Abstract
Multiple myeloma is characterized by the accumulation of terminally differentiated B cells in the bone marrow, due to increased proliferation and restricted apoptosis of the myelomatous clone. Here we have studied the participation of a novel mitogen-activated protein kinase (MAPK) route, the extracellular signal-regulated kinase 5 (Erk5) pathway, in the regulation of myeloma cell proliferation and apoptosis. Erk5 was expressed in cells isolated from patients and in myeloma cell lines. The myeloma growth factor interleukin 6 (IL-6) activated Erk5, and this activation was independent of Ras and Src. Expression of a dominant-negative form of Erk5 restricted the proliferation of myeloma cells and inhibited IL-6–dependent cell duplication. This dominant-negative form also sensitized myeloma cells to the proapoptotic action of dexamethasone and PS341. The latter compound caused a profound decrease in the amount of endogenous Erk5 and was less effective in inducing apoptosis when the level of Erk5 was increased by transfection of Erk5. These results place the Erk5 route as a new regulatory signaling pathway that affects multiple myeloma proliferation and apoptosis.
Introduction
Multiple myeloma (MM) is a clonal B-cell neoplasm characterized by the accumulation of terminally differentiated B cells (plasma cells [PCs]) in the bone marrow.1 Even though substantial advances in the treatment of MM have been made during recent years, complete eradication of the malignant clone is still not possible.1 Accordingly, new relevant areas of research focus on the incorporation of novel drugs into MM treatment, and the better knowledge of the mechanisms that control MM proliferation and survival.2 With respect to the latter, several studies have implicated the signal transducer and activator of transcription 3 (STAT3) and phosphatidylinositol 3′-kinase (PI3K)/Akt routes in the survival of MM cells.3 In addition, the mitogen-associated protein kinases (MAPKs) have been also involved in the control of the proliferation/survival of MM cells.3 Three classical MAPK pathways have been described in mammals.4-6 One, which includes the extracellular signal-regulated kinases 1 and 2 (Erk1 and Erk2), is activated by growth factors and has been linked to the stimulation of cell proliferation in several cellular systems,5,6 including MM cells.7 The 2 other MAPK routes, the p388 and the Jun N-terminal kinases (JNK) pathways,9 are mainly triggered by cytokine and stress stimuli, and their activation has been shown to regulate apoptotic responses.10
A recently characterized MAPK, the Big MAPK-1/Erk5, has recently been implicated in the control of proliferation,11-14 participates in cellular responses to oxidative and mechanical stresses,15,16 and regulates apoptotic responses.17 In vivo studies have indicated that Erk5 may support vascular endothelial viability in the adult animal18 and critically participates in embryogenesis, probably due to its role in proliferation and angiogenesis/vasculogenesis.19-21
Erk5 shares homology with Erk1/2 but is substantially higher in molecular weight, due to the presence of a C-terminal extension that is absent in Erk1/2.22,23 In analogy to Erk1/2, activation of Erk5 occurs by dual phosphorylation at the threonine (Thr218) and tyrosine (Tyr220) residues present in the TEY sequence of the activation loop.13 Phosphorylation at these sites is carried out by the upstream MAP/ERK kinase Mek5, which is specific for Erk5.22 How activated receptors couple to Erk5 is still unclear. In fact, growth factor receptors may cause Erk5 activation through Ras in certain cell lines,24,25 but not in others.13 In addition, other intracellular kinases, such as MEK kinase 2/3 (MEKK2/3),26 c-Cot,27 and c-Src28 may also regulate the activation of the Erk5 cascade. Signaling downstream of Erk5 involves phosphorylation of substrate targets as well as a direct action of Erk5 on gene expression, due to the presence of a transactivation domain in the unique C-terminus of Erk5.29 Important substrates of Erk5 are the serum and glucocorticoid-induced kinase,30 and transcription factors of the myocyte enhancer factor 2 (MEF2) family.31 The latter includes 4 proteins (MEF2A, B, C, and D),32 and 3 of them (MEF2A, C, and D) act as Erk5 substrates.33 Phosphorylation of MEF2 factors by Erk5 has been shown to regulate the expression of other transcription factors, such as c-Jun, that participate in cell cycle progression.34 In addition, MEF2 factors have been reported to mediate the antiapoptotic action of Erk5.35
Because of the important roles of the Erk5 route in the control of cell proliferation in several tissues and in tumoral cells, we investigated the expression and function of Erk5 in MM. Here, we show that MM cells express Erk5 and that this MAPK can be activated by IL-6. Expression of a dominant-negative form of Erk5 restricted the proliferative responses caused by IL-6 and favored drug-induced apoptosis of myeloma cells. Therefore, our data identify Erk5 as a novel route activated by IL-6 and indicate that Erk5 has multiple functions in MM, including the regulation of a proliferative and antiapoptotic response.
Materials and methods
Materials and immunochemicals
Cell culture media, sera, and penicillin-streptomycin were purchased from Gibco BRL (Gaithersburg, MD). Protein A-Sepharose was from Amersham-Pharmacia (Piscataway, NJ). Immobilon P membranes were from Millipore (Bedford, MA). PD98059, FPTIII, GGTI, PP2, Z-IETD-FMK, Z-LEHD-FMK, and Z-VAD-FMK were from Calbiochem (San Diego, CA). IL-6, epidermal growth factor (EGF), stem cell factor (SCF), tumor necrosis factor α (TNF-α) and insulin-like growth factor 1 (IGF-1) were from Strathmann (Hamburg, Germany). PS341 was from Millennium Pharmaceuticals (Cambridge, MA). Other generic chemicals were purchased from Sigma Chemical (St Louis, MO), Roche Biochemicals (Mannheim, Germany), or Merck (Darmstadt, Germany). Neuregulin (NRG) was generously provided by Dr Mark X. Sliwkowski (Genentech, San Francisco, CA). The anti-pMEK1 was from Cell Signaling (Beverly, MA). The anti-Erk1/2, anti-pErk1/2, anti–H-Ras, anti-Mek5, antitubulin, and anti-PY99 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-pAkt, anticaspase-3, and anti–c-Kit antibodies were from Becton Dickinson (San Diego, CA). The anti-HA antibody was from Roche Biochemicals. The anti-pMek5 antibody was generously provided by Dr N. Mody (University of Dundee, Scotland).36 The cyanine 3 (Cy3)–conjugated secondary antibody was from Jackson ImmunoResearch (West Grove, PA). The horseradish peroxidase (HRP)–conjugated secondary antibodies were from Bio-Rad (Hercules, CA). The anti-Erk5 antibodies have been described previously.12
Cell culture and transfections
The cell lines MM1S, MM1R (from Dr S. T. Rosen, Chicago, IL); MM144, OPM2 (from Dr S. Rudikoff, Bethesda, MD); NCIH929 (from Dr J. Teixidó, Madrid, Spain); U266, and RPMI8226 (from Dr W. Dalton, Tampa, FL) were cultured at 37°C in a humidified atmosphere in the presence of 5% CO2-95% air. Cells were grown in RPMI 1640 medium with l-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% (cell lines) or 20% (patient cells) fetal bovine serum (FBS). For the biochemical experiments, and unless otherwise indicated, myeloma cells were treated in complete media that had been with the cells for at least 24 hours.
Generation of retroviruses and infection
293T cells were plated in 60-mm–diameter dishes (1.8 × 106 cells in 3 mL Dulbecco modified Eagle medium [DMEM] with 10% FBS) and allowed to attach overnight. Five minutes prior to transfection, 25 μM chloroquine was added to each plate. The transfection solution contained DNA (2.5 μg pMDG-VSV, 5 μg pNGVL-MLV-gag-pol, 3 μg retroviral vector [pLZR-IRES-GFP, pLZR-HA-Erk5AEF-IRES-GFP, or pLZR-RasN17-IRES-GFP]), 61 μL 2 M CaCl2, and doubled-distilled H2O to 500 μL. After mixing, 0.5 mL of 2 × hepes-buffered saline solution (HBS) (pH 7.0) was added, and the solution was bubbled for 15 seconds. The HBS-DNA complex was dropped onto cells. Eight hours later, this medium was replaced with complete culture medium that was replaced 24 to 32 hour after transfection with 3 mL fresh virus-collecting medium. Twenty-four hours later, the supernatant from transfected cells was centrifuged at 1000g for 5 minutes. MM1S or MM144 cells were infected with 1 mL viral supernatants containing Polybrene at 6 μg/mL, to which 9 mL RPMI 1640 with 10% FBS medium was added 1 hour later. The following day, the medium was changed to overnight-infected MM cells.
Cell proliferation assays
The analysis of MM cell proliferation using the methylthiotetrazole (MTT) assay has been described previously.37 Four wells were analyzed for each condition, and the results are presented as the mean ± SD of quadruplicates of a representative experiment that was repeated at least twice. Annexin V–fluorescein isothiocyanate (FITC) measurements and analysis of apoptotic cell death were performed as described.37 Measurement of cell proliferation of myeloma cells cocultured with bone marrow stromal cells (BMSCs) was carried out as described previously.38 Briefly, BMSCs were plated in 96-well culture dishes (50 000/well) and allowed to reach confluence during 48 hours. Then, 30 000 myeloma cells were plated in RPMI 1640 containing 0.5% serum. Bromodeoxyuridine (BrdU) was added for the last 8 hours, and then BrdU uptake was measured using a commercial kit (Roche Biochemicals), following the manufacturer's instructions.
Reporter gene analysis
Plasmids encoding a luciferase gene driven by the wild-type Nur77 promoter (pMEF2-luc-wt that contains the -307 to -242 region of the Nur77 promoter), or a Nur77 promoter with mutated MEF2-binding sites (pMEF2-luc-mut)29,39 were provided by Dr A. Winoto (University of California, Berkeley, CA). These luciferase constructs were cotransfected with pRLSV40 Renilla vector to control for transfection efficiency, in a molar ratio of 1:40 (pRLSV40 versus pMEF2-luc-wt or pMEF2-luc-mut vectors). Then, 20 × 106 MM1S cells were transfected by electroporation with 5 μg of the indicator plasmid or the mutated version. Electroporation was performed in serum- and antibiotics-free media with a Gene Pulser II apparatus (Bio-Rad) using 0.4-cm gene pulser cuvettes (Bio-Rad) with settings of 250 V and 950 μF. Eighteen hours after transfection, cells were rinsed with phosphate-buffered saline (PBS) and harvested with Passive Lysis buffer (Promega, Madison, WI). The Dual Luciferase System (Promega) was used according to the manufacturer's protocol. Readings were taken in duplicate in a luminometer (BERTHOLD, Pforzheim, Germany), and experiments were repeated 3 times.
Immunoprecipitation, Western blotting, and immunofluorescence microscopy
Cells were collected and centrifuged at 10 000g for 2 minutes. Then cells were washed with PBS and lysed in ice-cold lysis buffer (140 mM NaCl,10 mM EDTA [ethylenediaminetetraacetic acid], 10% glycerol, 1% Nonidet P-40, 20 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.0, 1 μM pepstatin, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM sodium orthovana-date). Samples were centrifuged at 10 000g at 4°C for 10 minutes and supernatants were transferred to new tubes with the corresponding antibody and protein A-Sepharose. The rest of the immunoprecipitation and Western blotting protocol was carried out as described before.40
For immunofluorescence microscopy, myeloma cells were plated on poly-l-lysine–pretreated glass coverslips. Attached cells were subsequently washed with PBS and fixed in 2% P-formaldehyde for 30 minutes at room temperature. The rest of the protocol for the staining and confocal microscopy has been described.12
Ras binding to GST-RafRBD
Cells were stimulated for 15 minutes with 5 nM IL-6 and lysed with ice-cold lysis buffer containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5. After centrifugation at 12 000g for 10 minutes, cellular extracts were precipitated with 15 μg GST-RafRBD (gluthione-S-transferase-Raf Ras binding domain fusion protein) for 30 minutes at 4°C. Samples were then washed 3 times with lysis buffer, resuspended in 2 × sample buffer, and boiled for 5 minutes. The samples were run in 12% gels and Ras bound to the fusion protein was analyzed by Western blotting.
In vitro kinase analyses
Extracts from MM1S cells treated with IL-6 or IGF-1 were immunoprecipitated with the anti-Erk2 antibody, and immunocomplexes were washed 3 times with lysis buffer. A final wash was performed with kinase buffer (20 mM HEPES, pH 7.6, 20 mM MgCl2, 25 mM β-glycerophosphate). The immunocomplexes were then incubated for 30 minutes at 30°C with 2 μg GST-Elk in the kinase buffer containing 10 μM adenosine triphosphate (ATP), 1 μCi (γ-32P)ATP (0.037 MBq). Samples were electrophoresed in 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and bands in dried gels detected by autoradiography.
RT-PCR and quantitative PCR
RNA was isolated from MM1S cells, and an equal amount (2 μg) from the different samples was primed with poly-T. cDNA was synthesized with M-MLV reverse transcriptase (RT; Promega). The relative levels of gene expression were determined by real-time quantitative polymerase chain reaction (PCR) based on 2-step SYBR green I chemistry. As reference genes for normalization of the expression data, we used ABL and GAPDH. The primers used were: c-Jun: 5′-AAAAGTGAAAACCTTGAAAGCTCAG-3′ and 5′-CGTGGTTCATGACTTTCTGTTTAAG-3′;ABL: 5′-CCATGAGGTACTGGTCCCTTC-3′ and 5′-CGCCACTTAGAAAAGAGCGTC-3′; GAPDH: 5′-CAGGGCTGCTTTTAACTCTGGTAA-3′ and 5′-GGGTGGAATCATATTGGAACATGTA-3′; MEF2A: 5′-AGTCTCACTCTACCCCCCAGG-3′ and 5′-GCTACTCGGGAGGCTTGAGGT-3′; MEF2B: 5′-AGCACAAGGCAGACATGCAG-3′ and 5′-TGTCCACCAGGTTCTTCACG-3′; MEF2C: 5′-ACTTGAGCACACGCGGTACACC-3′ and 5′-TCCACCTGATTCAAACATGCAG-3′; MEF2D: 5′-CGCTCTTTGCCGTGACAAC-3′ and 5′-ACGGTCTGGGAACAGTGCTC-3′; MEKK2: 5′-AAACGGCTTCAGACCATCTGTC-3′ and 5′-CCATAGCCTTGTCCACTGATGA-3′; MEKK3: 5′-GAGAGCCCGAGGAGGTGTCT-3′ and 5′-GGACACAGCTGGTGGTAGCA-3′; c-cot: 5′-ACTGGTTGAGCCCAAGAGTTCA-3′ and 5′-GACTACAGGCATGCACCATCAC-3′.
Statistical analysis
The Student t test was used for comparisons between means. P < .01 was interpreted to denote statistical significance. The statistical analysis was performed using the SPSS 11.0 statistical package (SPSS, Chicago, IL).
Results
Expression of Erk5 in myeloma cells
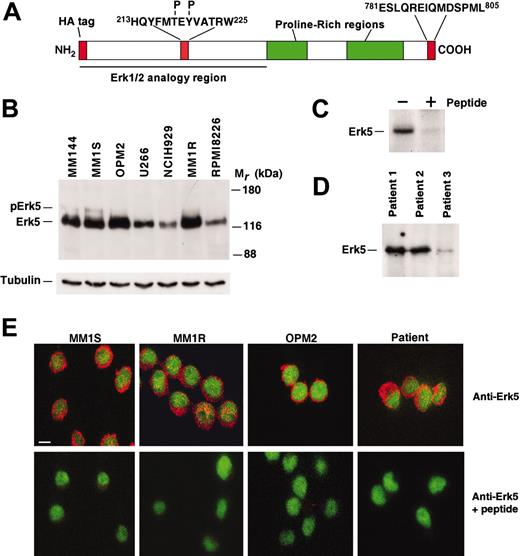
To investigate a possible role of Erk5 in MM biology, we first analyzed whether myeloma cells expressed this MAPK. For this purpose, lysates from different MM cell lines were prepared and immunoprecipitated with an antibody raised to the C-terminus of Erk5 (residues 781-805; Figure 1A; see also Esparis-Ogando et al12 ), and Erk5 expression was analyzed by Western blotting. As shown in Figure 1B, the antibody recognized 2 different bands. A faster migrating band of 120 kDa of Mr was present at different levels in all the cell lines analyzed. This band corresponds to a form of Erk5 that is devoid of phosphorylation at the TEY microdomain within the Erk5 activation loop12 and is considered to represent inactive Erk5. In addition, a slower migrating Erk5 form was also detected in MM1S (Figure 1B), although its amount was much lower. The Erk5 forms were absent when the antibody was preincubated with an excess of the peptide used for the raising of the antibody, indicating that the antibody specifically recognized Erk5 (Figure 1C). Erk5 was also expressed in bone marrow plasma cells from 3 patient samples examined (Figure 1D).
The subcellular distribution of Erk5 in myeloma cells was studied by immunofluorescence microscopy with the anti-Erk5 C-terminal antibody. Strong staining of Erk5 was observed in the cytosolic compartment (Figure 1E, top panels), and this staining was prevented by preincubation of the antibody with the Erk5 peptide (Figure 1E, bottom panel). On higher magnification analysis, some staining was also observed in the nucleus, where Erk5 accumulated in certain regions following a dotted pattern (data not shown).
Erk5 is activated by IL-6
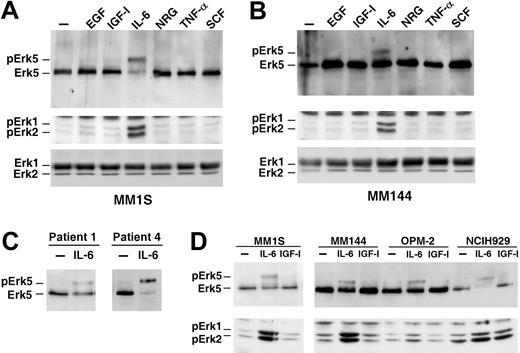
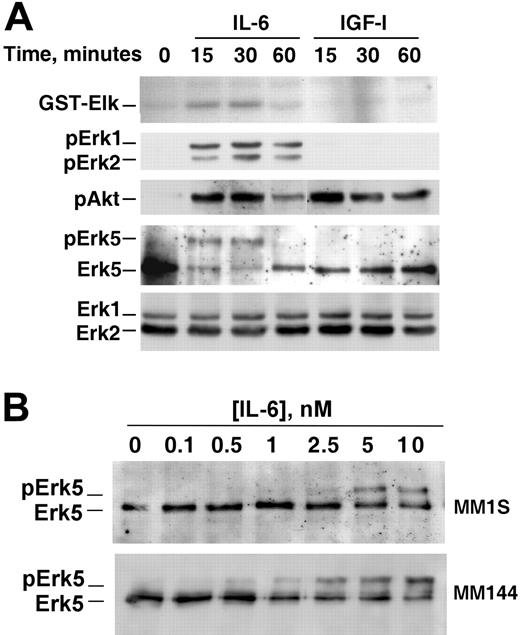
Because Erk5 is activated by factors that control cell proliferation/survival in other cell types,14 the activation of Erk5 by different cytokines known to affect these processes in MM cells was investigated. Treatment with IL-6 caused a retardation in the mobility of Erk5, indicative of Erk5 dual phosphorylation, in MM1S (Figure 2A, top panel) and in MM144 cell lines (Figure 2B, top panel), as well as in patient cells (Figure 2C). The dual Erk5 phosphorylation following IL-6 stimulation was confirmed by immunoprecipitation of MM cell lysates with an antibody that only detects phosphorylated Erk5 (data not shown). IL-6 also induced Erk1/2 phosphorylation in MM1S and MM144 cell lines (Figure 2A-B, middle panels), as well as phosphorylation of the Erk5 upstream element Mek5 (data not shown). Interestingly, IGF-1, which has been reported to stimulate MM cell proliferation,41,42 did not substantially induce Erk1/2 or Erk5 dual phosphorylation in MM1S, MM144, and OPM2 cells (Figure 2D). In NCIH929, IGF-1 also failed to substantially induce Erk5 phosphorylation; however, and in agreement with previously published data,43 IGF-1 activated Erk1/2 in these cells. Time-course experiments carried out in MM1S cells confirmed that IL-6 stimulated Erk1/2 phosphorylation and activity (the latter measured by in vitro kinase analyses using GST-Elk as a substrate; Figure 3A), whereas IGF-1 failed to induce any substantial increase in Erk1/2 phosphorylation or activity. However, both IL-6 and IGF-1 stimulated Akt phosphorylation (Figure 3A).
Expression of Erk5 in myeloma cells. (A) Schematic representation of Erk5, with the epitopes recognized by the anti-Erk5 (C-terminal), anti-pErk5, and anti-HA antibodies. (B) Expression of Erk5 in MM cell lines. Erk5 was immunoprecipitated from 1-mg cell extracts and detected by Western blotting with the anti–C-terminus antibody. The position of the Mr markers is indicated at the right. In parallel, and as a loading control, 20 μg protein from each of the cell extracts was analyzed for tubulin content by Western blotting. (C) Specificity of the anti-Erk5 antibody; 1 mg MM1S cell extracts was immunoprecipitated with the anti-Erk5 C-terminus antibody either in the absence or presence of 10 μg of the peptide used to raise the antibody. Erk5 was detected by Western blotting as described in panel B. (D) Expression of Erk5 in patient myeloma cells. Cells were isolated from myeloma patients, plated in complete media, and analyzed by Western blotting for Erk5. The proportion of malignant plasma cells was greater than 90% as indicated by phenotypic analysis before the experiment. (E) Immunofluorescent localization of Erk5 in myeloma cells. Cells were plated on poly-l-lysine–coated coverslips and immunostained with the anti-Erk5 C-terminal antibody that was previously preincubated with the antigenic peptide, where indicated. Erk5 is shown in red, together with a nuclear stain (SYBR green). Bar represents 10 μm.
Expression of Erk5 in myeloma cells. (A) Schematic representation of Erk5, with the epitopes recognized by the anti-Erk5 (C-terminal), anti-pErk5, and anti-HA antibodies. (B) Expression of Erk5 in MM cell lines. Erk5 was immunoprecipitated from 1-mg cell extracts and detected by Western blotting with the anti–C-terminus antibody. The position of the Mr markers is indicated at the right. In parallel, and as a loading control, 20 μg protein from each of the cell extracts was analyzed for tubulin content by Western blotting. (C) Specificity of the anti-Erk5 antibody; 1 mg MM1S cell extracts was immunoprecipitated with the anti-Erk5 C-terminus antibody either in the absence or presence of 10 μg of the peptide used to raise the antibody. Erk5 was detected by Western blotting as described in panel B. (D) Expression of Erk5 in patient myeloma cells. Cells were isolated from myeloma patients, plated in complete media, and analyzed by Western blotting for Erk5. The proportion of malignant plasma cells was greater than 90% as indicated by phenotypic analysis before the experiment. (E) Immunofluorescent localization of Erk5 in myeloma cells. Cells were plated on poly-l-lysine–coated coverslips and immunostained with the anti-Erk5 C-terminal antibody that was previously preincubated with the antigenic peptide, where indicated. Erk5 is shown in red, together with a nuclear stain (SYBR green). Bar represents 10 μm.
IL-6 activates Erk5 in myeloma cells. MM1S (A) or MM144 (B) cells were incubated for 10 minutes with 10 nM EGF, IGF-1, IL-6, NRG, TNF-α, or SCF, and Erk5 expression was analyzed by Western blotting. The action of the polypeptide factors on Erk1/2 phosphorylation was detected by Western blotting with anti-pErk1/2 antibodies, and the total amount of Erk1/2 was analyzed on cell extracts with an anti-Erk1/2 antibody. (C) Effect of IL-6 on patient myeloma cells. Cells were treated with IL-6 as described in panel A and Erk5 analyzed by Western blotting from anti-Erk5 immunoprecipitates. (D) Cells were serum-starved for 18 hours and then treated with IGF-1 (10 nM) or IL-6 (5 nM) and Erk5 and pErk1/2 were analyzed by Western blotting.
IL-6 activates Erk5 in myeloma cells. MM1S (A) or MM144 (B) cells were incubated for 10 minutes with 10 nM EGF, IGF-1, IL-6, NRG, TNF-α, or SCF, and Erk5 expression was analyzed by Western blotting. The action of the polypeptide factors on Erk1/2 phosphorylation was detected by Western blotting with anti-pErk1/2 antibodies, and the total amount of Erk1/2 was analyzed on cell extracts with an anti-Erk1/2 antibody. (C) Effect of IL-6 on patient myeloma cells. Cells were treated with IL-6 as described in panel A and Erk5 analyzed by Western blotting from anti-Erk5 immunoprecipitates. (D) Cells were serum-starved for 18 hours and then treated with IGF-1 (10 nM) or IL-6 (5 nM) and Erk5 and pErk1/2 were analyzed by Western blotting.
Ligands of EGF family, that act through ErbB receptors, activate Erk5 in other cellular systems,12,13 and have been shown to regulate proliferation of MM cells.44 Two of these ligands, EGF and NRG, failed to activate Erk5 in MM1S and MM144 cells. Furthermore, addition of EGF or NRG failed to induce activation of ErbB receptors in MM1S, MM144, and U266 cells (data not shown). SCF, an activator of the c-Kit receptor tyrosine kinase, which is present in malignant plasma cells from about 20% of the patients with MM,45 also failed to induce Erk5 activation in MM1S and MM144 cells (Figure 2A-B). IL-6–induced phosphorylation of Erk5 was maximal at concentrations of the cytokine of 5 nM and above and was noticeable at 1 nM (Figure 3B).
IL-6–induced Erk5 activation occurs through a Ras- and Src-independent pathway that is sensitive to classical MEK1/2 inhibitory compounds
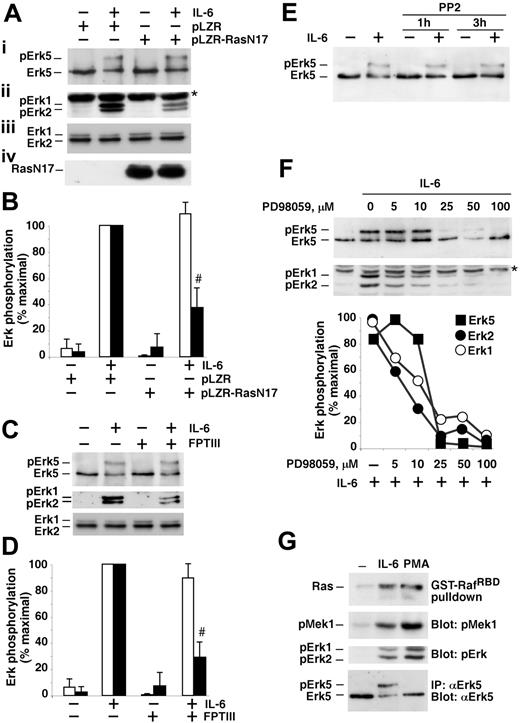
To investigate if Ras was involved in IL-6–induced Erk5 phosphorylation, MM1S cells were infected with a retroviral vector that contained a form of H-Ras (RasN17) that acts as a dominant-negative form.12,13 Western blotting with an anti–H-Ras antibody showed that the infected cells expressed the mutated RasN17 protein (Figure 4A, bottom panel). IL-6–induced phosphorylation of Erk5 was of similar magnitude in cells expressing RasN17 when compared to control cells (Figure 4A, top panel). In contrast, the phosphorylation of Erk1 and Erk2, which lie downstream of Ras, was significantly inhibited (Figure 4A, second panel). Quantitative analyses indicated that the presence of RasN17 resulted in a 60% inhibition of IL-6–induced Erk1/2 phosphorylation, without affecting Erk5 phosphorylation (Figure 4B). Another strategy used to assess whether Ras participated in Erk5 activation was based on the use of farnesyltransferase or geranylgeranyltransferase inhibitors. Pretreatment with the farnesyltransferase inhibitor, FPTIII, only marginally affected Erk5 phosphorylation in response to addition of IL-6 (Figure 4C, top panel), but decreased Erk1/2 phosphorylation (Figure 4C, middle panel). Quantitative analyses of these data showed the stronger effect of FPTIII on Erk1/2 phosphorylation, as compared to Erk5 phosphorylation (Figure 4D). Pretreatment with the geranylgeranyltransferase inhibitor, GGTI, did not significantly affect Erk5 activation by IL-6 (data not shown).
Src has been involved in the activation of Erk5 in other cellular systems.28,46 In MM cells treated with PP2, a compound that inhibits Src kinase activity, and Src-mediated Erk5 activation in other cell types,28,46 the compound was unable to prevent IL-6–induced Erk5 phosphorylation (Figure 4E), suggesting that Src did not play a major role in the activation of Erk5 by IL-6.
The homology between Erk1/2 and Erk5 led us to analyze whether inhibitors of the Erk1/2 route also acted as regulators of Erk5 activation in myeloma cells. Preincubation of MM1S cells with PD98059, a Mek1/2 inhibitor, impaired IL-6–induced phosphorylation of Erk1/2 and Erk5 (Figure 4F). Similar results were obtained when using U0126, a different Mek1/2 inhibitor (data not shown). As previously reported for other cell types,36 their inhibitory action on Erk1/2 was more potent than on Erk5. The fact that inhibitors of the activation of Erk1/2 affected the activation of Erk5 raised the possibility that the Mek1/2-Erk1/2 route could act upstream of the Erk5 route. To analyze this, we used an alternative treatment to activate the Mek1/2-Erk1/2 route. Protein kinase C (PKC) activation by phorbol esters, such as phorbol myristate acetate (PMA), has been reported to cause Erk1/2 activation by stimulation of Ras.47 In MM1S cells addition of PMA or IL-6 induced a clear activation of Ras (Figure 4G). The activation of Ras was followed by increased phosphorylation and activation of Mek1 and Erk1/2, and the degree of activation was higher in cells treated with the phorbol ester, as compared to cells that received IL-6 (Figure 4G and data not shown). However, PMA failed to induce Erk5 dual phosphorylation. These data exclude a link between the Mek1-Erk1/2 route and the Erk5 pathway. Furthermore, the fact that PMA activated Ras without causing Erk5 phosphorylation, together with the data using RasN17, clearly dissociate Ras activation from Erk5 phosphorylation in myeloma cells.
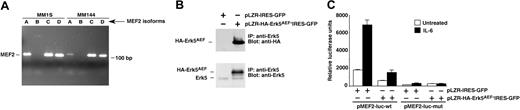
MEF2 factors are expressed in myeloma cells and act as downstream Erk5 mediators
Because of the reported role of MEF2 proteins in Erk5 signal transduction in other cellular systems,34,35 we investigated whether MEF2 transcription factors were expressed in MM cells and participated in Erk5 signal transduction. RT-PCR analyses showed that MM1S and MM144 cells expressed MEF2A, MEF2C, and MEF2D, whereas the expression of MEF2B was undetectable (Figure 5A). To analyze the potential participation of MEF2 proteins in Erk5 signaling in MM cells, we used a luciferase reporter system based on the Nur77 promoter, which contains MEF2-binding sites.29,39 In addition, to regulate Erk5 function we used a mutant HA-tagged version of Erk5 in which the TEY microdomain was changed to AEF, creating a form of Erk5 that acts as a dominant negative34 (HA-Erk5AEF, Figure 1A, see also Esparis-Ogando et al12 ). HA-Erk5AEF was subcloned into the retroviral vector pLZR-IRES-GFP and particles used to infect MM1S cells. Western blotting with the anti-HA antibody identified the HA-Erk5AEF form in cells infected with pLZR-HA-Erk5AEF-IRES-GFP, but not in pLZR-IRES-GFP–infected cells (Figure 5B, top panel). Western blotting using the anti-Erk5 antibody indicated that the expression of HA-Erk5AEF was above the levels of endogenous Erk5 (Figure 5B, bottom panel).
Time course and dose response of IL-6–induced Erk5 activation in myeloma cells. (A) Time course of the effect of IL-6 and IGF-1 on Erk1/2 activation and phosphorylation and Erk5 and Akt phosphorylation. MM1S cells were treated for the indicated times with the growth factors and cell extracts used for analyses of Erk1/2 activity using GST-Elk, Erk1/2, pErk1/2, or pAkt in Western blots of cell extracts. Erk5 was detected by Western blotting of anti-Erk5 immunoprecipitates. The panels show the results of an experiment that was repeated twice. (B) Dose effect of IL-6 on Erk5 phosphorylation. IL-6 was added for 15 minutes, and Erk5 was analyzed as in panel A.
Time course and dose response of IL-6–induced Erk5 activation in myeloma cells. (A) Time course of the effect of IL-6 and IGF-1 on Erk1/2 activation and phosphorylation and Erk5 and Akt phosphorylation. MM1S cells were treated for the indicated times with the growth factors and cell extracts used for analyses of Erk1/2 activity using GST-Elk, Erk1/2, pErk1/2, or pAkt in Western blots of cell extracts. Erk5 was detected by Western blotting of anti-Erk5 immunoprecipitates. The panels show the results of an experiment that was repeated twice. (B) Dose effect of IL-6 on Erk5 phosphorylation. IL-6 was added for 15 minutes, and Erk5 was analyzed as in panel A.
IL-6 activates Erk5 in myeloma cells through a Ras- and Src-independent route. (A) MM1S cells were infected with pLZR-IRES-GFP or pLZR-RasN17-IRES-GFP, and the expression of Ras was analyzed by Western blot of cell extracts using an anti–H-Ras antibody (iv). The effect of IL-6 on Erk5 (i) and pErk1/2 (ii) is shown, as well as the total Erk1/2 levels (iii). The asterisk indicates a nonspecific band that appears sometimes in the anti-pErk1/2 blots. (B) Quantitative analyses of the levels of Erk5 phosphorylation (□) or pErk1/2 (▪) in MM1S cells infected with pLZR-IRES-GFP or pLZR-RasN17-IRES-GFP, and treated, where indicated, with IL-6 (5 nM, 15 minutes). The results are represented as the mean ± SD of 3 different experiments, and considering as 100% the stimulation obtained with IL-6 in control infected cells. The number (#) sign indicates P < .01. (C) Effect of FPTIII on Erk5 phosphorylation in MM1S cells. Cells were preincubated with FPTIII (10 μM) for 24 hours and then treated with IL-6 for 15 minutes. Erk5, Erk1/2, and pErk1/2 were analyzed as described in A. (D) Quantitative analyses of the effect of FPTIII on Erk5 (□) and Erk1/2 (▪) phosphorylation. The results were quantitated as described for panel B. The number sign (#) indicates P < .01. (E) Effect of the Src inhibitor PP2 on IL-6–induced Erk5 activation. PP2 (20 μM) was added to MM1S cells for the times indicated and then cells were stimulated with IL-6 for 15 minutes. Erk5 was analyzed as described in A. (F) Action of PD98059 on IL-6–induced Erk5 and Erk1/2 phosphorylation. MM1S cells were preincubated with the indicated concentrations of PD98059 for 60 minutes, and then IL-6 added for an additional 15-minute period. Erk5 and pErk1/2 were analyzed by Western blotting as described in A, and the amount of phosphorylation quantitated and graphically represented at the bottom of each panel. (G) Action of IL-6 and PMA on Ras, Mek1, Erk1/2, and Erk5. MM1S cells were treated with IL-6 (5 nM) or PMA (1 μM) for 15 minutes and then lysates prepared for Ras pull-down using GST-RafRBD, pMek, or pErk1/2 analyses on Western blots of cell extracts, or Erk5 analysis on Western blots of anti-Erk5 immunoprecipitates. Results are representative of an experiment that was repeated 3 times.
IL-6 activates Erk5 in myeloma cells through a Ras- and Src-independent route. (A) MM1S cells were infected with pLZR-IRES-GFP or pLZR-RasN17-IRES-GFP, and the expression of Ras was analyzed by Western blot of cell extracts using an anti–H-Ras antibody (iv). The effect of IL-6 on Erk5 (i) and pErk1/2 (ii) is shown, as well as the total Erk1/2 levels (iii). The asterisk indicates a nonspecific band that appears sometimes in the anti-pErk1/2 blots. (B) Quantitative analyses of the levels of Erk5 phosphorylation (□) or pErk1/2 (▪) in MM1S cells infected with pLZR-IRES-GFP or pLZR-RasN17-IRES-GFP, and treated, where indicated, with IL-6 (5 nM, 15 minutes). The results are represented as the mean ± SD of 3 different experiments, and considering as 100% the stimulation obtained with IL-6 in control infected cells. The number (#) sign indicates P < .01. (C) Effect of FPTIII on Erk5 phosphorylation in MM1S cells. Cells were preincubated with FPTIII (10 μM) for 24 hours and then treated with IL-6 for 15 minutes. Erk5, Erk1/2, and pErk1/2 were analyzed as described in A. (D) Quantitative analyses of the effect of FPTIII on Erk5 (□) and Erk1/2 (▪) phosphorylation. The results were quantitated as described for panel B. The number sign (#) indicates P < .01. (E) Effect of the Src inhibitor PP2 on IL-6–induced Erk5 activation. PP2 (20 μM) was added to MM1S cells for the times indicated and then cells were stimulated with IL-6 for 15 minutes. Erk5 was analyzed as described in A. (F) Action of PD98059 on IL-6–induced Erk5 and Erk1/2 phosphorylation. MM1S cells were preincubated with the indicated concentrations of PD98059 for 60 minutes, and then IL-6 added for an additional 15-minute period. Erk5 and pErk1/2 were analyzed by Western blotting as described in A, and the amount of phosphorylation quantitated and graphically represented at the bottom of each panel. (G) Action of IL-6 and PMA on Ras, Mek1, Erk1/2, and Erk5. MM1S cells were treated with IL-6 (5 nM) or PMA (1 μM) for 15 minutes and then lysates prepared for Ras pull-down using GST-RafRBD, pMek, or pErk1/2 analyses on Western blots of cell extracts, or Erk5 analysis on Western blots of anti-Erk5 immunoprecipitates. Results are representative of an experiment that was repeated 3 times.
IL-6 increased luciferase activity in MM1S cells transfected with the MEF2 indicator plasmid pMEF2-luc-wt (Figure 5C). In cells expressing the dominant-negative form of Erk5, treatment with IL-6 caused a minimal increase in luciferase activity. IL-6 did not cause any significant change in luciferase activity in MM1S cells transfected with a mutated MEF2 indicator plasmid unable to bind MEF2 (pMEF2-luc-mut29 ). These results indicated that MEF2 factors were expressed by MM cells and that the Erk5-MEF2 axis may participate in the regulation of gene expression by IL-6 in MM cells.
Expression of dominant-negative Erk5 restricts MM proliferation and sensitizes MM cells to apoptosis
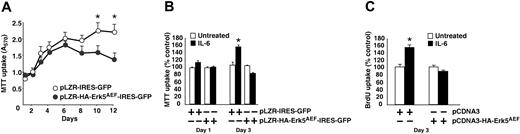
Because Erk5 has been involved in the control of cell proliferation in other cell types14 and was activated by IL-6 in myeloma cells, its potential role in MM proliferation was investigated. Expression of HA-Erk5AEF significantly reduced MTT uptakes of MM1S cells (Figure 6A). Furthermore, the MTT uptake of cells containing HA-Erk5AEF started to decline after 6 days in culture, suggestive of a reduction in cell number. The possible participation of Erk5 in IL-6–induced proliferation was also analyzed. IL-6 slightly increased MTT uptake of control MM1S cells after 24 hours, and this effect was clearly observed at 3 days of treatment (Figure 6B). In contrast, expression of HA-Erk5AEF resulted in a blockade of IL-6–induced proliferation of MM1S cells. That this effect was due to restricted proliferation of MM1S cells expressing HA-Erk5AEF with respect to control cells was confirmed by analysis of DNA synthesis by BrdU uptake experiments (Figure 6C).
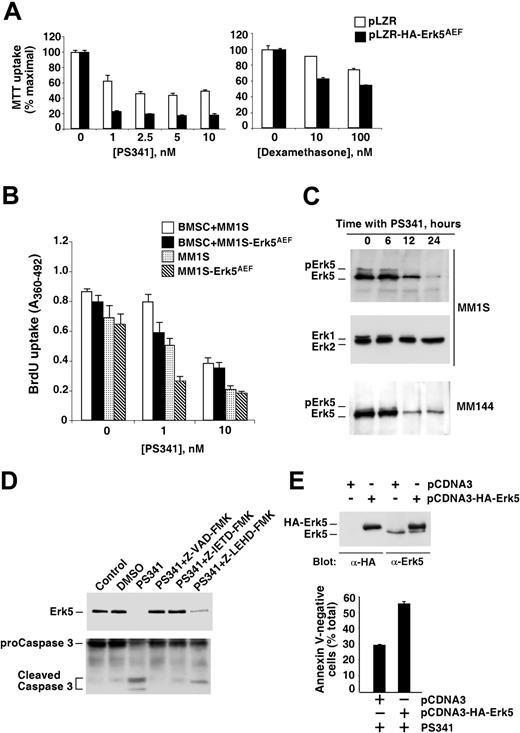
We investigated whether Erk5 could be implicated in the regulation of MM apoptosis. First, the sensitivity of MM cells expressing HA-Erk5AEF to PS341 and dexamethasone, 2 clinically relevant agents that induce MM apoptosis,1 was analyzed. Expression of HA-Erk5AEF significantly increased the sensitivity of MM cells to the apoptotic action of both PS341 and dexamethasone (Figure 7A). The increased sensitivity to PS341 was less evident when the myeloma cells were treated with this compound in the presence of bone marrow stromal cells (Figure 7B). Interestingly, PS341 caused a significant decrease in the amount of Erk5 in MM1S (Figure 7C, top panel) and MM144 cells (Figure 7C, bottom panel), without substantially affecting Erk1/2 levels (Figure 7C, middle panel). Dexamethasone also reduced the levels of Erk5 in MM1S cells (data not shown). PS341 has been shown to decrease the levels of the gp130 subunit of the IL-6 receptor through caspase activation.48 We analyzed whether an analogous mechanism participated in PS341-induced down-regulation of Erk5. As shown in Figure 7D, the pan-caspase inhibitor Z-VAD-FMK, as well as the caspase-8 inhibitor Z-IETD-FMK strongly inhibited PS341-induced Erk5 down-regulation, or caspase-3 processing. We reasoned that if PS341 decreased Erk5 and this was related to the sensitivity to apoptosis on treatment with this drug, then raising the levels of wild-type Erk5 could decrease the apoptotic action of PS341 in MM cells. To test this, we transfected MM1S cells with wild-type Erk5 and selected a pool of MM1S cells in which the endogenous amount of Erk5 was supplemented several-fold by exogenous HA-tagged Erk5 (Figure 7E). The action of PS341 was then tested on MM1S cells transfected with empty vector (pCDNA3) or with pCDNA3-HA-Erk5. As shown in Figure 7E, overexpression of HA-tagged wild-type Erk5 protected MM cells from PS341-induced apoptosis.
MEF2 participates in IL-6–Erk5 signal transduction. (A) Expression of MEF2 isoforms in MM1S and MM144 cells, analyzed by RT-PCR using primers specific for each of the MEF2 isoforms. (B) Expression of a dominant-negative form of Erk5 in MM1S cells. Cells were infected with retroviruses that included an empty or HA-Erk5AEF–containing vector, and the expression of the infected protein was analyzed by immunoprecipitation with the anti-Erk5 C-terminal antibody followed by blotting with anti-HA (top) or anti-Erk5 (bottom) antibodies. (C) Effect of the dominant-negative form of Erk5 on an MEF2 luciferase reporter gene. MM1S infected with either the empty retroviral vector or with the retroviral vector containing HA-Erk5AEF were electroporated with the indicated plasmid reporter gene and 24 hours later treated for 3 hours with IL-6 (5 nM; ▪) or not (□) as indicated. Cell extracts were prepared and analyzed for the relative luciferase activity as described in “Materials and methods.” Data are the mean ± SD of duplicates of an experiment repeated twice.
MEF2 participates in IL-6–Erk5 signal transduction. (A) Expression of MEF2 isoforms in MM1S and MM144 cells, analyzed by RT-PCR using primers specific for each of the MEF2 isoforms. (B) Expression of a dominant-negative form of Erk5 in MM1S cells. Cells were infected with retroviruses that included an empty or HA-Erk5AEF–containing vector, and the expression of the infected protein was analyzed by immunoprecipitation with the anti-Erk5 C-terminal antibody followed by blotting with anti-HA (top) or anti-Erk5 (bottom) antibodies. (C) Effect of the dominant-negative form of Erk5 on an MEF2 luciferase reporter gene. MM1S infected with either the empty retroviral vector or with the retroviral vector containing HA-Erk5AEF were electroporated with the indicated plasmid reporter gene and 24 hours later treated for 3 hours with IL-6 (5 nM; ▪) or not (□) as indicated. Cell extracts were prepared and analyzed for the relative luciferase activity as described in “Materials and methods.” Data are the mean ± SD of duplicates of an experiment repeated twice.
Discussion
Aberrant control of plasma cell proliferation/survival lies at the origin of MM and is responsible for the progression of this disease. Therefore, a better understanding of the molecular mechanisms that control these processes is critical for the development of biologically based therapies aimed at the eradication of the myelomatous cells. Because the Erk5 route has been shown to regulate the proliferation of other tumoral cells,14 we decided to analyze the role of this MAPK route in MM. Here, we describe the expression of Erk5 in MM cells and show the role of this kinase as a regulator of MM proliferation and survival. In addition, we demonstrate that Erk5 acts downstream of IL-6 in myeloma cells, adding this MAPK route to the complex IL-6 signal transduction scenario.
Erk5 was expressed at different levels in the MM cell lines and patient cells analyzed. Studies with different polypeptide factors known to have a role in MM biology showed that IL-6 acted as a regulator of Erk5. This cytokine plays an important function in MM cell proliferation and survival3 and had a clear stimulatory effect on Erk5 activation in both MM cell lines and patient cells. Activation of Erk5 by IL-6 occurs on binding of the cytokine to its heterodimeric cell-surface receptor, composed of the gp80 and gp130 subunits. Selectivity for the binding to IL-6 is provided by gp80 because gp130 can also function as a subunit of other cytokine receptors.49 gp130 is responsible for the initiation of the cytosolic signal transduction cascade. It is therefore likely that cytokines that use gp130 as part of their receptor signaling module may also activate the Erk5 route. In line with this is the finding that leukocyte inhibitory factor (LIF), a cytokine that also uses gp130, has been shown to provoke Erk5 activation in cardiomyocytes.50
Erk5 participates in MM proliferation. (A) Proliferation of MM1S (○) or MM1S cells expressing HA-Erk5AEF (•). Cells were plated at identical densities and MTT uptake measured at the indicated times. Data correspond to the mean from quadruplicates ± SD of an experiment that was repeated 3 times. *P < .01. (B) Action of HA-Erk5AEF on IL-6–induced MM1S proliferation. Cells were plated at identical densities and IL-6 (5 nM; ▪) was added or not (□) where indicated. One day or 3 days after the start of the experiment, MTT uptakes were measured. *P < .01. (C) Action of HA-Erk5AEF on IL-6–induced BrdU uptake. Control or HA-Erk5AEF–expressing MM1S cells were plated at 50 000 cells/well, and then IL-6 was (▪) added or not (□) for 3 days, where indicated. BrdU was added for the last 8 hours of the experiment, and then its uptake was measured as described in “Materials and methods.” Results are presented as the mean from quadruplicates ± SD.
Erk5 participates in MM proliferation. (A) Proliferation of MM1S (○) or MM1S cells expressing HA-Erk5AEF (•). Cells were plated at identical densities and MTT uptake measured at the indicated times. Data correspond to the mean from quadruplicates ± SD of an experiment that was repeated 3 times. *P < .01. (B) Action of HA-Erk5AEF on IL-6–induced MM1S proliferation. Cells were plated at identical densities and IL-6 (5 nM; ▪) was added or not (□) where indicated. One day or 3 days after the start of the experiment, MTT uptakes were measured. *P < .01. (C) Action of HA-Erk5AEF on IL-6–induced BrdU uptake. Control or HA-Erk5AEF–expressing MM1S cells were plated at 50 000 cells/well, and then IL-6 was (▪) added or not (□) for 3 days, where indicated. BrdU was added for the last 8 hours of the experiment, and then its uptake was measured as described in “Materials and methods.” Results are presented as the mean from quadruplicates ± SD.
In contrast to IL-6, IGF-1 failed to substantially activate Erk1/2 or Erk5 in most of the cell lines analyzed, with the exception of NCIH929 cells. IGF-1, however, was an excellent activator of the Akt route, even better than IL-6. These differences in signaling suggest that both factors may provoke their actions on MM cells through different intracellular routes. In line with this hypothesis is the fact that the Erk1/2 inhibitor PD98059 (that also inhibits the Erk5 route) prevented IL-6–induced MM proliferation,7 whereas it did not significantly affect IGF-1–induced MM proliferation.43 The latter effect of IGF-1 in MM cells is sensitive to the PI3K inhibitor LY294002 and to the mammalian target of rapamycin (mTOR) inhibitor rapamycin, indicating a predominant role of the Akt route in IGF-1–induced proliferation of MM cells.
Although in other cellular systems growth factor–induced Erk5 activation can be regulated by Ras,25 IL-6–induced Erk5 phosphorylation appeared to be Ras independent. Thus, expression of RasN17, or treatment with farnesyltransferase and geranylgeranyltransferase inhibitors, agents that prevent H and N (FTI) and K-Ras (GTI) activation,51 did not affect IL-6–induced Erk5 phosphorylation in myeloma cells. However, both treatments strongly affected the phosphorylation of Erk1/2, known to reside downstream of Ras. Because in other cell types RasN17 expression has been shown to prevent Erk5 activation,25 it is likely that the differences in the participation of Ras in Erk5 activation of MM cells may reflect a cell-specific mechanism. Another signaling molecule that has been linked to the activation of Erk5 is Src. In vascular endothelial cells, stresses such as stretching16 or oxidation28 cause an increase in Erk5 activation through a Src-dependent route, because the Erk5 response was sensitive to the Src inhibitor PP2. Analogous results were also observed in pulmonary cells treated with asbestos and preincubated with the Src antagonistic drug.46 However, in MM cells IL-6–induced Erk5 activation was insensitive to PP2, suggesting that Src may not be involved in this process. By contrast, PD98059 and U0126, which have been shown to inhibit the Erk1/2 upstream activating enzymes Mek1 and Mek2,36 also efficiently inhibited IL-6–induced Erk5 phosphorylation, as reported in other cellular systems.12,36 These results indicate that these compounds may target the Erk5-activating kinases, or that kinases participating in the Erk1/2 route may act upstream of Erk5. The latter appears quite unlikely, especially in light of the results obtained with PMA. This compound strongly activated Mek1 phosphorylation, as well as Erk1/2 phosphorylation and activity. Yet activation of this pathway was not followed by Erk5 phosphorylation, indicating that these 2 MAPK routes are not directly linked. Furthermore, because PMA also activated Ras, without a concomitant Erk5 phosphorylation, the data obtained with PMA further support the idea that Erk5 phosphorylation is also unlinked from Ras activation in MM cells.
Erk5 regulates MM apoptosis. (A) Expression of the dominant-negative form of Erk5 sensitizes MM cells to drug-induced apoptosis. Control (□) or HA-Erk5AEF–expressing (▪) MM1S cells were plated at identical densities and treated for 24 hours with the indicated concentrations of PS341 or dexamethasone. MTT uptake was carried out as described in the text. The results represent the mean ± SD of quadruplicates of an experiment repeated twice. (B) Effect of PS341 on myeloma cells in the presence of bone marrow stromal cells (BMSC). Control or HA-Erk5AEF–expressing MM1S cells were plated on wells previously coated, or not, with stromal cells. PS341 (at the indicated concentrations) was added together with the myeloma cells, and the experiment continued for 48 hours. BrdU was added for the last 8 hours. BrdU uptake was measured as described in “Materials and methods.” □ and ▪ indicate control and HA-Erk5AEF–expressing MM1S cells with BMSC, respectively; ▦ and ▧, the same cells without BMSC, respectively. The results represent the mean ± SD of quadruplicates. (C) Action of PS341 on the levels of Erk5. The cell lines shown were treated with 10 nM PS341 for the indicated times, and then Erk5 or Erk1/2 levels were analyzed by Western blotting. (D) Effect of different caspase inhibitors on PS341-induced Erk5 down-regulation. MM1S cells were treated with PS341 in the presence or absence of the different caspase inhibitors (Z-VAD-FMK, 100 μM; Z-IETD-FMK and Z-LEHD-FMK, 50 μM) for 8 hours, and then Erk5 expression was analyzed by Western blotting. An aliquot of the cell extract (30 μg) was used to analyze caspase-3 cleavage by Western blotting. DMSO represents dimethylsulfoxide; Z-VAD-FMK represents Z-Val-Ala-DL-Asp fluoromethylketone; Z-IETD-FMK represents Z-Ile-Glu-Thr-Asp fluoromethylketone; Z-LEHD-FMK represents Z-Leu-Glu-His-Asp fluoromethylketone. (E) Effect of Erk5 overexpression on PS341-induced cell death. Expression of wild-type HA-Erk5 was carried out by transfection of pCDNA3 or pCDNA3-HA-Erk5 into MM1S cells, followed by G418 selection. A pool of transfected MM1S cells was selected, and the expression of exogenous HA-Erk5 was detected by Western blotting with anti-HA or with anti-Erk5 (top). The bottom panel shows the effect of PS341 on control or HA-Erk5–overexpressing MM1S cells. Cells were treated for 24 hours with 10 nM PS341, and the amount of apoptotic cells analyzed by annexin V–FITC staining. Data are represented as the mean ± SD of duplicates of an experiment repeated twice.
Erk5 regulates MM apoptosis. (A) Expression of the dominant-negative form of Erk5 sensitizes MM cells to drug-induced apoptosis. Control (□) or HA-Erk5AEF–expressing (▪) MM1S cells were plated at identical densities and treated for 24 hours with the indicated concentrations of PS341 or dexamethasone. MTT uptake was carried out as described in the text. The results represent the mean ± SD of quadruplicates of an experiment repeated twice. (B) Effect of PS341 on myeloma cells in the presence of bone marrow stromal cells (BMSC). Control or HA-Erk5AEF–expressing MM1S cells were plated on wells previously coated, or not, with stromal cells. PS341 (at the indicated concentrations) was added together with the myeloma cells, and the experiment continued for 48 hours. BrdU was added for the last 8 hours. BrdU uptake was measured as described in “Materials and methods.” □ and ▪ indicate control and HA-Erk5AEF–expressing MM1S cells with BMSC, respectively; ▦ and ▧, the same cells without BMSC, respectively. The results represent the mean ± SD of quadruplicates. (C) Action of PS341 on the levels of Erk5. The cell lines shown were treated with 10 nM PS341 for the indicated times, and then Erk5 or Erk1/2 levels were analyzed by Western blotting. (D) Effect of different caspase inhibitors on PS341-induced Erk5 down-regulation. MM1S cells were treated with PS341 in the presence or absence of the different caspase inhibitors (Z-VAD-FMK, 100 μM; Z-IETD-FMK and Z-LEHD-FMK, 50 μM) for 8 hours, and then Erk5 expression was analyzed by Western blotting. An aliquot of the cell extract (30 μg) was used to analyze caspase-3 cleavage by Western blotting. DMSO represents dimethylsulfoxide; Z-VAD-FMK represents Z-Val-Ala-DL-Asp fluoromethylketone; Z-IETD-FMK represents Z-Ile-Glu-Thr-Asp fluoromethylketone; Z-LEHD-FMK represents Z-Leu-Glu-His-Asp fluoromethylketone. (E) Effect of Erk5 overexpression on PS341-induced cell death. Expression of wild-type HA-Erk5 was carried out by transfection of pCDNA3 or pCDNA3-HA-Erk5 into MM1S cells, followed by G418 selection. A pool of transfected MM1S cells was selected, and the expression of exogenous HA-Erk5 was detected by Western blotting with anti-HA or with anti-Erk5 (top). The bottom panel shows the effect of PS341 on control or HA-Erk5–overexpressing MM1S cells. Cells were treated for 24 hours with 10 nM PS341, and the amount of apoptotic cells analyzed by annexin V–FITC staining. Data are represented as the mean ± SD of duplicates of an experiment repeated twice.
The mechanism by which receptors couple to the Erk5 route has only partially been uncovered. Evidence indicates that Mek5 acts as the Erk5-activating kinase, and we have found that in fact this kinase is phosphorylated by IL-6 in MM cells. Mek5, in turn, can be activated by multiple upstream kinases, including c-cot,27 Mekk2,52 or Mekk3.26 Quantitative PCR analyses of the expression of c-cot, Mekk2, and Mekk3 indicated analogous expression of Mekk2 and Mekk3 in MM1S cells. These cells expressed very low levels of c-cot, suggesting that the latter probably does not act as an important intermediate in IL-6–induced Erk5 activation (R.L.-P. X.C.-V. and A.P., unpublished results, June 2004). On the other hand, expression of a dominant-negative form of Mekk3, created by mutating Lys391 to Trp391,26 did not affect IL-6–induced Erk5 phosphorylation (X.C.-V. and A.P., unpublished results, February 2005), indicating that Mekk3 activity may not be required for IL-6 to activate Erk5. It is therefore possible that MEKK2, or other kinases upstream of Mek5, may be intermediate in the action of IL-6 on Erk5. Additional knowledge into the Erk5 field will be required to identify the molecular components that link receptors to the Erk5 route.
Signaling downstream of Erk5 has been shown to include the regulation of the activity of several transcription factors, especially MEF2 family members. The latter factors have been shown to interact with Erk531 and are phosphorylated at particular sites by Erk5.33 Our analyses indicated that MEF2 proteins are expressed in MM cells and may participate as mediators in the action of Erk5. Whether MEF2 proteins act in the control of MM survival, as reported in other cell types,32,35 or in other aspects of MM biology will require future studies.
The use of a dominant-negative form of Erk5 uncovered multiple functions of this MAPK in MM biology. Expression of the dominant-negative form substantially affected IL-6–induced proliferation of MM1S cells. This is in line with a role of Erk5 in growth factor/cytokine-induced proliferation, as described in other systems.14 Several data indicated that Erk5 may also participate in the control of MM apoptosis. Expression of the dominant-negative form of Erk5 increased the sensitivity of MM cells to PS341 and dexamethasone, 2 compounds that effectively target MM cells.2 Furthermore, the levels of Erk5 were down-regulated by PS341. This effect appears to be analogous to the PS341-induced down-regulation of the gp130 subunit of the IL-6 receptor48 that occurs after caspase activation. In fact, the pan-caspase and caspase-8 inhibitors prevented PS341-induced Erk5 down-regulation. The down-regulation of Erk5 by PS341 was an unexpected and interesting finding that suggested that the decrease of Erk5 could be part of the action of PS341 in the induction of MM cell apoptosis. In fact, overexpression of wild-type Erk5 was found to confer resistance to PS341-induced cell death. Furthermore, preliminary data obtained in plasma cells from 2 MM patients expressing different levels of Erk5 indicated that low levels of endogenous Erk5 sensitize MM cells to TNF-regulated apoptosis-inducing ligand (TRAIL)–induced apoptosis (S.T., X.C.-V., and A.P., unpublished data, May 2004), suggesting that Erk5 levels may modulate apoptosis in response to TRAIL in myeloma cells. The mechanism by which Erk5 may exert this action in myeloma is unclear, but it is noteworthy that in endothelial cells Erk5 has been shown to phosphorylate Bad, preventing its apoptotic effect.17 In the future, and due to the resistance of MM cells to apoptosis, it appears attractive to analyze not only the mechanisms by which the Erk5 route may regulate such cellular process, but also how it could be exploited to improve the efficacy of treatments that cause MM cell death.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2004-08-2985.
Supported by grants from the Spanish Association Against Cancer (AECC), the International Myeloma Foundation, and the ISCIII-FISS through projects to A.P. (01/1060) and J.F.S.M., and through a Spanish Myeloma Network Program. X.C.-V. was supported by a fellowship from Glaxo-CSIC, and A.E.-O. and R.L.-P. from the AECC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal