Abstract
Homing of mast cell progenitors (MCps) to the mouse small intestine involves the interaction of α4β7 integrin with mucosal addressin cellular adhesion molecule-1 (MAdCAM-1). We now demonstrate the dependence of this process on CXC chemokine receptor 2 (CXCR2) and vascular cell adhesion molecule-1 (VCAM-1) using null strains and mice sublethally irradiated and bone marrow (BM) reconstituted (SIBR) with wild-type or null BM or with wild-type BM followed by administration of blocking antibody. The intestinal MCp concentration in CXCR2-/- mice was reduced by 67%, but was unaltered in CC chemokine receptor 2-/- (CCR2-/-), CCR3-/-, or CCR5-/- mice. SIBR mice given CXCR2-/- BM had an intestinal MCp concentration that was 76% less than that in BALB/c BM reconstituted mice. Antibody blockade of VCAM-1 or of CXCR2 in SIBR mice reduced intestinal MCp reconstitution, and mice lacking endothelial VCAM-1 also had a marked reduction relative to wild-type mice. Finally, the half-life of intestinal MCps in wild-type mice was less than one week on the basis of a more than 50% reduction by administration of anti-α4β7 integrin or anti-CXCR2. Thus, the establishment and maintenance of MCps in the small intestine is a dynamic process that requires expression of the α4β7 integrin and the α-chemokine receptor CXCR2.
Introduction
Mast cell progenitors (MCps) are derived from a bone marrow (BM) precursor and are disseminated hematogenously to peripheral tissues. Under the influence of the local microenvironment, they mature into different mast cell (MC) phenotypes broadly classified as mucosal MCs (MMCs) and connective tissue–type MCs (CT-MCs). These phenotypes are distinguished by their locations, distinctive staining characteristics, and differences in the protease and proteoglycan contents of their secretory granules.1-3 We demonstrated that the basal homing of MCps to the intestine is regulated by both the c-kit pathway and the CD49d/β7 (α4β7) integrin and is not influenced by T cells and other leukocytes.4 A deficiency in either c-kit or α4β7 integrin on MCps abrogates the resident intestinal population of both MCps and mature MCs. In antibody-blocking experiments with sublethally irradiated BM-reconstituted (SIBR) mice, mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1) was identified as an important counterligand for α4β7 integrin in coordinating intestinal homing of MCps.4 However, antibody to MAdCAM-1 was less efficient at blocking MCp reconstitution than was antibody to α4β7 integrin, suggesting a role for an additional counterligand.
Because vascular cell adhesion molecule-1 (VCAM-1) is also an endothelial counterligand for the α4β7 integrin,5 we assessed its impact on intestinal MCp homing using both antibody blocking and viable conditional mutants specifically lacking expression of VCAM-1 on endothelial cells and leukocytes.6 Since chemokine receptors provide signals that alter integrin affinities and, consequently, cellular migration in response to chemotactic gradients, we focused on the possible involvement of the chemokine receptors CCR2, CCR3, CCR5, and CXCR2, which have been found on different populations of MCs.7-10 We determined that CXCR2, but not CCR2, CCR3, or CCR5, is required for the migration of MCps into the intestinal reservoir. Furthermore, the administration to wild-type mice of blocking monoclonal antibody (mAb) to α4β7 integrin or of antibody to CXCR2 reveals that the constitutive intestinal MCps are subject to ongoing turnover with a resident half-life of less than 1 week.
Materials and methods
Animals and the sublethal irradiation and reconstitution protocol
Male mice 6 to 16 weeks old were used in these experiments. CXCR2-/- mice (C.129S2(B6)-Il8rbtm1Mwm)11 and their wild-type BALB/cJ controls; the CCR5-/- mice (B6;129P2-Ccr5tm1Kuz)12 and their wild-type B6129PF2/J controls; and wild-type C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CCR2-/- mice13 and monocyte chemoattractant protein-1-/- (MCP-1-/-) mice14 were maintained in the Dana Farber Cancer Institute animal facility and were compared with C57BL/6 mice. CCR3-/- mice15 were maintained in the Children's Hospital (Boston, MA) animal facility and were compared with BALB/c mice. VCAM-1-/- mice6 and the parental control strain, VCAM-1Flox, were maintained at the Medical College of Georgia. Reconstitution of the population of MCps in mice and inhibition of the reconstitution with blocking antibodies were performed as previously described.4 SIBR mice were irradiated in groups of 12 in a Gammacell 40 irradiator (Anatomic Energy of Canada, Ottawa) for 4.5 minutes. This treatment results in an average exposure of 5 Gy per animal. At 2 hours after irradiation, each mouse was intravenously injected with 1 × 107 BM cells isolated from syngeneic or congenic animals. We had previously demonstrated that SIBR mice that received wild-type BM had normal MCp concentrations in the BM after 4 days but required 10 to 12 days to reconstitute fully the MCp concentration in the small intestine.4 Therefore, MCp concentrations in the various tissues of mice receiving BM from null strains and from wild-type controls were analyzed after 14 days. Institutional Animal Care and Use Committee approval was obtained for all animal experiments, and the studies were carried out in accordance with the National Institutes of Health and Public Health Service guidelines for animal care.
Antibodies and the blocking of homing protocol
Monoclonal anti-KC antibody MAB453 (rat immunoglobulin G2a [IgG2a], 48415.111), anti–macrophage inflammatory protein-2 (MIP2) antibody MAB452 (rat-IgG2b, 40605.111), polyclonal anti–lipopolysaccharide-induced CXC chemokine (LIX) antibody AF433 (goat-IgG), and control polyclonal goat IgG (catalog no. AB-108-C) were obtained from R&D Systems (Minneapolis, MN). The monoclonal anti–VCAM-1 antibody 429 (MVCAM.A, rat IgG2a), monoclonal anti–MAdCAM-1 antibody MECA-367 (rat IgG2a,), anti-β1 integrin (9EG7, rat IgG2a), and anti-α4β7 integrin (DATK32, rat IgG2a) were obtained from Pharmingen (San Diego, CA). Affinity-purified antibody (Proteintech, Chicago, IL) against CXCR2 was generated in rabbits with the peptide Met-Gly-Glu-Phe-Lys-Val-Asp-Lys-Phe-Asn-Ile-Glu-Asp-Phe-Phe-Ser-Gly, which maps to the chemokine binding site.16 Normal rabbit IgG (catalog no. 42-530) was obtained from Antibodies Incorporated (Davis, CA). All antibodies containing sodium azide were dialyzed against Hanks balanced salt solution (HBSS) to eliminate the azide.
To evaluate the molecules important in tissue homing of MCps, approximately 25 μg of an isotype control or of a blocking antibody in 100 μL HBSS was administered intraperitoneally to SIBR BALB/c mice reconstituted with wild-type BALB/c BM cells. Injections were given every other day starting 2 days after SIBR, and MCp populations were evaluated on day 7 as previously described.4 This procedure minimizes the number of injections and examines the blocking on the linear upslope of the intestinal MCp reconstitution curve.
Homeostasis of the intestinal MCp pool was assessed with wild-type BALB/cJ mice that received 4 injections of saline, anti-β1 integrin, anti-α4β7 integrin, normal rabbit IgG, or anti-CXCR2. These antibodies (25 μg in 100 μL HBSS) were injected intraperitoneally every other day, and MCp numbers were evaluated 48 hours after the last injection.
Mononuclear cell preparation and MCp assessment
Mice were killed by CO2 asphyxiation and the small intestine, lungs, spleen, and BM were harvested. The entire organs were removed except for BM, where a single femur was taken from each mouse. Individual tissues from 2 mice were pooled, placed in 20 mL RPMI 1640 complete (RPMI 1640 containing 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μg/mL gentamicin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 10% heat-inactivated fetal calf serum [Sigma-Aldrich no. F2442, St. Louis, MO]), and processed essentially as previously described.4 Briefly, the intestines (flushed out and rinsed twice in HBSS) and lungs were finely chopped with scalpels and transferred separately to 50-mL plastic tubes with 30 mL RPMI 1640 complete plus 1 mg/mL collagenase Type 4 (Worthington, Lakewood, NJ). There were 3 enzymatic digestions carried out for approximately 20 minutes each at 37°C. The undigested tissue clumps were collected after each digestion period and were subjected to another enzymatic digestion, while the liberated cells were pelleted, resuspended in 44% Percoll (Sigma-Aldrich no. P1644, St Louis, MO), overlayed on a 67% Percoll layer, and spun at 400g for 20 minutes at 4°C. The cell collection procedure for BM and spleen omitted the digestion steps. BM was extruded from one femur of each animal using a 25-gauge syringe and 5 to 10 mL RPMI 1640 complete. Spleen cells were obtained from crushed whole spleens suspended in RPMI 1640 complete. The collected cells were pelleted and resuspended in 44% Percoll before centrifugation over 67% Percoll as described.
The mononuclear cells (MNCs) were harvested from the interfaces of the 3 digestions of the lung and intestine, pooled by separate tissue source, and washed in RPMI 1640 complete. The numbers of viable cells were determined by trypan blue dye exclusion with a hemocytometer. Cells were serially diluted in RPMI 1640 complete, and 100-μL samples of the MNC dilutions were added to each well of standard 96-well flat-bottomed microtiter plates (Corning no. 3596, Corning, NY). Typically, 24 wells were plated for each cell concentration. Intestinal or BM MNCs were plated starting at 5000 to 10 000 cells/well, and lung or spleen MNCs starting at 20 000 to 40 000 cells/well. Then, each well received 100 μL gamma-irradiated (30 Gy) splenic feeder cells plus cytokines (recombinant mouse interleukin-3 [IL-3] at 20 ng/mL and recombinant mouse stem cell factor [SCF] at 100 ng/mL).
The cultures were incubated in humidified 37°C incubators with 5% CO2 for 12 to 14 days, and positive wells containing mast cell colonies were identified and counted with an inverted microscope. The MC colonies were easily distinguished as large colonies of nonadherent small- to mediumsized cells.17,18 The MCp concentration is expressed as the number of MCps per 106 MNCs isolated from the tissue. The number of MCps/tissue is derived by multiplying the concentration of MCps by the MNC yield/organ.
RT-PCR of CXCR2 and CXCR2 ligands
Whole RNA was isolated from the lung, liver, intestine, and spleen of BALB/c and CXCR2-/- mice and from BM-derived MCs (BMMCs) of these strains after 4 weeks of culture in RPMI 1640 complete supplemented with IL-3 (10 ng/mL). Approximately 5- to 10-mm blocks of tissue or 5 × 105 BMMCs were homogenized in 1 mL Tri-Reagent (Sigma-Aldrich no. T9424) at room temperature over 5 minutes. Then, 0.2 mL chloroform was added. The homogenates were stored in the dark overnight at 4°C, followed by centrifugation at 12 000g for 15 minutes. The aqueous phase was collected and precipitated with 0.5 mL isopropanol. The precipitate was collected by centrifugation at 12 000g for 15 minutes, washed with 75% ethanol, centrifuged at 12 000g for 15 minutes, and resuspended in RNase-free H2O.19 Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on isolated tissue RNAs with an Advantage RT-for-PCR Kit (Clontech no. 639505, Palo Alto, CA) with oligo-dT18 primers. For each CXCR2 ligand and its receptor, PCR was performed with the Clontech Titanium Taq PCR Kit (no. 639210). All primers used spanned at least one exon-intron-exon boundary and included the following: neutrophil activating protein-2 (NAP2): 5′-ATCTCCCTTGTGAATGTG-3′, 5′-GTTGCAGAGGTTGCTTG-3′; LIX: 5′-ATTGATCGCTAATTTGGAGG-3′, 5′-ATCACAGGAGCTTCTGGATC-3′; MIP2: 5′-CCACTCTCAAGGGCGGTCAAA-3′, 5′-TACGATCCAGGCTTCCCGGGT-3′; KC: 5′-CGCTGCTGCTGCTGGCCACCA-3′, 5′-GGCTATGACTTCGGTTTGGGTGCAG-3′; CXCR2: 5′-CCTGACAGCTCCCAAGCCTT-3′, 5′-GGCAAGGTCAGGGCAAAGAA-3′; and actin: 5′-GTGGGCCGCTCTAGGCACCAA-3′, 5′-CTCTTTGATGTCACGCACGATTTC-3′. Primers were based on published sequences.20-27 Typical PCR conditions were as follows: an initial denaturation at 95°C for 1 minute followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 60 seconds, extension at 68°C for 1 minute, and a final extension at 68°C for 3 minutes using a PTC-200 (MJ Research, Reno, NV). The products were analyzed on 1% agarose gels.
Statistical analysis
Data were expressed as the mean ± SEM for values derived from 3 or more experiments and mean ± ½ range for 2 experiments. Significance was determined using a 2-tailed Student t test. Values of P < .05 were considered significant.
Results
Effect of CXCR2, CCR2, CCR3, and CCR5 deficiencies on basal MCp homing to the intestine
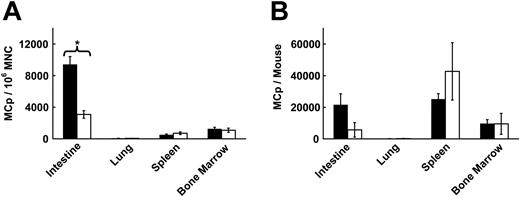
Immature BMMCs and cord blood–derived cultured human MCs express functional CXCR2, CCR2, CCR3, and CCR5.7-9 To evaluate the role of these receptors in MCp homing to small intestine, mouse strains with a targeted disruption of each of these receptors were examined for the concentrations of tissue MCps and for the absolute number of MCps per organ. CXCR2-/- mice displayed a marked reduction in intestinal MCp concentration (67% reduction, P < .001, Figure 1A) and total number (74% reduction, Figure 1B). The difference in total intestinal MCp number did not reach statistical significance (P = .06, n = 5). The CXCR2-/- and BALB/c control mice had similar concentrations and similar absolute numbers of MCps in their lungs and BM. The spleen MCp concentration was not different in these mice, but there was a 1.9-fold increase in the absolute MCp number in the spleen of the CXCR2-/- mice (Figure 1B). This increase was attributed to the increase in organ size and myeloid precursors, as noted by others.11,28 In contrast to the CXCR2-/- mice, there were no significant differences in the MCp concentration (Table 1) or the absolute number of MCps (data not shown) in the intestine of CCR3-/- or CCR5-/- animals compared with their controls. In 2 experiments, CCR2 deficiency had no impact on the MCp concentration (Table 1) or absolute number in the small intestine (data not shown), and mice lacking the CCR2 ligand MCP-1 also showed no diminution in intestinal MCp concentrations (Table 1).
MCp concentrations in the small intestine of chemokine receptor-deficient and chemokine-deficient mice and their wild-type controls
Mouse strains/wild-type control . | Intestine . | Spleen . | Bone marrow . |
|---|---|---|---|
| CCR2-/- | 1758 ± 621 (2) | ND | ND |
| C57BL/6J | 1819 ± 822 (2) | ND | ND |
| CCR3-/- | 8104 ± 2513 (3) | 208 (1) | 650 (1) |
| BALB/cJ | 7143 ± 3830 (3) | 237 (1) | 917 (1) |
| CCR5-/- | 946 ± 246 (3) | 161 (1) | 412 ± 79 (2) |
| B6.129(PF2) | 902 ± 336 (3) | 258 (1) | 529 ± 45 (2) |
| MCP-1-/- | 1523 (1) | ND | 424 (1) |
| C57BL/6J | 724 (1) | ND | 431 (1) |
Mouse strains/wild-type control . | Intestine . | Spleen . | Bone marrow . |
|---|---|---|---|
| CCR2-/- | 1758 ± 621 (2) | ND | ND |
| C57BL/6J | 1819 ± 822 (2) | ND | ND |
| CCR3-/- | 8104 ± 2513 (3) | 208 (1) | 650 (1) |
| BALB/cJ | 7143 ± 3830 (3) | 237 (1) | 917 (1) |
| CCR5-/- | 946 ± 246 (3) | 161 (1) | 412 ± 79 (2) |
| B6.129(PF2) | 902 ± 336 (3) | 258 (1) | 529 ± 45 (2) |
| MCP-1-/- | 1523 (1) | ND | 424 (1) |
| C57BL/6J | 724 (1) | ND | 431 (1) |
Values are the mean MCp concentration (± SEM for n = 3, or ½ range for n = 2) in the indicated tissue from the various mice. Deficient mice and their wild-type controls (listed directly below) were evaluated in parallel. The number of experiments is indicated in parentheses.
ND indicates not determined.
Reconstitution of MCp in SIBR mice with BM cells from CXCR2-/- or BALB/c mice
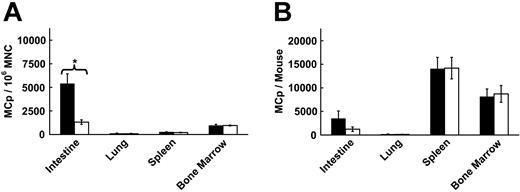
Our findings with the CXCR2-/- strain demonstrate that the receptor is important in the migration of MCp into the small intestine. However, they do not establish the requirement for CXCR2 expression by MCps. To confirm that CXCR2 must be expressed by the MCps to direct their homing to the small intestine, we depleted endogenous intestinal MCps in wild-type BALB/c mice by sublethal irradiation and reconstituted the mice with BM from either wild-type or CXCR2-/- mice. Compared with wild-type SIBR mice reconstituted with BALB/c BM, the intestinal MCp concentration of mice receiving BM from CXCR2-/- mice was 76% less (P < .01, Figure 2A) and the absolute number of intestinal MCps was 64% less (Figure 2B). In contrast, lung, spleen, and BM MCp populations showed no significant variation between mice reconstituted with either CXCR2-/- or BALB/c BM cells (Figure 2) whether evaluated by the MCp concentration or by absolute number of MCps/organ. The MCp concentration in sublethally irradiated mice that did not receive the adoptive transfer was minimal for all tissues (187 ± 122 MCps per 106 MNCs in the small intestine). Hence, the adoptively transferred BM from BALB/c and CXCR2-/- strains permitted the reconstitution of MCps rather than residual host hematopoietic stem cells.
Selective loss of MCps in the intestine of CXCR2-/- mice. (A) MCp concentrations in the intestine, lung, spleen, and BM of wild-type BALB/c mice (▪) and CXCR2-/- mice (□) evaluated in parallel. Concentrations are expressed as MCps per 106 MNCs isolated from the various tissues. Values are the mean ± SEM for 5 separate experiments, except for BM where values are the mean ± ½ range for 2 separate experiments. *Statistical significance, with P < .001 relative to BALB/c mice. (B) Absolute numbers of MCps per tissue for the same tissues shown in panel A.
Selective loss of MCps in the intestine of CXCR2-/- mice. (A) MCp concentrations in the intestine, lung, spleen, and BM of wild-type BALB/c mice (▪) and CXCR2-/- mice (□) evaluated in parallel. Concentrations are expressed as MCps per 106 MNCs isolated from the various tissues. Values are the mean ± SEM for 5 separate experiments, except for BM where values are the mean ± ½ range for 2 separate experiments. *Statistical significance, with P < .001 relative to BALB/c mice. (B) Absolute numbers of MCps per tissue for the same tissues shown in panel A.
Antibody blocking with anti-CXCR2 and antichemokine antibodies
Several ligands for the mouse CXCR2 receptor have been identified including MIP-2, KC, LIX, and NAP-2.21,29,30 However, no direct homolog to human IL-8 has been identified in the mouse.31 As a guide for antibody-blocking studies of ligands for CXCR2 that might account for its role in intestinal migration, we used RT-PCR in BALB/cJ mice. Steady-state levels of MIP-2, KC, and LIX mRNAs, but not NAP-2 mRNA, were detected in total RNA from the intestines of both CXCR2-/- and wild-type mice. All of the listed chemokines could be identified in the lung, liver, and spleen. The CXCR2 mRNA was also identified in the lung and spleen, but not in the liver or intestine. Steady-state expression of CXCR2 mRNA, as well as the mRNAs encoding each of the CXCR2 ligands, was also detected in total RNA obtained from mouse BMMCs. Tissues and BMMCs from CXCR2-/- mice revealed no qualitative differences in constitutive mRNA expression compared with BALB/c mice (data not shown).
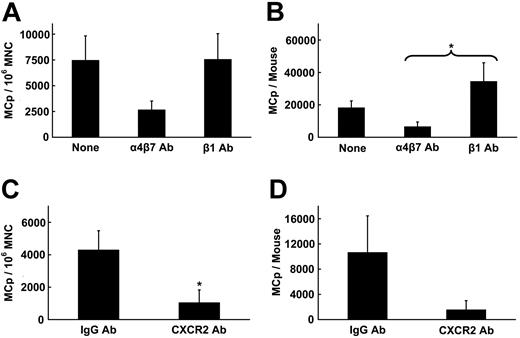
Since several of the known ligands for CXCR2 were expressed constitutively, we attempted to block basal intestinal migration of MCps in SIBR mice using a cocktail of the available antibodies to LIX, KC, and MIP-2. In 2 experiments, there was no inhibition of homing of MCps to the intestine. However, blockade of CXCR2 with affinity-purified rabbit anti-CXCR2 peptide IgG in SIBR mice inhibited reconstitution of the intestinal MCp concentration by 78% ± 0% (mean ± ½ range, n = 2) and the absolute number by 82% ± 5% relative to the values in mice injected with control IgG. Mice receiving anti-CXCR2 antibodies had a mean of 77 ± 13 MCps/106 MNCs, and 130 ± 22 total intestinal MCps/mouse, whereas mice receiving control Ig had 349 ± 63 MCps/106 MNCs and 734 ± 109 total intestinal MCps/mouse. As in mice receiving anti-α4β7 integrin mAbs, the inhibition in the intestine was accompanied by a 2-fold increase in both the concentration and in the absolute number of MCps found in the spleen (data not shown).
Reduced intestinal MCps in SIBR BALB/c mice receiving CXCR2-/- BM. (A) MCp concentrations in the intestine, lung, spleen, and BM in SIBR mice receiving BALB/c BM (▪) and SIBR mice receiving CXCR2-/- BM (□) evaluated in parallel. Values are the mean ± SEM for 4 separate experiments except BM, where values are the mean ± SEM for 3 separate experiments. *Statistical significance, with P < .01 relative to wild-type BM-reconstituted mice. (B) The absolute numbers of MCps per tissue for the same tissues shown in panel A.
Reduced intestinal MCps in SIBR BALB/c mice receiving CXCR2-/- BM. (A) MCp concentrations in the intestine, lung, spleen, and BM in SIBR mice receiving BALB/c BM (▪) and SIBR mice receiving CXCR2-/- BM (□) evaluated in parallel. Values are the mean ± SEM for 4 separate experiments except BM, where values are the mean ± SEM for 3 separate experiments. *Statistical significance, with P < .01 relative to wild-type BM-reconstituted mice. (B) The absolute numbers of MCps per tissue for the same tissues shown in panel A.
Reduced intestinal MCp reconstitution in SIBR mice by treatment with anti–MAdCAM-1 or anti–VCAM-1 and in VCAM-1-/- mice. (A-B) Bars represent the intestinal MCp concentrations (A) and the absolute number of intestinal MCps (B) in SIBR mice reconstituted with wild-type BM cells and treated with saline or antibody (Ab) directed against the indicated endothelial ligands. Values are the mean ± SEM for 4 experiments. (C-D) Bars represent the intestinal MCp concentrations (C) and the absolute number of intestinal MCps (D) in VCAM-1Flox and in VCAM-/- mice evaluated in parallel. Values are the mean ± SEM for 3 experiments.
Reduced intestinal MCp reconstitution in SIBR mice by treatment with anti–MAdCAM-1 or anti–VCAM-1 and in VCAM-1-/- mice. (A-B) Bars represent the intestinal MCp concentrations (A) and the absolute number of intestinal MCps (B) in SIBR mice reconstituted with wild-type BM cells and treated with saline or antibody (Ab) directed against the indicated endothelial ligands. Values are the mean ± SEM for 4 experiments. (C-D) Bars represent the intestinal MCp concentrations (C) and the absolute number of intestinal MCps (D) in VCAM-1Flox and in VCAM-/- mice evaluated in parallel. Values are the mean ± SEM for 3 experiments.
Effect of endothelial/leukocyte VCAM-1 deficiency on basal MCp homing to the intestine
We previously demonstrated an absolute requirement for the α4β7 integrin for directing the basal migration of MCps into the small intestine. However, based on incomplete antibody blocking by anti–MAdCAM-1 in SIBR mice, endothelial MAdCAM-1 is only partially required.4 An alternative endothelial ligand for α4β7 is VCAM-1,5 which has been identified on the endothelial surface of the intestine under both basal and inflamed conditions.32 To test whether VCAM-1 contributes to intestinal MCp homing, SIBR mice were given antibody directed against MAdCAM-1 or VCAM-1 and evaluated 7 days after reconstitution. Compared with SIBR mice given saline, in mice given anti–VCAM-1, the MCp concentration in the small intestine was reduced by 50% ± 12% (mean ± SEM, n = 4, Figure 3A) and the total number of MCps was reduced by 60% ± 15% (Figure 3B). In comparison, in mice given anti–MAdCAM-1, the MCp concentration in the small intestine was reduced by 65% ± 15% (mean ± SEM, n = 4) and the total number of MCps in the intestine was reduced by 84% ± 7%.
To confirm these results, intestinal MCp numbers were evaluated in mice lacking VCAM-1. Mice that are completely deficient in VCAM-1 do not survive through embryogenesis due to defects in the placenta and in cardiac development.33 We therefore used a conditional mutant that deletes VCAM-1 expression by endothelial cells and leukocytes without affecting mouse viability.6 The VCAM-1-/- mice are generated by intercrossing Tie2Cre mice with floxed VCAM-1 transgenic mice (VCAM-1Flox), both of which have been backcrossed to C57BL/6 mice. We compared the number of MCps in the intestine of VCAM-1-/- mice with the VCAM-1Flox parental strain. Compared with the VCAM-1Flox mice, the MCp concentration in the intestine of the VCAM-1-/- mice was reduced by 61% ± 9% (mean ± SEM, n = 3, Figure 3C), while the total number of MCps was reduced by 46% ± 15% (Figure 3D). No reduction in MCp concentration was observed in the lung, spleen, or BM of VCAM-1-/- mice (data not shown).
Dynamic nature of the intestinal MCp reservoir in normal mice
The identification of molecules responsible for tissue-specific homing to the small intestine and the observation that the loss of MCps in this tissue after irradiation is reversed only after reconstitution of the recipient's BM suggest that MCp homing to the small intestine is a normal and ongoing homeostatic process. If this hypothesis is correct, an antibody capable of blocking homing should deplete intestinal MCp. Thus, wild-type BALB/c mice were treated every other day for 1 week with the anti-α4β7 integrin antibody DATK32 that was previously demonstrated to block homing in SIBR mice, with an isotype-matched anti-β1 integrin antibody that had no effect in SIBR mice,4 or with antibody to CXCR2, and their intestinal MCp populations were evaluated 2 days after the fourth injection. Compared with saline-injected normal mice, mice receiving antibody directed against β1 integrins had no reduction in the concentration or total number of intestinal MCps, whereas in mice receiving anti-α4β7 integrin antibody the MCp concentration was reduced by 66% ± 8% (mean ± SEM, n = 4, Figure 4A) and the absolute number was reduced by 66% ± 20% (Figure 4B). Compared with mice given control IgG, the MCp concentration in mice given anti-CXCR2 was reduced by 82% ± 11% (mean ± SEM, n = 3, P < .02, Figure 4C) and the absolute number was reduced by 92% ± 6% (Figure 4D). This reduction by either the anti-α4β7 or the anti-CXCR2 antibody was tissue specific as there was an increase in the absolute number of MCps in the spleen (n = 3) (data not shown). This finding with antibody blocking in wild-type mice is consistent with our previous findings of an increase in MCps in the lung in SIBR mice after blocking intestinal reconstitution with anti-α4β7 integrin antibody4 and argues against cytotoxic depletion of MCp by these antibodies. Mice receiving only 2 injections of anti-α4β7 had no reduction in intestinal MCp concentrations when enumerated 2 days after the second injection (data not shown). The more than 50% reduction in the total number of MCps in the mice given anti-α4β7 integrin or anti-CXCR2 antibody suggests that the half-life of MCp in the intestinal pool is less than 1 week.
Reduced intestinal MCp reservoir in wild-type mice by treatment with anti-α4β7 integrin and anti-CXCR2 antibodies. (A-B) Bars represent the intestinal MCp concentrations (A) and the absolute number of intestinal MCps (B) in mice treated with saline or antibody directed against the indicated integrins. Values are the mean ± SEM for 4 experiments. *Statistical significance (P < .05) relative to mice treated with anti-β1 integrin antibody. (C-D) Bars represent the intestinal MCp concentrations (C) and the absolute number of intestinal MCps (D) in mice treated with normal rabbit IgG or antibody directed against the chemokine receptor CXCR2. Values are the mean ± SEM for 3 experiments. *Statistical significance (P < .05) relative to mice treated with normal rabbit IgG.
Reduced intestinal MCp reservoir in wild-type mice by treatment with anti-α4β7 integrin and anti-CXCR2 antibodies. (A-B) Bars represent the intestinal MCp concentrations (A) and the absolute number of intestinal MCps (B) in mice treated with saline or antibody directed against the indicated integrins. Values are the mean ± SEM for 4 experiments. *Statistical significance (P < .05) relative to mice treated with anti-β1 integrin antibody. (C-D) Bars represent the intestinal MCp concentrations (C) and the absolute number of intestinal MCps (D) in mice treated with normal rabbit IgG or antibody directed against the chemokine receptor CXCR2. Values are the mean ± SEM for 3 experiments. *Statistical significance (P < .05) relative to mice treated with normal rabbit IgG.
Discussion
The homing of immature MCps to the small intestine depends on the α4β7 integrin interacting at least in part with the endothelial ligand MAdCAM-1.4 We now report that the transendothelial movement of MCps into intestinal tissue under basal conditions requires that MCp progenitors express CXCR2 and that the α4β7 integrin also interacts with endothelial VCAM-1. A mouse strain conditionally deficient in endothelial VCAM-1 had a marked reduction in intestinal MCps, and a role for VCAM-1 was supported by the effect of blocking antibody in the reconstitution of irradiated wild-type mice with wild-type BM (Figure 3). Since antibody blocking of either MAdCAM-14 or VCAM-1 reduced reconstitution of intestinal MCps, it appears that both of the endothelial ligands of α4β7 integrins participate in the homing of the MCps into the small intestine. In addition, the restricted distribution under basal conditions of these endothelial counterligands provides the basis for the tissue-specific homing of MCps via the α4β7 integrin.32,34
Tissue-specific homing of leukocytes involves addressins, integrins, and chemokines in a process in which the latter molecules up-regulate the affinity of the integrins on the leukocyte to achieve firm adhesion to the endothelium and then direct migration into the appropriate tissue.35 The recognition that an MCp uses the α4β7 in partnership with either MAdCAM-1 or VCAM-1 for transendothelial migration to the small intestine led to an initial screen of candidate chemokine receptors regulating MCp homing to this tissue among available null strains. The CXCR2 chemokine receptor was identified by the low numbers of intestinal MCps in the null strain (Figure 1) compared with normal numbers in the CCR2, CCR3, and CCR5 null strains (Table 1). The essential role of CXCR2 in directing MCp migration to the small intestine was confirmed and shown to be due to autologous expression of this receptor on the MCp by the poor reconstitution obtained by adoptive transfer of CXCR2-/- BM compared with wild-type BM, into MCp-depleted recipients (Figure 2). Blocking antibody to CXCR2 reduced reconstitution in irradiated recipients and depleted intestinal MCps in wild-type mice (Figure 4). In contrast, splenic MCps in these mice were increased somewhat, suggesting that the spleen, which should be in equilibrium with the blood, was reflecting increased numbers in the circulation and indicating that the inhibition was not due to cytotoxicity of the antibodies. Although we were able to demonstrate the transcriptional expression of several CXCR2 ligands, we were unable to block MCp homing with a combination of antibodies directed to these ligands. Several explanations are possible. There may be an as-yet-unidentified ligand that is either critical to or additive to the other ligands in directing the migration of the MCps to the small intestine. Alternatively, the affinities or amounts of antibodies injected were simply insufficient. While these results do not identify the ligands that interact with CXCR2 and regulate tissue-specific migration, the selectively decreased numbers of small intestine MCps relative to control animals in CXCR2 null mice (Figure 1), in SIBR BALB/c mice reconstituted with CXCR2 null BM (Figure 2), in SIBR BALB/c mice reconstituted with BALB/c BM and injected with anti-CXCR2 antibody, and in wild-type BALB/c mice injected with anti-CXCR2 antibody (Figure 4) indicate that CXCR2 plays a critical role in establishing the MCp pool in the small intestine.
The marked reduction in intestinal MCps and mature MCs in β7 integrin null mice4,36 and the pattern of reconstitution of the progenitor pool in SIBR mice suggests that homing is a constitutive, ongoing process. This view is supported by our findings that BM reconstitution of the MCp population in irradiated recipients begins with engraftment of recipient BM and not with the intestine. The recipient BM was fully reconstituted for MCps by day 4, whereas the small intestine still lacked these cells and began reconstitution only after this time point.4 Furthermore, when administration of blocking antibody to α4β7 was delayed for up to 4 days after provision of the donor BM, reconstitution of intestinal MCps was suppressed as effectively as when a blocking schedule began on the day of BM administration. That donor BM was processed in recipient BM so as to enter the MC lineage before distribution to the small intestine argues for a continuing constitutive supply of MCps from BM to peripheral tissues. The ongoing nature of the peripheral distribution of MCps to the intestine was verified by the ability of antibodies to α4β7 integrin and to CXCR2 to reduce the size of the intestinal MCp pool of wild-type BALB/c mice by more than 50% over a 1-week period of administration (Figure 4). Antibody directed against the β1 integrin or control Ig did not cause any reduction in the same period. Thus, MCps in the intestine represent a dynamic population of cells with a short tissue half-life that appears to undergo constant replenishment.
The pathways that regulate the basal homing of MCps to the intestine appear to be dominated by autologous c-kit, α4β7 integrin, and CXCR2. That the targeted disruption of phosphoinositide-3 kinase (PI3K) results in the absence of mature intestinal MCs suggests an additional requirement.37 Moreover, SCF activation of c-kit acting through PI3K appears to stimulate the migration of BMMCs through the α4 integrin.38 In rat cardiac-derived endothelial cells, activation of CXCR2 with its ligand LIX results in activation of PI3K and amplification of cytokine production.39 Furthermore, in lymphoblastoid JY cells stably transfected with human CXCR2, stimulation with the CXCR2 ligand IL-8 resulted in an α4β7-dependent transient adhesion to VCAM-1.40 Together, these data suggest that circulating MCps that express CXCR2 and c-kit could respond to the respective ligands for each via PI3K so as to up-regulate the affinity of the α4β7 integrin for its endothelial ligands, MAdCAM-1 and VCAM-1. This pathway allows for tissue-specific migration signals to sustain the intestinal pool of MCps. Our model receives additional support from the findings that MCps in the small intestine of wild-type mice can be depleted directly by administration of blocking antibody to α4β7 integrin or to CXCR2 over a 1-week period.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-09-3578.
Supported by grants from the National Institutes of Health: AI-057991, HL-036110, AI-031599, and AI-047379.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal