Abstract
Cellular oxygen partial pressure is sensed by a family of prolyl-4-hydroxylase domain (PHD) enzymes that modify hypoxia-inducible factor (HIF)α subunits. Upon hydroxylation under normoxic conditions, HIFα is bound by the von Hippel-Lindau tumor suppressor protein and targeted for proteasomal destruction. Since PHD activity is dependent on oxygen and ferrous iron, HIF-1 mediates not only oxygen- but also iron-regulated transcriptional gene expression. Here we show that copper (CuCl2) stabilizes nuclear HIF-1α under normoxic conditions, resulting in hypoxia-response element (HRE)-dependent reporter gene expression. In in vitro hydroxylation assays CuCl2 inhibited prolyl-4-hydroxylation independently of the iron concentration. Ceruloplasmin, the main copper transport protein in the plasma and a known HIF-1 target in vitro, was also induced in vivo in the liver of hypoxic mice. Both hypoxia and CuCl2 increased ceruloplasmin (as well as vascular endothelial growth factor [VEGF] and glucose transporter 1 [Glut-1]) mRNA levels in hepatoma cells, which was due to transcriptional induction of the ceruloplasmin gene (CP) promoter. In conclusion, our data suggest that PHD/HIF/HRE-dependent gene regulation can serve as a sensory system not only for oxygen and iron but also for copper metabolism, regulating the oxygen-, iron- and copper-binding transport proteins hemoglobin, transferrin, and ceruloplasmin, respectively. (Blood. 2005;105:4613-4619)
Introduction
The hypoxia-inducible factor 1 (HIF-1) is an ubiquitously expressed transcriptional master regulator of many genes regulating mammalian oxygen homeostasis.1 Among others, the corresponding gene products are involved in erythropoiesis, iron metabolism, angiogenesis, control of blood flow, glucose uptake and glycolysis, pH regulation, and cell-cycle control.2 HIF-1 is a α1β1 heterodimer specifically recognizing the HIF-binding site within cis-regulatory hypoxia response elements.3 Under normoxic conditions, the von Hippel-Lindau tumor suppressor protein (pVHL) targets the HIF-1α subunit for rapid ubiquitination and proteasomal degradation.4 Binding of the pVHL tumor suppressor protein requires the modification of HIF-1α by prolyl-4-hydroxylation at prolines 402 and 564 of human HIF-1α.5-8 A family of 3 oxygen- and iron-dependent prolyl-4-hydroxylases called PHD1, PHD2, PHD3, or HPH3, HPH2, HPH1, respectively, has been shown to hydroxylate HIFα.9,10 A fourth member, called PH-4, regulates HIF-1α in overexpression conditions only.11 Thus, limited oxygen supply prevents HIFα hydroxylation and degradation.12 This unusual mechanism of protein regulation provides the basis for the very rapid HIF-1α response to hypoxia.13 In addition to protein stability, oxygen-dependent C-terminal asparagine hydroxylation of HIF-1α by factor inhibiting HIF (FIH) prevents transcriptional cofactor recruitment, thereby fine-tuning HIF-1 activity following a further decrease in oxygen availability.14,15
Among the HIF-1 targets are the genes encoding transferrin, transferrin receptor, heme oxygenase-1, and ceruloplasmin, which coordinately regulate iron metabolism.16-20 Increased iron uptake, release from the liver, plasma transport, and uptake in the bone marrow are essential to sustain the erythropoietic function of erythropoietin, the prototype HIF-1 target. Ceruloplasmin is a multicopper plasma protein containing ferroxidase activity necessary for Fe3+ saturation of transferrin.21 Hereditary aceruloplasminemia in humans as well as targeted deletion of the ceruloplasmin gene (Cp) in mice results in iron metabolism disorders characterized by anemia, hepatic iron overload, and neurodegeneration, demonstrating a tight connection between copper and iron metabolism.22-26
Iron deficiency has been known for more than a decade to induce erythropoietin gene expression and HIF-1α protein stabilization.27 Nowadays, these results are most likely explained by inactivation of the iron-dependent protein hydroxylases PHD1 to 3 and FIH.12 Iron deficiency also results in mRNA induction of ceruloplasmin by HIF-1-dependent promoter activation and subsequent transcriptional up-regulation of the Cp gene.20,28 These results suggest that PHDs and FIH not only function as oxygen sensors but also display iron-sensing properties. Whereas transition metal ions such as Co2+ and Ni2+ stabilize HIF-1α by inhibiting PHD function, it is unknown whether copper salts also interfere with PHD function. In a screen for agents that modulate HIF-1 transcriptional activity, we found that Cu2+ can induce HIF-1-dependent reporter gene induction. Detailed analysis of this finding suggests that free Cu2+ can induce ceruloplasmin synthesis in a HIF-1-dependent way.
Materials and methods
Cell lines and cell culture
All cell lines were cultured in Dulbecco modified Eagle medium (high glucose) as described previously.29 Oxygen partial pressures in the hypoxic workstation (InVivO2-400; Ruskinn Technology, Leeds, United Kingdom) or in the incubator (Model 3319; Forma Scientific, Illkirch, France) were either 140 mmHg (20% O2 vol/vol, normoxia) or 7 mmHg (1% O2 vol/vol, hypoxia). Stable transfection with the plasmid pH3SVL led to HIF-dependent luciferase reporter cell lines designated HRCHO5 (derived from CHO Chinese hamster ovary cells) or HRB5 (derived from Hep3B human hepatoma cells), respectively, as described before.30-32 The luciferase reporter gene pH3SVL is driven by a simian virus 40 (SV40) promoter and contains a total of 6 HIF-1 DNA-binding sites derived from the transferrin gene.16
Cell proliferation/viability assays
Cell proliferation/viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) conversion assay as described before.33 Absorbances at 570 nm were determined in a 96-well photometer and noncellular background was subtracted. Data were normalized to the MTT conversion activity of solvent-treated normoxic control cells, which were arbitrarily defined as 1.
Reporter gene assays
Stably transfected hypoxia reporter cell lines were seeded in triplicates into 96-well dishes at a density of 104/well. After incubation overnight, freshly dissolved CuCl2 or CuCl in phosphate-buffered saline (PBS) was added at the concentrations indicated and the cells were exposed to normoxic or hypoxic conditions for 24 hours. In some experiments, the kinase inhibitors LY294002, PD98059, or PD169316 (Calbiochem, VWR International, Schwalbach, Germany) dissolved in dimethyl sulfoxide (DMSO), or DMSO alone, were added in addition to 100 μM CuCl2. Firefly luciferase reporter gene activity was determined as described by the manufacturer (Promega, Madison, WI) using a microplate luminometer (Berthold, Regensdorf, Switzerland). Results were normalized to the solvent-treated normoxic control values, which were arbitrarily defined as 1.
Transient transfections
Hep3B cells were transiently transfected with the CP promoter firefly reporter construct pGL-Cp477420 by the calcium phosphate coprecipitation method.34 CP enhancer constructs, either containing a wild-type or a mutant HRE, were cloned into pGL3Prom (Promega) as described previously.20 Semiconfluent cells on a 10-cm dish were cotransfected with 20 μg of pGL-Cp4774 together with 0.2 μg of the renilla luciferase control construct pRL-SV40 (Promega) for 16 hours. Cells were detached with 2 mM EDTA (ethylenediaminetetraacetic acid; pH 8.0) in PBS, distributed onto 2 24-well dishes, and allowed to recover for 8 hours. Freshly prepared CuCl2 in PBS or PBS alone was added and the cells were incubated for 24 hours under normoxic or hypoxic conditions. Luciferase activities were determined using the dual-luciferase kit (Promega) as described previously.
Protein extraction and immunoblot analyses
Cells were treated with CuCl2 or the iron chelator ciclopirox olamine (CPX)32 at the concentrations indicated for 6 or 24 hours. For stability experiments, 20 μg/mL cycloheximide was added following 6 hours hypoxic induction with or without CuCl2 and the cells were allowed to reoxygenate. Combined cytoplasmic and nuclear extracts of cultured cells were prepared using 0.4 M NaCl and 0.1% Nonidet P-40 (NP-40) in extraction buffer as described previously.29 Protein concentrations were determined by the Bradford method using bovine serum albumin (BSA) as a standard.35 For immunoblot analysis, cellular protein (100 μg or 50 μg in stability experiments) was electrophoresed through 5% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred onto nitrocellulose membranes (Amersham, Freiburg, Germany) by semidry blotting (BioRad, München, Germany). Membranes were stained with Ponceau S (Sigma, Buchs, Switzerland) to confirm equal protein loading and transfer. HIF-1α was detected using a mouse monoclonal anti-HIF-1α immunoglobulin G1 (IgG1; Transduction Laboratories, Heidelberg, Germany) followed by a goat anti-mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-β-actin antibodies were from Sigma. Chemiluminescence detection of horseradish peroxidase-coupled secondary antibodies was performed by incubation of the membranes with 100 mM Tris (tris(hydroxymethyl)aminomethane)-HCl (pH 8.5), 2.65 mM H2O2, 0.45 mM luminol, and 0.625 mM coumaric acid (all purchased from Sigma) for 1 minute followed by exposure to X-ray films (Fuji, Düsseldorf, Germany).
HIF-1α immunofluorescence
Immunofluorescence analysis was performed as described before.29 Briefly, cells were fixed with formaldehyde for 10 minutes, washed with PBS, permeabilized with Triton X-100, and rinsed again with PBS. After blocking nonspecific binding sites with 3% BSA in PBS for 30 minutes, the cells were incubated for 1 hour with mouse monoclonal anti-HIF-1α IgG1 (Transduction Laboratories) diluted 1:10 in 3% BSA in PBS, followed by a fluorescein isothiocyanate (FITC)-coupled secondary anti-mouse (Dako, Copenhagen, Denmark) antibody diluted 1:100 with 3% BSA in PBS. After extensive washings with PBS, the slides were mounted in mowiol and analyzed by fluorescence microscopy (Axioplan 2000, equipped with an Axiovision digital camera and Axiovision software; Carl Zeiss Vision, Mannheim, Germany).
In vitro prolyl-4-hydroxylation assay
Prolyl-4-hydroxylation activity was determined as recently described in detail elsewhere.36 Briefly, a biotinylated peptide containing Pro564 of HIF-1α was bound to NeutrAvidin-coated 96-well plates and incubated with partially purified PHD1, PHD2, or PHD3 in the presence of 2-oxoglutarate, FeSO4, and ascorbate for 1 hour. After washing, thioredoxin-tagged pVHL in complex with elongins B and C was allowed to bind to the hydroxylated peptide, followed by detection of the thioredoxin tag by primary antithioredoxin antibodies and secondary horseradish peroxidase-coupled anti-mouse antibodies (Sigma) using the TMB (3,3′,5,5′-tetramethylbenzidine) substrate kit (Pierce, Bonn, Germany). MBP-PHD2 (amino acids 196-426) was expressed and purified by using the pMAL system according to the instructions provided by the manufacturer (New England BioLabs, Frankfurt, Germany). PHD1 and PHD3 were expressed with an N-terminal 6His-Tag in Sf9 insect cells as described elsewhere.36,37
mRNA quantification
Exposure of mice in triplicate to 7.5% oxygen for 0, 24, 48, and 72 hours and excision of their livers was described earlier.38 Total RNA from frozen mouse liver tissue or cultured Hep3B cells was isolated and analyzed by Northern blotting as described previously.16 Hybridization probes were obtained by restriction digestion and gel isolation followed by labeling with the random-primed method.16 The mouse ceruloplasmin cDNA39 was a kind gift of J. Gitlin (St Louis, MO) and the ribosomal protein L28 cDNA was cloned from a HepG2 cDNA library.40 Radioactive signals were detected by phosphoimaging and quantitated using QuantityOne software (Bio-Rad). Human ceruloplasmin, vascular endothelial growth factor (VEGF), glucose transporter-1 (Glut-1) and L28 were determined by real-time polymerase chain reaction (PCR). Briefly, 2 μg of total RNA was reverse transcribed with Superscript III according to the manufacturer's instructions (Invitrogen, Basel, Switzerland). Real-time PCR was performed in duplicates with 1% of the cDNA reaction mixture using a SybrGreen Q-PCR reagent kit on a MX3000P PCR cycler according to the manufacturer's instructions (Stratagene, Amsterdam, the Netherlands). Dilution series of the corresponding plasmids were used to obtain standard curves. Primers: ceruloplasmin, hCpfwd 5′-gattaattggccccctgatt-3′ and hCprev 5′-tgcattgtgaggccttgtag-3′; VEGF, hVEGF165fwd 5′-gaggagggcagaatcatcac-3′ and hVEGF165rev 5′-aggcccacagggattttcttgtc-3′; Glut-1, hGlut1fwd 5′-tcactgtgctcctggttctg-3′ and hGlut1rev 5′-cctgtgctcctgagagatcc-3′; and L28, hL285.1 5′-gcatctgcaatggatggt-3′ and hL283.1 5′-tgttcttgcggatcatgtgt-3′.
CuCl2-dependent regulation of a HRE-driven reporter gene. CHO chinese hamster ovary (A) and Hep3B human hepatoma (B-C) cell lines stably transfected with an HRE-containing luciferase reporter gene were treated for 24 hours with the CuCl2 (A-B) or CuCl (C) concentrations indicated. (A-B) MTT assays were used to assess Cu2+ toxicity. Shown are mean values ± SEM of n = 3 (all MTT assays), n = 7 (hypoxic Hep3B), n = 8 (normoxic Hep3B), or n = 10 (CHO) independent experiments. (C) A representative experiment performed in quadruplicate is shown as mean values ± SEM.
CuCl2-dependent regulation of a HRE-driven reporter gene. CHO chinese hamster ovary (A) and Hep3B human hepatoma (B-C) cell lines stably transfected with an HRE-containing luciferase reporter gene were treated for 24 hours with the CuCl2 (A-B) or CuCl (C) concentrations indicated. (A-B) MTT assays were used to assess Cu2+ toxicity. Shown are mean values ± SEM of n = 3 (all MTT assays), n = 7 (hypoxic Hep3B), n = 8 (normoxic Hep3B), or n = 10 (CHO) independent experiments. (C) A representative experiment performed in quadruplicate is shown as mean values ± SEM.
Results
CuCl2 can activate a hypoxia-response element-containing reporter gene
We previously established a Chinese hamster ovary (CHO) cell line, called HRCHO5, which was stably transfected with a HIF-1-dependent luciferase reporter gene containing multiple hypoxia-response elements (HREs).30 Apart from hypoxia, reporter activity in these cells could also be induced by cobalt and nickel salts as well as by iron chelation.30,32 Unexpectedly, we found that CuCl2 dose-dependently induced reporter gene induction approximately 4.1-fold at 500 μM CuCl2 in CHO cells (Figure 1A). The human hepatoma Hep3B cell line is known to synthesize the plasma proteins transferrin and ceruloplasmin, both of which are induced in a HIF-dependent manner upon iron depletion of these cells.16,20 We thus stably transfected Hep3B cells with the HIF-dependent reporter gene like previously used to generate the HRCHO5 cell line. When the resulting cell line (termed HRB5) was treated with various doses of CuCl2 for 24 hours, reporter gene expression increased up to 14-fold at 125 μM CuCl2 (Figure 1B, top graph). Hypoxia alone (24 hours of 1% O2) induced reporter gene activity 11-fold (Figure 1B, bottom graph) and the combined treatment with 1% O2 and 125 μM CuCl2 resulted in a 118-fold reporter gene induction. The CuCl2 dose-response relationship revealed a relatively narrow window of active CuCl2 concentrations. Since we suspected CuCl2 cytotoxicity at higher concentrations, MTT conversion assays were performed to analyze cell viability (ie, the combined decrease in cell growth and/or the increase in cell death). A dose-dependent reduction of MTT conversion activity was found in both cell lines (Figure 1A-B). At CuCl2 concentrations that maximally induced reporter gene expression, MTT conversion was still 60% in CHO and 67% in Hep3B cells, respectively, when compared with untreated cells. Hypoxic conditions did not significantly alter MTT conversion activity in Hep3B cells. In addition to Cu2+, Cu+ also was able to induce reporter gene activity (Figure 1C), which might be due to the disproportionation of cuprous ions to cupric ions and copper in aqueous solutions. Other metal ions tested (Al3+, Ca2+, Fe2+, Fe3+, Gd2+, Mg2+, Mn2+, Se2+, and Zn2+) did not alter reporter gene activity in this type of assay (data not shown).
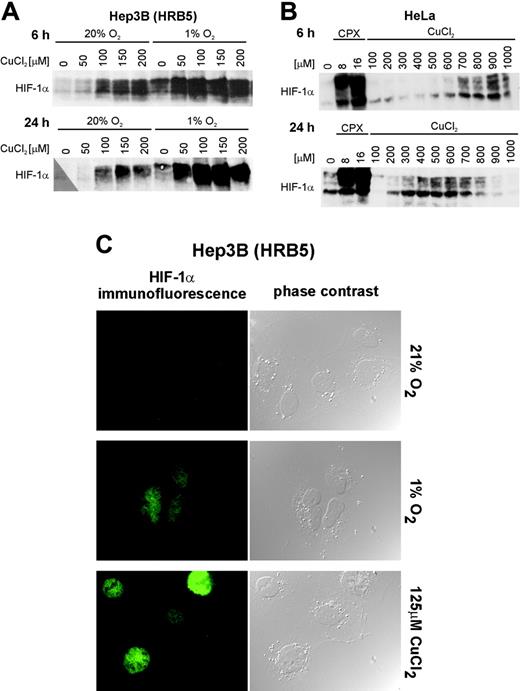
Nuclear HIF-1α protein induction following treatment with CuCl2. Hep3B hepatoma (subline HRB5) (A) and HeLa cervical carcinoma (B) cell lines were treated with the CuCl2 concentrations indicated for 6 and 24 hours. HIF-1α protein was detected by immunoblotting using a monoclonal anti-HIF-1α antibody. Hypoxia (A) and the iron chelator CPX (B) were used as positive controls. (C) Hep3B hepatoma cells (subline HRB5) were exposed to normoxic (20% O2) or hypoxic (1% O2) conditions or to 125 μM CuCl2 for 24 hours. The cells were prepared for indirect immunofluorescence analysis as described in “Materials and methods.” Original magnification, × 630.
Nuclear HIF-1α protein induction following treatment with CuCl2. Hep3B hepatoma (subline HRB5) (A) and HeLa cervical carcinoma (B) cell lines were treated with the CuCl2 concentrations indicated for 6 and 24 hours. HIF-1α protein was detected by immunoblotting using a monoclonal anti-HIF-1α antibody. Hypoxia (A) and the iron chelator CPX (B) were used as positive controls. (C) Hep3B hepatoma cells (subline HRB5) were exposed to normoxic (20% O2) or hypoxic (1% O2) conditions or to 125 μM CuCl2 for 24 hours. The cells were prepared for indirect immunofluorescence analysis as described in “Materials and methods.” Original magnification, × 630.
Nuclear HIF-1α protein induction by CuCl2
The most likely explanation for the induction of reporter gene expression by CuCl2 is the activation of the HIF system. We therefore analyzed whether CuCl2 treatment leads to the stabilization of the HIF-1α protein. As shown by immunoblotting (Figure 2A), CuCl2 treatment strongly induced HIF-1α protein stability in Hep3B cells after 6 and 24 hours. Both the range of CuCl2 concentrations and the combined effects with hypoxia are consistent with the reporter gene data, especially after 24 hours of induction. To examine whether Cu2+ also stabilizes HIF-1α in a nonhepatic cell line, human cervical carcinoma HeLa cells were treated with CuCl2. As shown in Figure 2B, HIF-1α was also stabilized in HeLa cells, though at a considerably higher CuCl2 concentration and not as strongly as with the iron chelator CPX that was included as positive control in these experiments. Of note, the window of active CuCl2 concentrations was shifted to lower values after prolonged incubation, suggesting an overlap with time-dependent cytotoxicity of CuCl2.
Molecular mechanism of HIF-1α protein stabilization by CuCl2. (A) Hep3B hepatoma reporter cells (subline HRB5) were treated with or without 100 μM CuCl2 for 24 hours in the presence or absence of the indicated kinase inhibitors under normoxic conditions. Shown are mean induction factors of luciferase activity ± SD of n = 3 independent experiments. (B) Hep3B cells were kept under hypoxic conditions in the presence or absence of 150 μM CuCl2 before the addition of 20 μM cycloheximide (CHX) to block translation. Following reoxygenation for the indicated time periods, HIF-1α levels were determined by immunoblotting as in Figure 2. Subsequent detection of β-actin served as control for equal loading.
Molecular mechanism of HIF-1α protein stabilization by CuCl2. (A) Hep3B hepatoma reporter cells (subline HRB5) were treated with or without 100 μM CuCl2 for 24 hours in the presence or absence of the indicated kinase inhibitors under normoxic conditions. Shown are mean induction factors of luciferase activity ± SD of n = 3 independent experiments. (B) Hep3B cells were kept under hypoxic conditions in the presence or absence of 150 μM CuCl2 before the addition of 20 μM cycloheximide (CHX) to block translation. Following reoxygenation for the indicated time periods, HIF-1α levels were determined by immunoblotting as in Figure 2. Subsequent detection of β-actin served as control for equal loading.
We next determined whether CuCl2 leads to a nuclear translocation of stabilized HIF-1α protein. Therefore, the Hep3B subline HRB5 was treated with 1% O2 or 125 μM CuCl2 for 24 hours. As shown by indirect immunofluorescence analysis, HIF-1α protein accumulated exclusively within the nucleus, excluding the nucleoli, following hypoxia or CuCl2 treatment but not in normoxic control cells (Figure 2C).
CuCl2 stabilizes HIF-1α under normoxic conditions
To gain more insight into the molecular mechanisms by which CuCl2 induces and activates HIF-1α, the phosphatidylinositol 3 (PI3)-kinase and mitogen-activated protein kinase (MAPK)-kinase signaling pathways, which are known to be responsible for normoxic HIF-1α induction by a large number of stimuli, were investigated. Therefore, the PI3-kinase inhibitor LY294002, the MAPK kinase-1 (MEK-1) inhibitor PD98059, and the p38 kinase inhibitor PD169316 were used to specifically block these pathways. However, as shown in Figure 3A, these 3 kinase inhibitors had no significant impact on reporter gene induction by 100 μM CuCl2, suggesting that other mechanisms are responsible for Cu2+-dependent HIF-1α induction.
In contrast to the kinase pathways that induce HIF-1α protein by translational up-regulation, hypoxia is known to induce HIF-1α by preventing its normoxic degradation. Thus, we induced HIF-1α accumulation by hypoxia in Hep3B cells for 6 hours in the presence or absence of 150 μM CuCl2. Thereafter, cycloheximide was added to block de novo translation of HIF-1α and reoxygenation was allowed for up to 135 minutes. As shown in Figure 3B, the continued presence of CuCl2 clearly delayed HIF-1α degradation, suggesting that CuCl2 stabilizes HIF-1α protein rather than inducing its production. In contrast, β-actin protein levels remained unaffected by this treatment, demonstrating a specific inhibition of HIF-1α degradation.
CuCl2 inhibits HIF-1α prolyl-4-hydroxylation independent of Fe2+ concentration
CuCl2-dependent stabilization of HIF-1α might be due to inhibition of HIF-1α prolyl-4-hydroxylation by PHD isoenzymes. Indeed, using a recently developed cell-free in vitro assay, we found that CuCl2 strongly inhibited hydroxylation of a peptide containing HIF-1α Pro564 by PHD1, PHD2, and PHD3, respectively (Figure 4A).
To gain more insight into the mechanism by which CuCl2 inhibits PHD activity, we repeated the prolyl-4-hydroxylation assays in the presence of limited concentrations (1 μM) or a vast excess (100 μM) of FeSO4. As shown in Figure 4B, PHD3 activity was inhibited by CuCl2 with an IC50 value of approximately 2.3 μM. However, the inhibitory concentration at 50% (IC50) value did not significantly change when Fe2+ was present in excess, suggesting that iron oxidation and/or competitive replacement from the active center is not the cause for CuCl2-dependent inhibition of PHD activity.
Induction of ceruloplasmin mRNA by hypoxia and Cu2+
Cu2+-dependent induction of HIF-1α offers the intriguing possibility that the Cu2+-binding protein ceruloplasmin itself is regulated by Cu2+ in a HIF-1-dependent manner. Thus, we further analyzed ceruloplasmin regulation, a known HIF-1 target gene inducible by hypoxia and iron depletion in in vitro cultured cells.20,28 First, we determined ceruloplasmin mRNA levels in livers of mice exposed to 7.5% O2 for up to 3 days by Northern blotting (Figure 5A). Liver ceruloplasmin mRNA levels significantly (t test, P < .05) increased 1.7-fold and 1.5-fold from days 1 and 2 to day 3, respectively. Only nonsignificant changes occurred during the first 2 days of exposure (Figure 5B). These data provide the first in vivo evidence that prolonged hypoxia can induce the mouse Cp gene.
Inhibtion of PHD activity by CuCl2 in vitro. PHD activity was measured in the prescence of oxygen, 2-oxoglutarate, and ascorbate by the binding of a purified pVHL-elonginB-elonginC complex to a HIF-1α-derived peptide bound onto microtiter plates as described in “Materials and methods.” (A) PHD enzyme-free assays using a nonhydroxylated peptide or 40 nM of a hydroxyproline-containing peptide served as negative and positive controls (black bars labeled with HIF-P564 and HIF-P564OH, respectively). PHD1 and PHD3 were obtained from insect cells as 6His fusion proteins and PHD2 was expressed in bacteria as MBP fusion protein. Representative experiments performed in triplicate are shown as mean values ± SD. (B) PHD3-dependent HIF-1α Pro564 hydroxylation activity was determined in the presence of either 1 or 100 μM FeSO4. Shown are mean values ± SD of n = 3 independent experiments. OD indicates optical density.
Inhibtion of PHD activity by CuCl2 in vitro. PHD activity was measured in the prescence of oxygen, 2-oxoglutarate, and ascorbate by the binding of a purified pVHL-elonginB-elonginC complex to a HIF-1α-derived peptide bound onto microtiter plates as described in “Materials and methods.” (A) PHD enzyme-free assays using a nonhydroxylated peptide or 40 nM of a hydroxyproline-containing peptide served as negative and positive controls (black bars labeled with HIF-P564 and HIF-P564OH, respectively). PHD1 and PHD3 were obtained from insect cells as 6His fusion proteins and PHD2 was expressed in bacteria as MBP fusion protein. Representative experiments performed in triplicate are shown as mean values ± SD. (B) PHD3-dependent HIF-1α Pro564 hydroxylation activity was determined in the presence of either 1 or 100 μM FeSO4. Shown are mean values ± SD of n = 3 independent experiments. OD indicates optical density.
We next determined mRNA concentrations in cultured Hep3B cells (subline HRB5) exposed to hypoxic conditions for up to 3 days by real-time reverse transcription (RT)-PCR. As shown in Figure 6, ceruloplasmin as well as the prototype oxygen-regulated VEGF and Glut-1, but not L28 control mRNA, are induced by hypoxia at all time points examined, confirming that ceruloplasmin is hypoxia-inducible in Hep3B cells. Of note, 150 μM CuCl2 also induced ceruloplasmin, VEGF, and Glut-1, but not L28 mRNA, in Hep3B cells and the combination with hypoxia further enhanced these mRNA levels (Figure 6). Maximal ceruloplasmin induction by CuCl2 was 5.5-fold after 72 hours of treatment under normoxic conditions.
Ceruloplasmin mRNA levels in hypoxic mouse liver. (A) Northern blotting of liver RNA derived from mice exposed to 7.5% O2 for 0 to 3 days. Hybridization signals obtained with a ribosomal protein L28 probe served as a control for loading and blotting efficiency. (B) Phosphoimager quantification of band intensities shown in panel A. Mean ceruloplasmin to L28 mRNA ratios ± SD of n = 3 mice for each time point are given.
Ceruloplasmin mRNA levels in hypoxic mouse liver. (A) Northern blotting of liver RNA derived from mice exposed to 7.5% O2 for 0 to 3 days. Hybridization signals obtained with a ribosomal protein L28 probe served as a control for loading and blotting efficiency. (B) Phosphoimager quantification of band intensities shown in panel A. Mean ceruloplasmin to L28 mRNA ratios ± SD of n = 3 mice for each time point are given.
Induction of ceruloplasmin mRNA by hypoxia and CuCl2 in Hep3B hepatoma cells. Real-time PCR quantification of reverse transcribed ceruloplasmin, VEGF, Glut-1, and L28 mRNA levels in Hep3B cells treated with 150 μM CuCl2 under normoxic or hypoxic conditions for the indicated time periods. Shown are mRNA copy numbers relative to the L28 control as measured in duplicate.
Induction of ceruloplasmin mRNA by hypoxia and CuCl2 in Hep3B hepatoma cells. Real-time PCR quantification of reverse transcribed ceruloplasmin, VEGF, Glut-1, and L28 mRNA levels in Hep3B cells treated with 150 μM CuCl2 under normoxic or hypoxic conditions for the indicated time periods. Shown are mRNA copy numbers relative to the L28 control as measured in duplicate.
Ceruloplasmin promoter regulation by CuCl2. (A) Hep3B hepatoma cells were transiently cotransfected with a ceruloplasmin promoter-firefly luciferase reporter gene construct together with a renilla luciferase control reporter gene driven by the SV40 promoter. Transfected cells were treated with the indicated CuCl2 concentrations under normoxic or hypoxic conditions. After 24 hours, firefly luciferase reporter gene activity was determined and divided by the renilla luciferase values to correct for differences in transfection efficiency. Shown are mean relative luciferase activities ± SEM of 2 transfections performed in quadruplicate. (B) Hep3B cells were transfected with HRE wild-type and HRE mutant ceruloplasmin enhancer SV40 promoter constructs, or the SV40 promoter alone, driving firefly luciferase expression. Following 24 hours of 150 μM CuCl2 or 1% oxygen, luciferase induction factors compared with the untreated controls were determined. A representative experiment performed in triplicate is shown as mean values ± SEM.
Ceruloplasmin promoter regulation by CuCl2. (A) Hep3B hepatoma cells were transiently cotransfected with a ceruloplasmin promoter-firefly luciferase reporter gene construct together with a renilla luciferase control reporter gene driven by the SV40 promoter. Transfected cells were treated with the indicated CuCl2 concentrations under normoxic or hypoxic conditions. After 24 hours, firefly luciferase reporter gene activity was determined and divided by the renilla luciferase values to correct for differences in transfection efficiency. Shown are mean relative luciferase activities ± SEM of 2 transfections performed in quadruplicate. (B) Hep3B cells were transfected with HRE wild-type and HRE mutant ceruloplasmin enhancer SV40 promoter constructs, or the SV40 promoter alone, driving firefly luciferase expression. Following 24 hours of 150 μM CuCl2 or 1% oxygen, luciferase induction factors compared with the untreated controls were determined. A representative experiment performed in triplicate is shown as mean values ± SEM.
The ceruloplasmin gene promoter is activated by Cu2+
Cu2+-dependent HIF-1α protein and ceruloplasmin mRNA induction suggested that ceruloplasmin gene expression is regulated by HIF-1-dependent promoter activation. We thus transiently transfected Hep3B cells with a ceruloplasmin promoter-firefly luciferase reporter gene containing a functional HRE within a 4774 base pair (bp) fragment upstream of the ATG translational start codon.20 A cotransfected constitutive renilla luciferase reporter gene served to normalize for differences in transfection efficiency. Following 24 hours of treatment with CuCl2, reporter gene activity dose-dependently increased up to 4-fold at 200 μM CuCl2 (Figure 7A). Hypoxia alone induced reporter gene activity 3.5-fold and the combination of CuCl2 with hypoxia further increased reporter gene activity. However, CuCl2 concentrations greater than 200 μM were considerably more toxic under hypoxic conditions as could be judged from the decrease in cell density and renilla reporter gene activity (not shown).
The involvement of HIF-1 was further analyzed by using the HRE-containing fragment of the ceruloplasmin -3429 to -3639 upstream region linked to the constitutive SV40 promoter. As shown in Figure 7B, the presence of this fragment was sufficient to induce firefly luciferase activity approximately 5-fold by 150 μM CuCl2 or 1% O2 after 24 hours of treatment. Point mutation of this HRE reduced but did not completely inhibit induction by CuCl2 or hypoxia in these experiments, suggesting that other elements might also be involved in CuCl2-dependent activation of ceruloplasmin gene expression.
Discussion
Molecular mimicry of hypoxia by distinct transition metal ions has been known since 1988, when Bunn and coworkers reported that HepG2 and Hep3B hepatoma cell lines increase erythropoietin gene expression not only under hypoxic conditions but also following treatment with Co2+ or Ni2+ and to a lesser extent Mn2+ salts.41 However, Cu2+ has not been found to induce erythropoietin gene expression in these early experiments. In our study, we demonstrated that Cu2+ can functionally stabilize HIF-1α, leading to target gene induction in hepatoma cells by a mechanism likely to involve the inhibition of prolyl-4-hydroxylation.
Apart from Co2+, Ni2+, or Cu2+, several other transition metals have been reported to induce HIF-1α, including vanadate,42 arsenite,43 and chromium.44 A common mechanism by which these transition metals interfere with PHD function is unknown. Salnikow and colleagues reported that intracellular ascorbate required for PHD activity might be depleted by Co2+ and Ni2+.45 Reactive oxygen species, PI3K/Akt, p38, p70S6K,1 PKCδ, and adenosine monophosphate (AMP) kinases have been reported to be involved in transition metal-dependent HIF-1α induction.42-44,46-48 However, several kinase pathway inhibitors did not affect CuCl2-dependent HIF-1 induction in our hands. Moreover, all of these mechanisms are based on an intact cellular environment, but in our experiments we could demonstrate a direct inhibition of PHD activity in a cell-free system in vitro with an IC50 value of approximately 2.3 μM and full inhibition at approximately 10 μM CuCl2. These values are somewhat lower than the approximately 150 μM CuCl2 required to obtain maximal induction of HIF-1α in hepatoma cells. However, cell culture conditions, including the Cu2+-binding capacity of serum proteins present in the fetal calf serum (FCS), cannot be directly compared with in vitro assays performed with different kinetics and purified proteins in a cell-free environment. Over all, our data suggest that inhibition of PHD activity is the most likely mechanism by which CuCl2 induced HIF-1α.
Spectroscopic and structural studies on 2-oxoglutarate-dependent dioxygenases showed that Cu2+ can substitute active-site Fe2+ in this class of enzymes.12 However, under our experimental conditions Cu2+ does probably not compete for the active site of HIF-PHD3 because even the presence of a vast excess of ferrous iron did not reverse the inhibitory function of CuCl2. Although it cannot be excluded that Cu2+ ions bind to the active site of PHD with considerably higher affinity compared with Fe2+, it appears to be more likely that other, yet unknown mechanisms are responsible for the inhibition of HIF PHDs by transition metal ions.
Recently, van Heerden and coworkers demonstrated that rainbow trout exposed to CuSO4 induce HIF-1α protein in their gills.49 These results confirm that Cu2+ salts can also induce HIF-1α protein in vivo. van Heerden and coworkers attributed their results to an increased oxygen consumption leading to regional tissue hypoxia. However, our data suggest that a direct inhibition of HIF-1α prolyl-4-hydroxylation by Cu2+ salts might also contribute to the accumulation of HIF-1α protein. Whereas the fish were exposed to water containing 1.65 μM CuSO4,49 optimal CuCl2 concentrations in our experiments were between 100 and 200 μM for hepatoma cells. This concentration range is very similar to the widely used CoCl2 that generally stabilizes HIF-1α in the range between 50-150 μM.41,50 Of note, under normal conditions the “free” intracellular copper ion concentration is less than 1 atom per cell.51 Due to its toxicity, intracellular copper is tightly regulated by copper-scavenging metallochaperones.52 Their copper-binding capacity either needs to be saturated before “free” copper is available to interfere with the PHDs or the copper-bound metallochaperones might be directly involved in PHD regulation. However, the precise molecular mechanisms acting between extracellular transition metal concentrations and intracellular PHD target protein regulation remain unknown.
Liver-derived ceruloplasmin is the main copper-binding plasma transport protein and functions as a ferroxidase essential for the oxidation of Fe2+ before it can be bound to transferrin as Fe3+.21 Previous studies demonstrated that ceruloplasmin is transcriptionally regulated by HIF-1 under hypoxic conditions in vitro.20 Here, we demonstrated that ceruloplasmin mRNA expression is also induced in the liver of hypoxic mice in vivo. Because both ceruloplasmin and apotransferrin facilitate iron release from liver cells and macrophages,53-56 the coordinate induction of their transcription under hypoxic conditions is likely required to match the increased iron demand of erythropoietic precursor cells in the bone marrow following stimulation by erythropoietin.
The finding that Cu2+ inhibits PHD activity provides the intriguing possibility that the concentration of free Cu2+ regulates the expression of its binding protein in the liver in a HIF-dependent manner. Indeed, when CuSO4 was administered intravenously to copper-deficient pigs, an increase in plasma ceruloplasmin was observed which preceded the increase in plasma iron.57 Other known HIF-1 target genes induced by Cu2+ in vivo include VEGF which is increased at wound sites topically treated with CuSO4.58 These data are consistent with our findings and support a physiological role of HIF-1 in Cu2+-dependent gene regulation, extending the sensory functions of the PHD family beyond oxygen sensing.
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-10-3980.
Supported by grants from the Medical Faculty of the University of Leipzig (formel.1-22 to F.M.), Bundesministerium für Bildung und Forschung (BMBF; NBL3-01ZZ0106 to F.M. and NBL3 to D.M.K.), Deutsche Forschungsgemeinschaft (DFG; Ka1269/5-1 to D.M.K.), Schweizerischer Nationalfonds (SNF; 3100A0-104219 to R.H.W.) and Stiftung für wissenschaftliche Forschung an der Universität Zürich (to R.H.W.).
F.M. and T.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank J. D. Gitlin and P. L. Fox for the generous gifts of plasmids, and B. Stier, U. Lang, C. Franke, B. Dübel, K. Krauss, and P. Spielmann for excellent technical assistance.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal