Abstract
Mast cells play a central role in inflammatory and immediate-type allergic reactions by secreting a variety of biologically active substances, including sphingosine-1 phosphate (S1P). Sphingosine kinase 1 (SphK1) and formation of S1P, which leads to transactivation of S1P receptors and their downstream signaling pathways, regulates mast-cell functions initiated by cross-linking of the high-affinity immunoglobulin E (IgE) receptor FcϵRI. Surprisingly, overexpression of SphK1 in rat basophilic leukemia (RBL)-2H3 mast cells impaired degranulation as well as migration toward antigen. These effects were reversed by serum withdrawal, yet the increased formation and secretion of S1P were the same as in the presence of serum. Nonetheless, serum increased localization of SphK1 at the plasma membrane. This restricted formation of S1P induced internalization and desensitization of S1P receptors on the surface of mast cells as determined by confocal immunofluorescence microscopy, aberrant S1P receptor signaling, and lack of S1P receptor coupling to G proteins. Serum starvation, which significantly reduced membrane-associated SphK1 activity, restored S1P receptor functions. Our results have important implications for mast-cell migration and degranulation as well as for the biologic functions of the S1P receptors on cells that are circulating in the bloodstream. (Blood. 2005;105:4736-4742)
Introduction
Sphingosine-1 phosphate (S1P) is a pluripotent lysophospholipid signaling molecule that has been implicated in regulation of diverse cellular processes.1-5 S1P is produced by the phosphorylation of sphingosine catalyzed by 2 sphingosine kinase isozymes, SphK1 and SphK2. Many of the biologic responses of S1P are mediated by binding and signaling through a family of 5 differentially expressed G-protein-coupled receptors (GPCRs) known as S1P1-5.1,2 There is also evidence that S1P can serve as an intracellular messenger.1,6 Interest in the functions of S1P in the immune system has increased recently due to the discovery that the immunosuppressive drug FTY720 is phosphorylated by SphKs and functions as an S1P mimetic to induce sequestration of T lymphocytes in thymus and lymph nodes.7-10 Adaptive immunity depends on circulation of T and B cells between secondary lymphoid organs to monitor antigens. Studies with mice whose hematopoietic cells lack S1P1 have established that this S1P receptor is essential for lymphocyte recirculation.11
Mast cells play a central role in inflammatory and immediatetype allergic reactions by secreting a variety of biologically active substances including histamine, eicosanoids, proteolytic enzymes, and many chemokines and cytokines. Activation of SphK1 and formation of S1P in mast cells induced by cross-linking of the high-affinity immunoglobulin E (IgE) receptor FcϵRI is important for calcium release by an inositol-1,4,5-P3 (InsP3)-independent pathway,12,13 as well as for degranulation12-15 and chemotaxis toward antigen.15 S1P, which is also secreted by allergically stimulated mast cells,14,15 is involved in degranulation and leukotriene release.14 In contrast, high intracellular concentrations of sphingosine in mast cells inhibit FcϵRI-mediated leukotriene synthesis and cytokine production by preventing activation of extracellular signal-related kinase 1/2 (ERK1/2) and activator protein-1 (AP-1)-dependent transcription.14 Thus, SphK1 is pivotal to the activation of signaling cascades initiated at FcϵRI by modulating the balance of the counterregulatory lipids sphingosine and S1P.
Recent studies have shown that S1P secreted by mast cells can then transactivate S1P1 and/or S1P2, the only S1P receptors expressed by these cells.15 Although transactivation of S1P1 and Gi signaling are important for cytoskeletal rearrangements and migration of mast cells toward antigen, they are not involved in FcϵRI-triggered degranulation.15 In contrast, activation of S1P2 is required for degranulation but also inhibits mast-cell motility.15 The yin-yang effects of these 2 receptors may serve as an important mast-cell physiologic regulator: mast cells migrate up an antigen gradient due to activation of S1P1, whereas S1P2 expression is up-regulated by antigen,15 resulting in cessation of migration and allowing degranulation at sites of inflammation.
We previously showed that down-regulation of SphK1 impaired motility and degranulation of bone marrow-derived mast cells as well as rat basophilic leukemia (RBL)-2H3 cells. To further explore the role of SphK1 in mast-cell biology, SphK1 was overexpressed in RBL-2H3 cells. Unexpectedly, this strongly inhibited chemotaxis toward antigen as well as S1P and also sharply decreased IgE-triggered degranulation. In this study, we explored the conundrum of how both overexpression as well as down-regulation of SphK1 can produce the same phenotype.
Materials and methods
Reagents
Lipids were from Biomol Research Laboratory (Plymouth Meeting, PA). [γ-32P] adenosine triphosphate ([γ-32P]ATP; 3000 Ci/mmol [110 TBq-mmol]) was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). Alkaline phosphatase from bovine intestinal mucosa, Type VII-NT, was from Sigma (St Louis, MO). Dinitrophenyl (DNP)-specific mouse IgE was kindly provided by Dr J. Rivera. DNP human serum albumin (DNP-HSA, antigen [Ag]; Sigma) was diluted in phosphate-buffered saline (PBS) prior to use. Serum was obtained from Biofluids (Rockville, MD). Polyclonal anti-S1P1 and monoclonal anti-S1P2 antibodies directed against C-terminal peptides were purchased from Exalpha (Watertown, MA). Monoclonal anti-green fluorescent protein (GFP) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture and transfection
RBL-2H3 cells (CRL-2256; ATCC, Manassas, VA) were grown as monolayer cultures in Eagle minimum essential medium (EMEM; Biofluids, Gaithersburg, MD) supplemented with 15% heat-inactivated fetal bovine serum (FBS). Because RBL-2H3 cells can only be transfected at very low efficiency, cells were transfected with GFP-tagged SphK1, which has previously been shown to retain its normal functions,6 using a Gene Pulser (BioRad, Hercules, CA) as previously described.15 Cells expressing vector-GFP or SphK1-GFP were collected by fluorescence-activated cell sorting with an EPICS 753 argon dual laser flow cytometer (Beckman Coulter Elite ESP, Fullerton, CA) and pooled clones were isolated by culturing in the presence of 1 mg/mL G418 (Life Technologies, Gaithersburg, MD). For all experiments, stable transfectants were cultured for 24 hours in EMEM in the presence of 15% serum or in its absence.
Measurement of S1P
IgE-sensitized RBL-2H3 cells (3 × 106) were washed and resuspended in 1 mL of EMEM/bovine serum albumin (BSA) (0.1%). Lipids were extracted from cells and supernatants and mass levels of S1P were determined exactly as described previously.16
Sphingosine kinase assay
Cells were harvested and lysed by freeze-thawing as previously described.15 SphK1 activity was determined in the presence of 50 μM sphingosine in 0.25% Triton X-100 and [γ-32P]ATP (10 μCi [0.37 MBq]; 1 mM) containing MgCl2 (10 mM).17 Radioactive spots corresponding to S1P were quantified with an FX Molecular Imager (BioRad).
FcϵRI aggregation
RBL-2H3 cells were sensitized with anti-DNP IgE in Tyrode buffer in the presence or absence of serum overnight, washed, and stimulated with 100 ng/mL DNP-HSA (Ag) for 1 hour in EMEM for all assays except degranulation and calcium measurements, where cells were washed and stimulated with Ag in Tyrode buffer containing 0.05% BSA.
Degranulation
Secretion of granules into Tyrode-BSA (0.05%) buffer was determined by measuring the release of the granule marker β-hexosaminidase with a colorimetric assay in which the production of p-nitrophenol from p-nitrophenyl-N-acetyl-β-D-glucosaminide is measured.15 Values were expressed as the percentage of total cellular β-hexosaminidase that was released into the medium.
Chemotaxis
Chemotaxis was measured with a modified Boyden chamber and polycarbonate filters (25 × 80 mm, 8-μM pore size). Chemoattractants were added to the lower chamber and cells were added to the upper chamber at 5 × 104 cells/well in serum-free EMEM. After 3 hours, unless otherwise indicated, nonmigratory cells on the upper membrane surface were mechanically removed and the cells that traversed and spread on the lower surface of the filter were fixed and stained with Diff-Quik (Fisher Scientific, Pittsburgh, PA). The migrated cells were counted with an Olympus BH-2 microscope equipped with a 10×/0.25 objective (Olympus, Melville, NY).15 Each data point is the average number of cells in 4 random fields, each counted twice, and is the average ± standard deviation (SD) of 3 individual wells.
Western blotting
RBL-2H3 cells were scraped in lysis buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4; 150 mM NaCl; 0.1% Triton X-100; 1.5 mM MgCl2; 1 mM EDTA [ethylenediaminetetraacetic acid]; 2 mM sodium orthovanadate; 4 mM sodium pyrophosphate; 100 mM NaF; 1 mM PMSF [phenylmethylsulfonyl fluoride]; 5 μg/mL leupeptin; 5 μg/mL aprotinin). Equal amounts of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transblotted to nitrocellulose, blocked with 5% BSA in 0.5% Tween 20 for 2 hours at room temperature, and then incubated with primary antibodies: anti-S1P1 and anti-S1P2 (1 μg/mL); antibodies against phosphorylated ERK1/2 (pERK1/2), pp38, and tubulin (1:1000; Cell Signaling, Beverly, MA). Horseradish peroxidase-conjugated secondary antibody was added in tris(hydroxymethyl)aminomethane (Tris)-buffered saline containing 5% BSA in 0.5% Tween 20 and immunoreactive signals were visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and exposed to Kodak X-Omat film (Rochester, NY).
Immunofluorescence and confocal microscopy
To visualize endogenous S1P1, fixed cells were incubated for 1 hour at room temperature in 5% serum/2% milk/0.05% Tween 20 in PBS and then for 12 hours at 4°C with S1P1 antibody (1:100) in PBS with 1% serum and 0.05% Tween 20. After incubating for 2 hours with Texas-Red-conjugated secondary antibody (Molecular Probes, Eugene, OR), cells were examined by confocal fluorescence microscopy (LSM 510; Zeiss, Thornwood, NY) with a 60×/1.4 oil immersion objective. Quantitative image analysis was performed using LSM 510 image processing software (Zeiss). At least 50 cells were examined in each experiment.
GTPγS binding
Activation of specific G proteins was determined from agonist-induced increases in G-protein binding of guanosine-5′-O-(3-thiotriphosphate) (GTPγS) as previously described with slight modifications.18 Briefly, cells were scraped and homogenized in 20 mM HEPES (pH 7.4) containing 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 2 mM 1.4 dithiothreitol, and 0.25 M sucrose. To prepare plasma membrane fractions, homogenates were centrifuged at 1000g for 10 minutes to remove nuclei and unbroken cells. Supernatants were then spun at 17 000g for 10 minutes to remove heavy membrane fractions (endoplasmic reticulum [ER], endosomes, and mitochondria). Finally, supernatants from this fraction were centrifuged at 100 000g for 60 minutes to isolate plasma membranes. Plasma membranes were solubilized at 4°C in 20 mM HEPES (pH 7.4) buffer containing 0.5% CHAPS (3[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate). The solubilized membranes were incubated for 20 minutes at 37°C with 100 nM 35S-labeled GTPγS in 10 mM HEPES (pH 7.4) in the presence or absence of S1P. Ten volumes of 100 mM Tris-HCl (pH 8.0) containing 10 mM MgCl2, 100 mM NaCl, and 20 mM GTP were then added and the membranes were incubated on ice in plastic dishes precoated with specific antibodies to Gαi3 or Gαq. After washing with PBS, the radioactivity in each well was counted by liquid scintillation.
Calcium measurements
Cells were grown in the absence or presence of serum to near confluence in 10-cm plates and washed with Tyrode buffer supplemented with 0.1% BSA and 250 μM sulfinpyrazone. Cells were then trypsinized, washed twice with Tyrode-BSA containing sulfinpyrazone, and resuspended at 1 × 106 cells/mL. Cells were then loaded with 5 μM of the Ca2+ indicator Fura red-AM (Molecular Probes) for 1 hour at 37°C, washed, and incubated for 30 minutes at 37°C. Intracellular calcium was measured with a spectrofluorometer (SLM Series 2; AMINCO, Rochester, NY) in a 3-mL stirred quartz cuvette using excitation and emission wavelengths of 488 and 630 nm, respectively. For FcϵRI-induced calcium release, cells were incubated with 1 μg/mL anti-DNP IgE during the 30-minute incubation at 37°C following Fura red-AM loading.
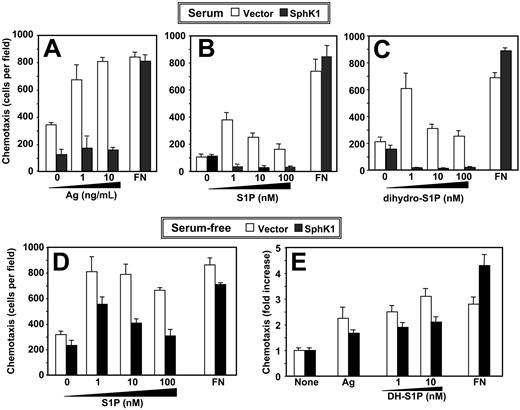
Chemotaxis of RBL-2H3 cells toward antigen or S1P is inhibited by expression of SphK1. (A) IgE-sensitized vector-expressing (□) or SphK1-expressing (▪) RBL-2H3 cells cultured in the presence of serum were allowed to migrate toward the indicated concentrations of DNP-HSA or fibronectin (FN; 20 μg/mL) for 3 hours. (B-C) RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) cultured in the presence of serum were allowed to migrate toward the indicated concentrations of S1P (B), dihydro-S1P (C), or fibronectin (20 μg/mL) for 2 hours. Data are the means ± SD of triplicate determinations. Similar results were obtained in 3 additional experiments. (D-E) Effect of SphK1 expression on chemotaxis of serum-starved RBL-2H3 cells. RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were serum-starved overnight and allowed to migrate toward the indicated concentrations of S1P (D), dihydro-S1P (DH-S1P) (E), or fibronectin (20 μg/mL) for 2 hours. Duplicate cultures were also sensitized with IgE and then allowed to migrate toward DNP-HSA (1 ng/mL) for 3 hours (E). Data are the means ± SD of triplicate determinations and are expressed as fold increases compared with vehicle-treated cells. Similar results were obtained in 2 additional experiments.
Chemotaxis of RBL-2H3 cells toward antigen or S1P is inhibited by expression of SphK1. (A) IgE-sensitized vector-expressing (□) or SphK1-expressing (▪) RBL-2H3 cells cultured in the presence of serum were allowed to migrate toward the indicated concentrations of DNP-HSA or fibronectin (FN; 20 μg/mL) for 3 hours. (B-C) RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) cultured in the presence of serum were allowed to migrate toward the indicated concentrations of S1P (B), dihydro-S1P (C), or fibronectin (20 μg/mL) for 2 hours. Data are the means ± SD of triplicate determinations. Similar results were obtained in 3 additional experiments. (D-E) Effect of SphK1 expression on chemotaxis of serum-starved RBL-2H3 cells. RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were serum-starved overnight and allowed to migrate toward the indicated concentrations of S1P (D), dihydro-S1P (DH-S1P) (E), or fibronectin (20 μg/mL) for 2 hours. Duplicate cultures were also sensitized with IgE and then allowed to migrate toward DNP-HSA (1 ng/mL) for 3 hours (E). Data are the means ± SD of triplicate determinations and are expressed as fold increases compared with vehicle-treated cells. Similar results were obtained in 2 additional experiments.
Statistical analysis
Experiments were repeated at least 3 times with consistent results. For each experiment, data from triplicate samples were calculated and expressed as means ± SD. Statistics were performed using SigmaStat statistical software version 2.0 (Statistical Solutions, Saugus, MA).
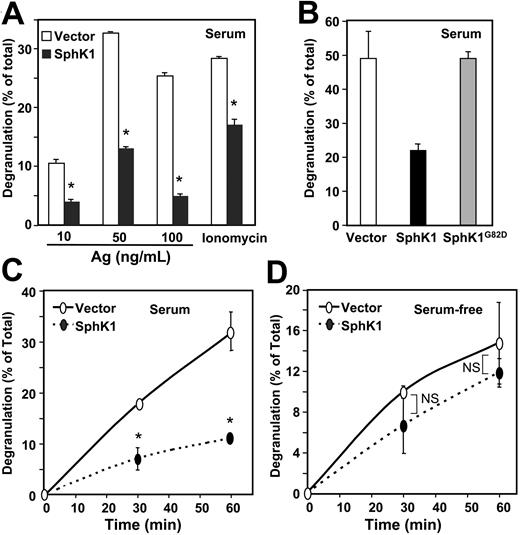
Expression of SphK1 reduces degranulation. (A) IgE-sensitized RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were cultured in the presence of 15% serum (A-B) and then treated for 1 hour with the indicated concentrations of DNP-HSA (Ag) or 1 μM ionomycin. Degranulation was assessed by measuring release of β-hexosaminidase. (B) IgE-sensitized RBL-2H3 cells expressing vector (□), SphK1 (▪), or catalytically inactive SphK1 (▦; SphK1G82D) were treated for 1 hour with Ag (100 ng/mL) and degranulation was measured. (C-D) Effect of serum on SphK1-modulated degranulation. IgE-sensitized vector-overexpressing (○) or SphK1-overexpressing (•) RBL-2H3 cells were cultured for 24 hours in the presence of 15% serum (C) or in the absence of serum (D) and then degranulation was induced by treatment with DNP-HSA (100 ng/mL) for the indicated times. *P < .05; Student t test. NS indicates not significantly different. Data are means ± standard deviation (SD) of triplicate determinations.
Expression of SphK1 reduces degranulation. (A) IgE-sensitized RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were cultured in the presence of 15% serum (A-B) and then treated for 1 hour with the indicated concentrations of DNP-HSA (Ag) or 1 μM ionomycin. Degranulation was assessed by measuring release of β-hexosaminidase. (B) IgE-sensitized RBL-2H3 cells expressing vector (□), SphK1 (▪), or catalytically inactive SphK1 (▦; SphK1G82D) were treated for 1 hour with Ag (100 ng/mL) and degranulation was measured. (C-D) Effect of serum on SphK1-modulated degranulation. IgE-sensitized vector-overexpressing (○) or SphK1-overexpressing (•) RBL-2H3 cells were cultured for 24 hours in the presence of 15% serum (C) or in the absence of serum (D) and then degranulation was induced by treatment with DNP-HSA (100 ng/mL) for the indicated times. *P < .05; Student t test. NS indicates not significantly different. Data are means ± standard deviation (SD) of triplicate determinations.
Results
Expression of SphK1 inhibits motility of RBL-2H3 cells
We previously discovered that chemotaxis of mast cells toward antigen, which plays an important role in immune responses at inflammation sites, is blocked by the SphK inhibitor N, N-dimethylsphingosine and by down-regulation of SphK1 expression.15 Paradoxically, overexpression of SphK1 completely inhibited chemotaxis of RBL-2H3 cells toward antigen (Figure 1A). Furthermore, these cells did not migrate toward S1P (Figure 1B). These effects appeared to be specific, as haptotactic migration toward fibronectin was not significantly altered. Because overexpression of SphK1 inhibited chemotactic motility of MCF-7 cells in the absence of detectable S1P secretion, it was suggested that the inhibitory effects on motility were likely mediated through intracellular actions of S1P rather than through cell-surface S1P receptors.19 To distinguish between intracellular and extracellular actions, we used an S1P analog, dihydro-S1P, which binds to all of the S1P receptors but has no known intracellular actions,1 as a chemoattractant. Dihydro-S1P stimulated motility of vector-transfected RBL-2H3 cells cultured in the presence of serum in a similar concentration-dependent manner as S1P (Figure 1C). Migration toward dihydro-S1P was also markedly inhibited by expression of SphK1 (Figure 1C). Remarkably, when cells were deprived of serum for 24 hours prior to chemotaxis assays, inhibition of motility caused by SphK1 expression was reduced and the cells regained the ability to migrate toward S1P (Figure 1D), dihydro-S1P, and antigen (Figure 1E), albeit not to the same extent as chemotaxis induced by these chemoattractants in vector transfectants.
SphK1 expression inhibits FcϵRI-mediated degranulation
We recently showed that cross-linking of the IgE receptor on mast cells translocates and stimulates SphK1, increasing S1P formation and release and, subsequently, activation of S1P receptors.15 Moreover, N, N-dimethylsphingosine or down-regulation of SphK1 reduced IgE-triggered degranulation of mast cells,13-15 suggesting that formation of S1P is important in this process. Surprisingly, we found that overexpression of SphK1 inhibited rather than enhanced antigen-induced degranulation assessed by hexosaminidase release (Figure 2A). Furthermore, it also reduced degranulation induced by the calcium ionophore ionomycin (Figure 2A). Overexpression of SphK1G82D, a catalytically inactive mutant,20 had no effect on degranulation (Figure 2B). Although in the presence of serum the rate of degranulation of SphK1 expressing cells was dramatically lower than that of vector transfectants (Figure 2C), interestingly, the rate of degranulation was almost the same when the transfected cells were cultured in the absence of serum (Figure 2D).
SphK1 expression enhances S1P secretion
Previous studies demonstrated that mast cells secrete S1P.14,15 Thus, it was of interest to examine whether the differences observed when RBL-2H3 cells expressing SphK1 were cultured in serum-containing medium could be due to enhanced synthesis and secretion of S1P. SphK1 activity was higher in both vector and SphK1 transfectants cultured in the presence of serum (Figure 3A), consistent with studies showing that growth factors stimulate SphK1.22-24 Expression of SphK1 also increased intracellular levels of S1P by 4-fold (Figure 3B), similar to what has been reported in other cell types.23,25 The increase did not mirror the greater than 600-fold increase in SphK1 activity measured in vitro, suggesting that formation of S1P is probably substrate limited. Moreover, S1P levels were not significantly different in cells cultured in the absence or presence of serum (Figure 3B). Furthermore, secretion of S1P, which was increased approximately 3-fold by overexpression of SphK1, was also similar in cells cultured in the presence or absence of serum (Figure 3C).
Expression of SphK1 increases intracellular S1P and its secretion. (A-C) RBL-2H3 cells stably expressing vector or SphK1-GFP were cultured for 24 hours in the absence (□) or presence of 15% serum (▦) and SphK1 activity was measured (Ai). (Aii) Equal amounts of cell-lysate proteins were also separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-GFP antibody. Blots were stripped and reprobed with antitubulin to show equal loading. Ratios of SphK1/tubulin intensities in the absence and presence of serum are 0.85 and 0.87, respectively, determined with NIH ImageJ.21 Mass levels of cellular (B) and secreted S1P (C) were measured in duplicate cultures as described in “Measurement of S1P.” *P < .05 by Student t test. (D-E) SphK1 is not secreted by RBL-2H3 cells. (D) RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence (-) or presence (+) of 15% serum for 24 hours and (D) SphK1 activity was determined in cells and media as described in “Sphingosine kinase assay.” Data are means ± SD of triplicate determinations. (E) Equal amounts of proteins were analyzed by immunoblotting with anti-GFP antibody. Blots were stripped and then reprobed with antiactin antibody as a loading control. Note the presence of actin in both vector and SphK1 supernatants. The double-headed arrow indicates SphK1-GFP. (F) Longer exposure of a portion of the immunoblot shown in panel E.
Expression of SphK1 increases intracellular S1P and its secretion. (A-C) RBL-2H3 cells stably expressing vector or SphK1-GFP were cultured for 24 hours in the absence (□) or presence of 15% serum (▦) and SphK1 activity was measured (Ai). (Aii) Equal amounts of cell-lysate proteins were also separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-GFP antibody. Blots were stripped and reprobed with antitubulin to show equal loading. Ratios of SphK1/tubulin intensities in the absence and presence of serum are 0.85 and 0.87, respectively, determined with NIH ImageJ.21 Mass levels of cellular (B) and secreted S1P (C) were measured in duplicate cultures as described in “Measurement of S1P.” *P < .05 by Student t test. (D-E) SphK1 is not secreted by RBL-2H3 cells. (D) RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence (-) or presence (+) of 15% serum for 24 hours and (D) SphK1 activity was determined in cells and media as described in “Sphingosine kinase assay.” Data are means ± SD of triplicate determinations. (E) Equal amounts of proteins were analyzed by immunoblotting with anti-GFP antibody. Blots were stripped and then reprobed with antiactin antibody as a loading control. Note the presence of actin in both vector and SphK1 supernatants. The double-headed arrow indicates SphK1-GFP. (F) Longer exposure of a portion of the immunoblot shown in panel E.
SphK1 is not secreted by RBL-2H3 cells
It has been suggested that SphK1 is secreted by several types of cells and produces extracellular S1P that then activates cell-surface S1P receptors.26,27 Thus, we next examined the possibility that elevated levels of S1P in the medium from RBL-2H3 cells were mediated by secretion of SphK1. Although the medium of cells overexpressing SphK1 had more SphK activity than the medium from vector transfectants, this represented only about 2% of the total cellular SphK1 of the cells (Figure 3D). Moreover, in agreement with previous studies demonstrating that growth factors do not stimulate secretion of SphK1,23 the SphK activity detected in the media was unaffected by serum (Figure 3D). In agreement, Western blotting revealed that there were only trace amounts of SphK1 protein in the medium (Figure 3E-F). However, it should be noted that actin protein was also detected in the medium (Figure 3E), suggesting that extracellular SphK1 is derived from dead or broken cells rather than secreted.
Serum induces translocation of SphK1 to the plasma membrane
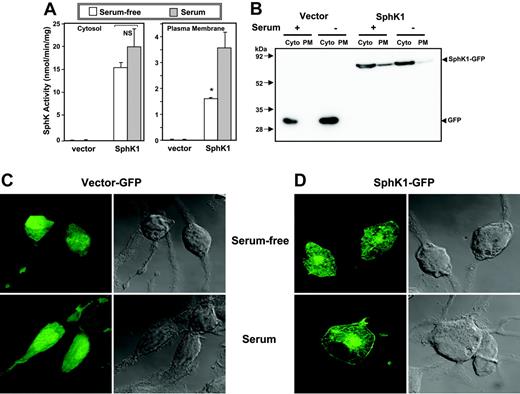
A common feature of activation of SphK1 by a number of stimuli is its translocation to the plasma membrane where its substrate sphingosine resides.13,28,29 It has been suggested that this results in spatially restricted formation of S1P leading to transactivation of its receptors.30 Previous studies with human bone marrow-derived mast cells13 and RBL-2H3 cells15 have shown that SphK1 is primarily cytosolic and is rapidly translocated to the plasma membrane after aggregation of FcϵRI. In agreement, the majority of the SphK activity in serum-starved SphK1-overexpressing RBL-2H3 cells was cytosolic (Figure 4A). However, culturing the cells in the presence of serum enhanced membrane-associated SphK1 activity by more than 2-fold (Figure 4A). Immunoblot analysis also demonstrated that culturing in the presence of serum induced translocation of SphK1 from the cytosol to the plasma membrane fraction (Figure 4B). Moreover, examination of the localization of SphK1 in these cells by confocal microscopy revealed that SphK1 was expressed predominantly in the cytosol of serum-starved cells (Figure 4C) and was translocated to the plasma membrane by serum (Figure 4D).
SphK1 expression attenuates ERK1/2 and p38 activation and calcium release induced by S1P
To understand the puzzle of why SphK1-overexpressing cells cultured in the presence of serum behave so differently than cells cultured in the absence of serum, we examined signaling pathways known to be activated by S1P. In many cell types, including mast cells, binding of S1P to S1P1 and S1P2 leads to activation of ERK1/2 and p38, respectively.2,31 Indeed, S1P activated ERK1/2 (Figure 5A) and p38 (Figure 5C) in vector-transfected RBL-2H3 cells cultured in serum-free media, as measured by Western blotting with phosphospecific antibodies. Expression of SphK1 drastically curtailed the ability of S1P to stimulate ERK1/2 and p38 (Figure 5A,C). Serum reduced S1P-induced activation of ERK1/2 (Figure 5B) and p38 (Figure 5D), which was decreased even further by expression of SphK1.
Inhibition of SphK1 by a specific inhibitor12 or down-regulation of its expression13 diminish FcϵRI-triggered mast-cell degranulation and calcium release from intracellular stores. Thus, we next examined the effect of SphK1 on calcium mobilization. In agreement with several previous studies,12-14 both antigen and S1P mobilized calcium from internal sources in RBL-2H3 cells (Figure 5E). Again, SphK1 rather than augmenting calcium release, significantly blunted the calcium responses to S1P. Calcium release induced by IgE cross-linking was also significantly reduced, albeit to a lesser extent than by S1P (Figure 5E).
Since overexpression of SphK1 decreases S1P receptor-mediated events in RBL-2H3 cells, it was of interest to examine the effects of SphK1 on expression of endogenous S1P1 and S1P2, the only S1P receptors present on these cells.15 While there appeared to be higher levels of S1P1 in cells grown in serum, as determined by Western blotting, levels of S1P2 were not significantly different (Figure 5F). Importantly, SphK1 overexpression did not alter protein levels of either receptor (Figure 5F).
SphK1 expression induces internalization of S1P1 receptor
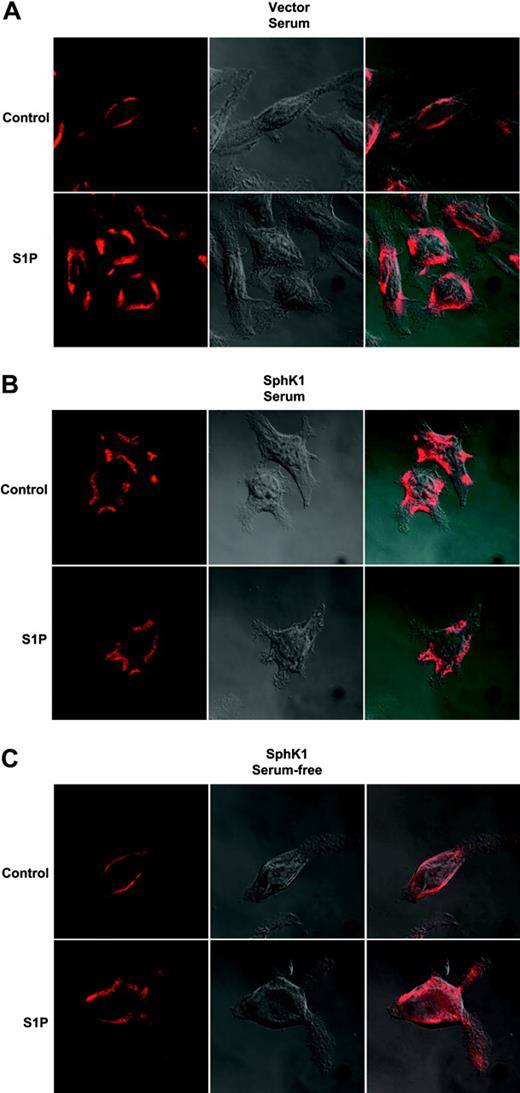
Ligand binding of GPCRs results in receptor phosphorylation and subsequent internalization.32 This internalization is necessary not only for signal termination and degradation and recycling of the receptor but also for certain signaling events including mitogen-activated protein kinase (MAPK) activation.32 Previously, we demonstrated that S1P induced internalization of endogenous S1P1 in RBL-2H3 cells.15 We next examined the possibility that this receptor might be constitutively activated and internalized due to increased formation of S1P at the plasma membrane by SphK1 expression and translocation. Endogenous S1P1 in vector-transfected cells grown in the presence of serum was localized predominantly on the cell surface and, when treated with S1P, was rapidly internalized (Figure 6A). In contrast, endogenous S1P1 in SphK1-overexpressing RBL-2H3 cells grown in serum-containing media appeared to be constitutively internalized (Figure 6B). Additionally, S1P treatment did not alter its localization. However, 24 hours after serum withdrawal from these cells, S1P1 expression was restored to the cell surface and it could then be internalized by treatment with S1P (Figure 6C).
Serum induces translocation of SphK1 to the plasma membrane. (A) RBL-2H3 cells stably expressing vector or SphK1 were cultured for 24 hours in the absence (□) or presence (▦) of 15% serum. Cells were homogenized and centrifuged at 1000g to remove unbroken cells and then at 100 000g for 1 hour to obtain cytosol (Cyto) and plasma membrane (PM) fractions. SphK1 activity was measured as described in “Sphingosine kinase assay.” *P < .05; Student t test. (B) Equal amounts of proteins were analyzed by immunoblotting with anti-GFP antibody. (C-D) Duplicate cultures of vector-GFP- or SphK1-GFP-expressing cells were fixed and stained with anti-GFP and examined by confocal fluorescence microscopy (left panels) or by differential interference contrast (DIC; right panels) to visualize the cells.
Serum induces translocation of SphK1 to the plasma membrane. (A) RBL-2H3 cells stably expressing vector or SphK1 were cultured for 24 hours in the absence (□) or presence (▦) of 15% serum. Cells were homogenized and centrifuged at 1000g to remove unbroken cells and then at 100 000g for 1 hour to obtain cytosol (Cyto) and plasma membrane (PM) fractions. SphK1 activity was measured as described in “Sphingosine kinase assay.” *P < .05; Student t test. (B) Equal amounts of proteins were analyzed by immunoblotting with anti-GFP antibody. (C-D) Duplicate cultures of vector-GFP- or SphK1-GFP-expressing cells were fixed and stained with anti-GFP and examined by confocal fluorescence microscopy (left panels) or by differential interference contrast (DIC; right panels) to visualize the cells.
Overexpression of SphK1 reduces S1P receptor coupling to Gi and Gq
Given that signaling downstream of S1P receptors is attenuated in SphK1-overexpressing RBL-2H3 cells, it was important to examine the ability of S1P to activate its receptors. In plasma membrane fractions from vector transfectants cultured in the presence of serum, S1P stimulated specific GTPγS binding to Gi3, the major Gi isoform of RBL-2H3 cells,33 whereas expression of SphK1 nearly eliminated this response to S1P (Figure 7A). Since S1P1 couples solely to Gi, whereas S1P2 also couples to Gq,34 S1P-dependent Gq-specific GTPγS binding was used as a measure of S1P2 receptor activation. Similar to Gi3, S1P induced Gq-specific GTPγS binding in RBL-2H3 cells overexpressing vector but not SphK1 (Figure 7B). Nevertheless, serum deprivation for 24 hours restored the ability of SphK1-transfected RBL-2H3 cells to respond to S1P as measured by both Gi3- (Figure 7C) and Gq-specific (Figure 7D) GTPγS binding.
Effect of SphK1 expression on signaling pathways. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence (A,C) or presence of 15% serum (B,D) for 16 hours. Cells were then treated with S1P (100 nM) for 2 or 10 minutes. Thirty micrograms (A,C) or 60 μg (B,D) of cell-lysate proteins were analyzed by Western blotting with phospho-ERK1/2 (A,B) and phospho-p38 (C,D) antibodies. Blots were stripped and reprobed with antitubulin to show equal loading. Similar results were obtained in 2 additional experiments. (E) SphK1 overexpression attenuates calcium mobilization in response to S1P. RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were cultured in the presence of serum and loaded with the calcium indicator Fura red-AM for 1 hour at 37°C as described in “Calcium measurements.” Changes in [Ca2+]i after treatment with either S1P (100 nM) or IgE/Ag (100 ng/mL) were measured spectrofluorometrically. Data are expressed as means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. *P < .01; Student t test. (F) Effect of SphK1 expression on S1P receptor levels. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence or presence of 15% serum for 24 hours. Equal amounts of cell-lysate proteins were examined by immunoblotting with anti-S1P1 or anti-S1P2 antibodies. Blots were stripped and reprobed with antitubulin antibody to show equal loading.
Effect of SphK1 expression on signaling pathways. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence (A,C) or presence of 15% serum (B,D) for 16 hours. Cells were then treated with S1P (100 nM) for 2 or 10 minutes. Thirty micrograms (A,C) or 60 μg (B,D) of cell-lysate proteins were analyzed by Western blotting with phospho-ERK1/2 (A,B) and phospho-p38 (C,D) antibodies. Blots were stripped and reprobed with antitubulin to show equal loading. Similar results were obtained in 2 additional experiments. (E) SphK1 overexpression attenuates calcium mobilization in response to S1P. RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were cultured in the presence of serum and loaded with the calcium indicator Fura red-AM for 1 hour at 37°C as described in “Calcium measurements.” Changes in [Ca2+]i after treatment with either S1P (100 nM) or IgE/Ag (100 ng/mL) were measured spectrofluorometrically. Data are expressed as means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. *P < .01; Student t test. (F) Effect of SphK1 expression on S1P receptor levels. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence or presence of 15% serum for 24 hours. Equal amounts of cell-lysate proteins were examined by immunoblotting with anti-S1P1 or anti-S1P2 antibodies. Blots were stripped and reprobed with antitubulin antibody to show equal loading.
Discussion
SphK1 is a critical component determining the balance of the counterregulatory sphingolipid metabolites sphingosine and S1P, which have opposing actions on many mast-cell functions.13-15 Much of the evidence implicating SphK1 and synthesis of S1P in mast-cell functions, including degranulation and chemotaxis, has come from the use of specific inhibitors and down-regulation of its expression.12-15 We previously showed that FcϵRI triggering of mast cells activates and translocates SphK1 to the plasma membrane, which leads to generation and secretion of S1P. Consequently, S1P transactivates S1P1 and Gi signaling central for cytoskeletal rearrangements and migration of mast cells toward antigen but not for degranulation. Transactivation of the other mast-cell S1P receptor, S1P2, was important for degranulation.15 These results re-emphasized the importance of S1P in mast-cell biology.
Effect of expression of SphK1 on internalization of endogenous S1P1. RBL-2H3 cells stably expressing vector (A) or SphK1 (B) were cultured in the presence of 15% serum for 24 hours and then treated with vehicle or S1P (100 nM) for 30 minutes. Cells were fixed, incubated with anti-S1P1 primary antibody (1:100), and examined by confocal microscopy after staining with Texas Red-conjugated secondary antibody or by DIC to visualize the cells. (C) RBL-2H3 cells stably expressing SphK1 were cultured in serum-free medium for 24 hours and then treated as described for panels A and B. Right panels show merged pictures. Representative images from over 30 cells examined are shown.
Effect of expression of SphK1 on internalization of endogenous S1P1. RBL-2H3 cells stably expressing vector (A) or SphK1 (B) were cultured in the presence of 15% serum for 24 hours and then treated with vehicle or S1P (100 nM) for 30 minutes. Cells were fixed, incubated with anti-S1P1 primary antibody (1:100), and examined by confocal microscopy after staining with Texas Red-conjugated secondary antibody or by DIC to visualize the cells. (C) RBL-2H3 cells stably expressing SphK1 were cultured in serum-free medium for 24 hours and then treated as described for panels A and B. Right panels show merged pictures. Representative images from over 30 cells examined are shown.
Surprisingly, however, we found that overexpression of SphK1, rather than enhancing degranulation and chemotaxis, had similar inhibitory effects on these processes as diminishing its activity, even though, as expected, overexpression of SphK1 resulted in significantly greater production of intracellular as well as released S1P. These results suggested that S1P signaling might be aberrant in SphK1-overexpressing cells, particularly when cultured in the presence of serum. Several lines of evidence indicate that this may be due to restricted formation of S1P at the plasma membrane resulting in activation and subsequent internalization and desensitization of S1P receptors. First, in RBL-2H3 cells overexpressing SphK1 and cultured in serum, more SphK1 protein and sphingosine phosphorylating activity are plasma membrane associated than in the absence of serum and the S1P1 receptor was internalized, even in the absence of added S1P. This is in sharp contrast to vector transfectants grown in serum, where S1P1 was localized predominantly to the cell surface and was internalized in response to S1P. Second, GTPγS binding was in accord with these results: while S1P stimulated specific GTPγS binding to Gi and Gq in membranes from vector cells, the responses to S1P were blunted in membranes from SphK1 transfectants, indicating a decreased presence of S1P receptors coupled to these G proteins. Moreover, serum starvation restored the ability of SphK1-overexpressing cells to migrate toward S1P and antigen and to degranulate similar to vector cells. Indeed, in the absence of serum, the S1P1 receptor was relocalized predominantly to the cell surface. Additionally, unlike SphK1 cells grown in serum-containing media, S1P1 internalization was clearly evident in serum-deprived cells following S1P treatment. Finally, they also recovered their specific GTPγS binding to Gi and Gq in response to S1P. We previously showed that S1P2 expression was required for degranulation.15 Thus, overexpression of SphK1 could suppress degranulation induced by antigen or ionomycin due to down-regulation of S1P2.
Effect of serum and SphK1 expression on activation of S1P receptors. Membranes were isolated from vector-expressing (□) and SphK1-expressing (▪) RBL-2H3 cells cultured in the presence (A,B) or absence (C,D) of 15% serum for 24 hours and [35S]GTPγS binding was determined without or with the indicated concentrations of S1P for 30 minutes. Aliquots were added to wells precoated with Gαi3 antibody (A,C) or Gαq antibody (B,D), incubated for 2 hours at room temperature, and bound radioactivity determined. Data are expressed as percent of controls incubated with [35S]GTPγS and vehicle. Values are means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. Statistical significance was determined by Student t test; *P < .05.
Effect of serum and SphK1 expression on activation of S1P receptors. Membranes were isolated from vector-expressing (□) and SphK1-expressing (▪) RBL-2H3 cells cultured in the presence (A,B) or absence (C,D) of 15% serum for 24 hours and [35S]GTPγS binding was determined without or with the indicated concentrations of S1P for 30 minutes. Aliquots were added to wells precoated with Gαi3 antibody (A,C) or Gαq antibody (B,D), incubated for 2 hours at room temperature, and bound radioactivity determined. Data are expressed as percent of controls incubated with [35S]GTPγS and vehicle. Values are means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. Statistical significance was determined by Student t test; *P < .05.
Others have proposed that in some cell types, SphK1 is constitutively secreted and can generate S1P in the extracellular media.26,27 Although SphK1 activity was detected in cell supernatants from SphK1-overexpressing RBL-2H3 cells, this activity is likely due to broken cells, as GFP was also present in the supernatant of GFP-vector transfectants. Additionally, the cytoskeletal protein actin was also observed in media and, in agreement with the established cytoprotective effect of SphK1,1,35 there was less actin in the medium from cells overexpressing SphK1 compared with vector transfectants.
It has been suggested that translocation of SphK1 from the cytosol to the plasma membrane is necessary for agonist-stimulated activation of the enzyme.29 In RBL-2H3 cells expressing SphK1, serum withdrawal for 24 hours significantly reduced plasma membrane-associated SphK1 activity. Surprisingly however, in contrast to the reduction in SphK1 activity, serum deprivation did not cause appreciable changes in cellular S1P levels or its secretion. Taken together, our results suggest that the release of S1P from cells is not sufficient to induce transactivation of the S1P receptors. Instead, formation of S1P by SphK1 at the plasma membrane in a highly localized manner is important for activation of its receptors. This is consistent with a previous study reporting that S1P receptor transactivation by platelet-derived growth factor (PDGF) occurs without detectable secretion of S1P despite translocation of SphK1 to the plasma membrane.30
Another possible explanation for these observations that cannot be excluded is that serum contains suboptimal S1P levels for receptor activation. When cells are cultured with serum, the serum-borne S1P together with the secreted S1P may provide sufficient extracellular S1P to activate and internalize its receptors. It is still puzzling why culturing RBL-2H3 cells in 15% serum, which contains 40 nM S1P,16 a concentration above reported Kd values for S1P receptors, does not result in internalization and desensitization of endogenous S1P1. Our observation that serum can increase S1P1 expression may provide a possible explanation, as increased synthesis of S1P1 could compensate for loss due to internalization. It is also possible that S1P in serum is not completely available to ligate S1P receptors or that serum-bound S1P is rapidly degraded by RBL-2H3 cells and thus serum-induced internalization of S1P1 could be a transient event. Alternatively, overexpressed SphK1 might associate with receptors for serum factors that are only minimally associated with endogenous SphK1. Nevertheless, the observation that S1P1 is not down-regulated by serum could have important implications for the biologic functions of the S1P1 receptors on cells that are circulating in the bloodstream, including lymphocytes, monocytes, and basophils. If these receptors in vivo were down-regulated due to continuous exposure to S1P in blood, they would be unable to signal and regulate important physiologic processes, such as lymphocyte homing and trafficking.11,36
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-12-4686.
Supported by NIH grant AIS0094 (S.S.). Confocal microscopy was supported in part by NIH grant P30 CA16059.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Karnam S. Murthy for assistance with the GTPγS binding assays and helpful discussions.


![Figure 5. Effect of SphK1 expression on signaling pathways. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence (A,C) or presence of 15% serum (B,D) for 16 hours. Cells were then treated with S1P (100 nM) for 2 or 10 minutes. Thirty micrograms (A,C) or 60 μg (B,D) of cell-lysate proteins were analyzed by Western blotting with phospho-ERK1/2 (A,B) and phospho-p38 (C,D) antibodies. Blots were stripped and reprobed with antitubulin to show equal loading. Similar results were obtained in 2 additional experiments. (E) SphK1 overexpression attenuates calcium mobilization in response to S1P. RBL-2H3 cells stably expressing vector (□) or SphK1 (▪) were cultured in the presence of serum and loaded with the calcium indicator Fura red-AM for 1 hour at 37°C as described in “Calcium measurements.” Changes in [Ca2+]i after treatment with either S1P (100 nM) or IgE/Ag (100 ng/mL) were measured spectrofluorometrically. Data are expressed as means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. *P < .01; Student t test. (F) Effect of SphK1 expression on S1P receptor levels. RBL-2H3 cells stably expressing vector or SphK1 were cultured in the absence or presence of 15% serum for 24 hours. Equal amounts of cell-lysate proteins were examined by immunoblotting with anti-S1P1 or anti-S1P2 antibodies. Blots were stripped and reprobed with antitubulin antibody to show equal loading.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-12-4686/5/m_zh80120580050005.jpeg?Expires=1765899131&Signature=dvGk5BosfI~loL0Ezv1hbX7yz~jtFeORcW4HYu1gpJrigg8Up1kT74YoX2fAIjLMAs0P4qEZgnztS3PeyHyA~wXt47Jj1IYKbIUbxFWU8DdcqQcHqoIA2jxfBFHJuGcup0JjwSqXnUHxV3fELBxn6Q3f81qGrIBscwepfnWF25BRvQmCd0azxI-6Ecb3~SzvZonxRefKtJqNZBAx2a51zofY0fdRKX7Eu0WtSx8i6xjvc5ZlxoBdxJ2Ixe18FjAtkHVRvyR36leasM7EYtl25gxxkD-0oi2mMNKSq~qF2CHyHWzlEkLPW0q1NbG6QbG579dT7ulZjGZ6Kh7KUEf7xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Effect of serum and SphK1 expression on activation of S1P receptors. Membranes were isolated from vector-expressing (□) and SphK1-expressing (▪) RBL-2H3 cells cultured in the presence (A,B) or absence (C,D) of 15% serum for 24 hours and [35S]GTPγS binding was determined without or with the indicated concentrations of S1P for 30 minutes. Aliquots were added to wells precoated with Gαi3 antibody (A,C) or Gαq antibody (B,D), incubated for 2 hours at room temperature, and bound radioactivity determined. Data are expressed as percent of controls incubated with [35S]GTPγS and vehicle. Values are means ± SD of triplicate determinations. Similar results were obtained in 2 additional experiments. Statistical significance was determined by Student t test; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-12-4686/5/m_zh80120580050007.jpeg?Expires=1765899131&Signature=v9hKogaDtS3SxWsTqNFDnYsZeF6otCC~r7mcDf4dld7lieHzSeQIWS0S~CBp2lDvSVYMzC0UTq-xNtuiV1A1zeZUPaK0mrYvfaWCXcZ8SxLiGZfxQoq10NE3q2j9i2FQMFxSIWtYa3X43bzOGRlm77iUiyJfS96jz80JgoweiJZWENYzpc23FIgOpig0u1jnC6x1DLc6A8U5KBLdTGKSXcmY33fmz5VSp3q67aThWOQaNy3dQc6J7OTHA7Je8G8OOKZpdv1Fumq-e5gdFsTJwsRH4113P98F7TTjsDt1ATyk0CUY6ZXvaVbNQAm1R~W-hY9N-~b2xHhGqHXfp3mFDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal