preserved FoxP3 expression (Figure 6B). Surprisingly, CD4+CD25+ T cells expanded in the absence of rapamycin lost their suppressive function in vitro (Figure 6A). Although it has been previously shown that CD4+CD25+ T cells can be expanded in vitro for 1 week with αCD3 and αCD28 mAbs and high doses of IL-2 without losing their suppressive function,27 it is possible that repeated activation and culture of CD4+CD25+ T cells in medium and high doses of IL-2 result in an overgrowth of activated CD4+CD25+ effector T cells rather than an expansion of CD4+CD25+ Tr cells.
Overall, these data demonstrate that rapamycin selectively expands the naturally occurring CD4+CD25+FoxP3+ Tr cells normally present in the naive splenic CD4+ T-cell compartment and that CD4+CD25+ Tr cells repetitively activated for 3 weeks in the presence of high doses of IL-2 preserve their phenotype and in vitro suppressive function only when cultured in the presence of rapamycin.
CD4+CD25+FoxP3+ Tr cells expanded ex vivo by rapamycin prevent allograft rejection in vivo
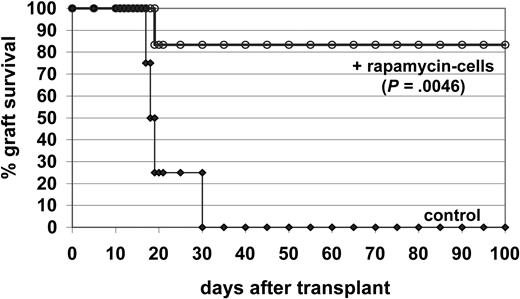
The ability of CD4+CD25+FoxP3+ Tr cells expanded ex vivo by rapamycin to suppress an immune response in vivo was tested in a model of allogeneic pancreatic islet transplantation. Rapamycin-exposed Balb/c CD4+ T cells were collected at the end of the third round of in vitro stimulation and were subsequently injected into diabetic Balb/c mice receiving allogeneic pancreatic islets from C57BL/6 mice. Untreated control diabetic mice rejected the allograft within 30 days after transplantation. Conversely, 5 of 6 mice receiving transplants and injected with rapamycin-exposed T cells did not reject the allograft (Figure 7), clearly demonstrating the potent in vivo suppressive activity of CD4+CD25+FoxP3+ Tr cells expanded in vitro by rapamycin.
Response of CD4+CD25- T cells activated in the presence of rapamycin. (A) The same experiment described in Figure 3A (left panel) with cells from DO11.10 tg mice was performed using CD4+CD25- T cells cultured for 3 weeks with APCs plus OVA (medium cells) or APCs plus OVA plus rapamycin (rapamycin cells). The cultured cells were added in equal number to naive KJ1-26+CFSE+ cells (105:105) and proliferation was monitored by CFSE dilution. One representative experiment of 3 is presented. FACS profile of the cells used before culture (starting population) is shown on top. (B) Relative levels of mRNA FoxP3 were determined by real-time quantitative RT-PCR in CD4+ (top) or CD4+CD25- (bottom) T cells repetitively activated in vitro with or without rapamycin. The amounts of FoxP3 mRNA are expressed relative to that in splenocytes depleted of CD4+CD25+ T cells (which was given an arbitrary value of 1). Relative levels of mRNA FoxP3 in the cells before culture (▪) are also indicated. One representative experiment of 3 is presented.
Response of CD4+CD25- T cells activated in the presence of rapamycin. (A) The same experiment described in Figure 3A (left panel) with cells from DO11.10 tg mice was performed using CD4+CD25- T cells cultured for 3 weeks with APCs plus OVA (medium cells) or APCs plus OVA plus rapamycin (rapamycin cells). The cultured cells were added in equal number to naive KJ1-26+CFSE+ cells (105:105) and proliferation was monitored by CFSE dilution. One representative experiment of 3 is presented. FACS profile of the cells used before culture (starting population) is shown on top. (B) Relative levels of mRNA FoxP3 were determined by real-time quantitative RT-PCR in CD4+ (top) or CD4+CD25- (bottom) T cells repetitively activated in vitro with or without rapamycin. The amounts of FoxP3 mRNA are expressed relative to that in splenocytes depleted of CD4+CD25+ T cells (which was given an arbitrary value of 1). Relative levels of mRNA FoxP3 in the cells before culture (▪) are also indicated. One representative experiment of 3 is presented.
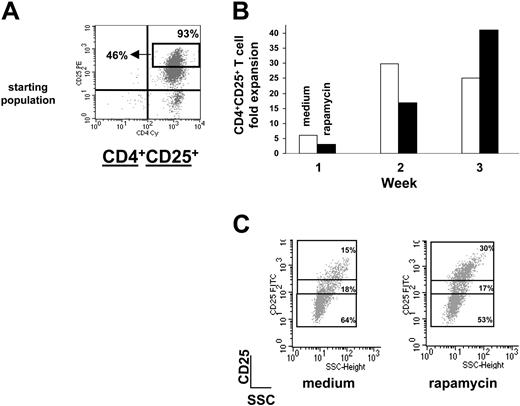
Rapamycin expands a sorted population of CD4+CD25+Tr cells. (A) CD4+CD25+ T cells isolated from spleens of Balb/c mice were sorted by FACS and the FACS profile of sorted CD4+CD25+ T cells is shown. Sorted cells were 93% CD4+CD25+ among which 46% were CD4+CD25bright T cells. (B) Fold expansion of Balb/c CD4+CD25+ T cells 1, 2, and 3 weeks after culture in the presence of αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium, □) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin, ▪) was evaluated by direct cell counts. (C) After 3 rounds of stimulation with αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin), Balb/c CD4+CD25+ T cells were left resting for 1 week with no further stimulation in the presence of low-dose IL-2 (50 U/mL). After 7 days, cells were analyzed by FACS. Cells were gated on CD4+CD25+ cells and numbers represent percentages of the 3 different CD25+ subsets (ie, bright, dim, and low). FITC indicates fluorescein isothiocyanate.
Rapamycin expands a sorted population of CD4+CD25+Tr cells. (A) CD4+CD25+ T cells isolated from spleens of Balb/c mice were sorted by FACS and the FACS profile of sorted CD4+CD25+ T cells is shown. Sorted cells were 93% CD4+CD25+ among which 46% were CD4+CD25bright T cells. (B) Fold expansion of Balb/c CD4+CD25+ T cells 1, 2, and 3 weeks after culture in the presence of αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium, □) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin, ▪) was evaluated by direct cell counts. (C) After 3 rounds of stimulation with αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin), Balb/c CD4+CD25+ T cells were left resting for 1 week with no further stimulation in the presence of low-dose IL-2 (50 U/mL). After 7 days, cells were analyzed by FACS. Cells were gated on CD4+CD25+ cells and numbers represent percentages of the 3 different CD25+ subsets (ie, bright, dim, and low). FITC indicates fluorescein isothiocyanate.
Sorted CD4+CD25+ Tr cells exposed to rapamycin suppress proliferation of syngeneic naive CD4+ T cells and preserve FoxP3 expression. (A) The same experiment described in Figure 3A (right) with cells from Balb/c mice was performed using CD4+CD25+ T cells cultured for 3 weeks with αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium cells) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin cells). The cultured cells were added in equal number to naive CD4+ T cells isolated from Balb/c mice (105:105) and proliferation was monitored by CFSE dilution. (B) Relative levels of mRNA FoxP3 were determined by real-time quantitative RT-PCR in Balb/c CD4+CD25+ T cells repetitively activated in vitro with or without rapamycin. The amounts of FoxP3 mRNA are expressed as relative to amounts in splenocytes depleted of CD4+CD25+ T cells (which was given an arbitrary value of 1). Relative levels of mRNA FoxP3 in the cells before culture (start) are also indicated.
Sorted CD4+CD25+ Tr cells exposed to rapamycin suppress proliferation of syngeneic naive CD4+ T cells and preserve FoxP3 expression. (A) The same experiment described in Figure 3A (right) with cells from Balb/c mice was performed using CD4+CD25+ T cells cultured for 3 weeks with αCD3 plus αCD28 plus 1000 U/mL IL-2 (medium cells) or αCD3 plus αCD28 plus 1000 U/mL IL-2 plus rapamycin (rapamycin cells). The cultured cells were added in equal number to naive CD4+ T cells isolated from Balb/c mice (105:105) and proliferation was monitored by CFSE dilution. (B) Relative levels of mRNA FoxP3 were determined by real-time quantitative RT-PCR in Balb/c CD4+CD25+ T cells repetitively activated in vitro with or without rapamycin. The amounts of FoxP3 mRNA are expressed as relative to amounts in splenocytes depleted of CD4+CD25+ T cells (which was given an arbitrary value of 1). Relative levels of mRNA FoxP3 in the cells before culture (start) are also indicated.
Discussion
A direct effect of rapamycin on CD4+CD25+ Tr cells has been recently investigated. Tian et al showed that a short course of rapamycin treatment in normal rats enriches for the CD4+CD25+ T cells.28 However, no direct demonstration that the expanded cells were T cells with regulatory properties was provided. In addition, Hering and colleagues demonstrated an increase in CD4+CD25+ donor-specific Tr cells in pancreatic-islet transplant recipients treated with αCD3 mAb and rapamycin.29 However, no direct evidence that the expansion of CD4+CD25+ Tr cells was due to either rapamycin or αCD3 mAb was provided. Our data demonstrate that rapamycin selectively expands in vitro CD4+CD25+ FoxP3+ Tr cells, which retain their suppressive ability both in vitro and in vivo. This mode of action of rapamycin may be also important in vivo and account for some of the immunomodulatory effects of this compound. On the other hand, rapamycin is not a bona fide immunosuppressant because it does not interfere with AICD and proliferation of CD4+ T cells. However, it is important to point out that our results do not exclude that rapamycin may have an inhibitory effect on T-cell differentiation (Anna Mondino et al, personal communication, October 2003) and cytokine production,30 thus favoring selective expansion of Tr over differentiation of T effector cells. Indeed, it is possible that IL-2R signaling through mTOR is required for differentiation of T effector cells, whereas Tr cells may use a different signaling pathway, namely the Janus kinase/signal transducer and activator of transcription 5 (JAK/STAT5) pathway.31
Suppressive activity of CD4+CD25+FoxP3+ Tr cells. Diabetic Balb/c mice received transplants under the kidney capsule with pancreatic β-islets purified from C57BL/6 mice. Mice were not treated (control n = 4), or injected the day before the transplantation with 5 × 106 CD4+ T cells isolated from Balb/c mice and activated for 3 weeks in the presence of rapamycin (rapamycin cells, n = 6). Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was higher than 250 mg/dL. Kaplan-Meier survival curves were compared by the log-rank test.
Suppressive activity of CD4+CD25+FoxP3+ Tr cells. Diabetic Balb/c mice received transplants under the kidney capsule with pancreatic β-islets purified from C57BL/6 mice. Mice were not treated (control n = 4), or injected the day before the transplantation with 5 × 106 CD4+ T cells isolated from Balb/c mice and activated for 3 weeks in the presence of rapamycin (rapamycin cells, n = 6). Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was higher than 250 mg/dL. Kaplan-Meier survival curves were compared by the log-rank test.
Although the precise molecular mechanism of action of rapamycin on Tr cells is still unknown, its ability to ex vivo expand CD4+CD25+FoxP3+ Tr cells can be used in cellular immunotherapy protocols for the cure of T-cell-mediated diseases.
Prepublished online as Blood First Edition Paper, March 3, 2005; DOI 10.1182/blood-2004-10-3932.
Supported by the Italian Telethon Foundation and Juvenile Diabetes Research Foundation Grant JT-O1.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Elena Draghici (HSR-TIGET) for performing pancreaticislet transplants, Eleonora Tresoldi (HSR-TIGET) for helpful technical assistance in the FoxP3 analysis, and Anna Mondino (DIBIT S. Raffaele Scientific Institute, Milano, Italy) for helpful scientific discussions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal